Chemical Characterization of a Renoprotective Metabolite from Termite-Associated Streptomyces sp. RB1 against Cisplatin-Induced Cytotoxicity
Abstract
:1. Introduction
2. Results
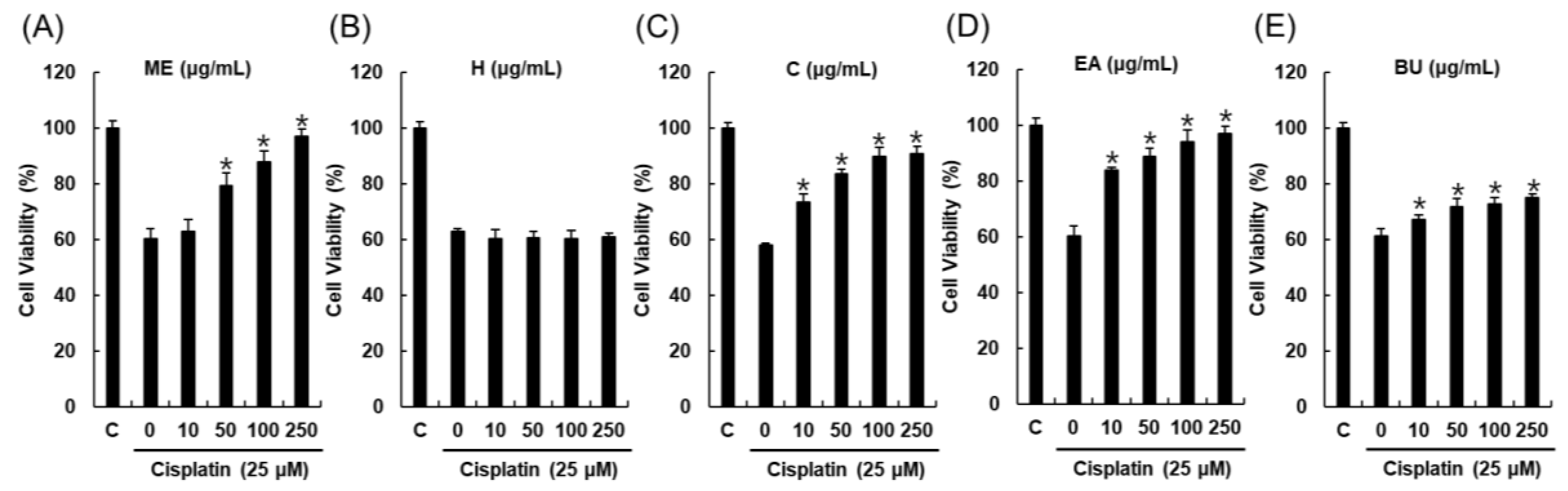
2.1. Bioactivity-Guided Fractionation and Isolation of a Renoprotective Metabolite
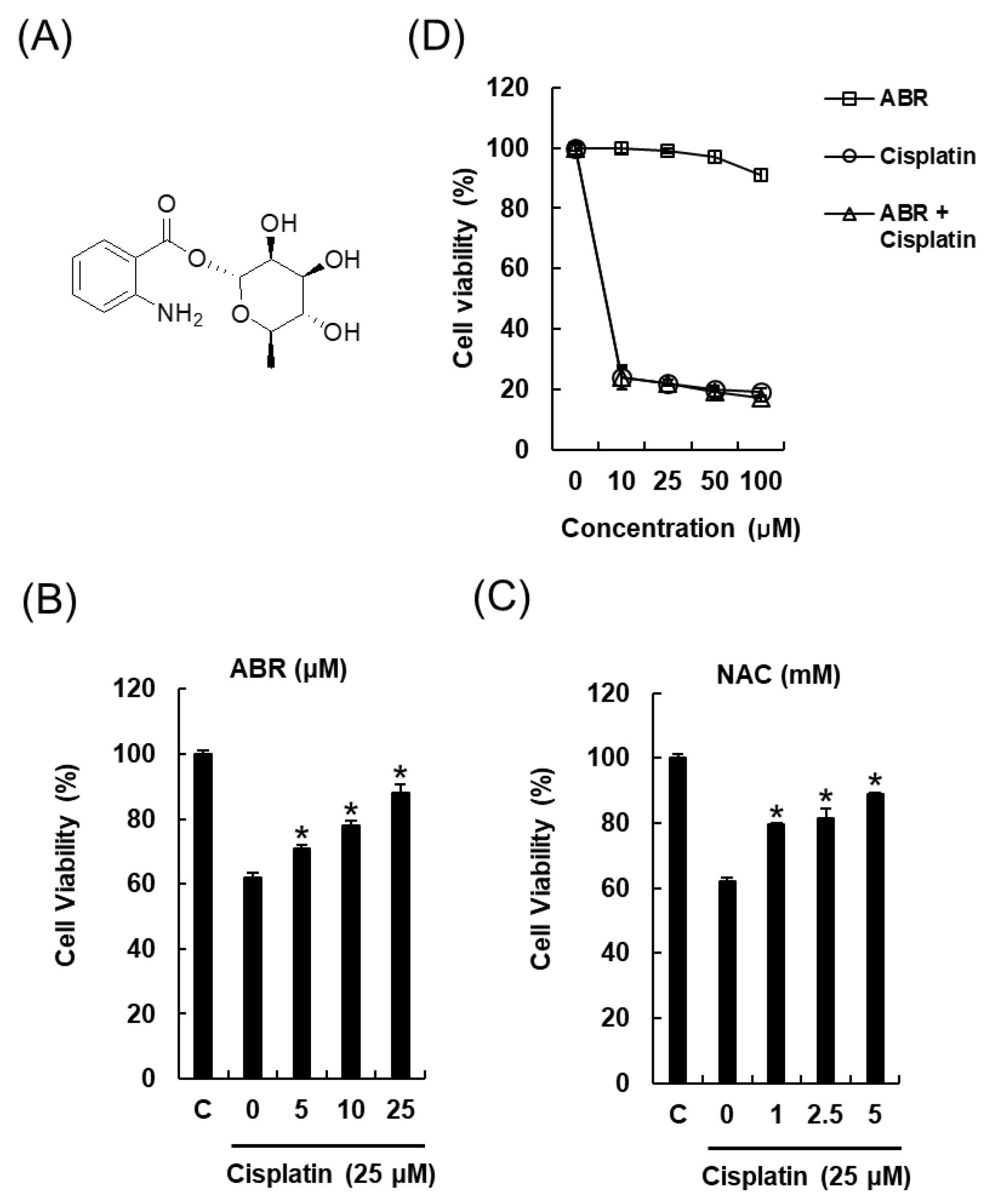
2.2. Protective Effects of 1-O-(2-aminobenzoyl)-α-l-rhamnopyranoside (ABR) in LLC-PK1 Cells Exposed to 25 μM of Cisplatin for 24 h by MTT Assay
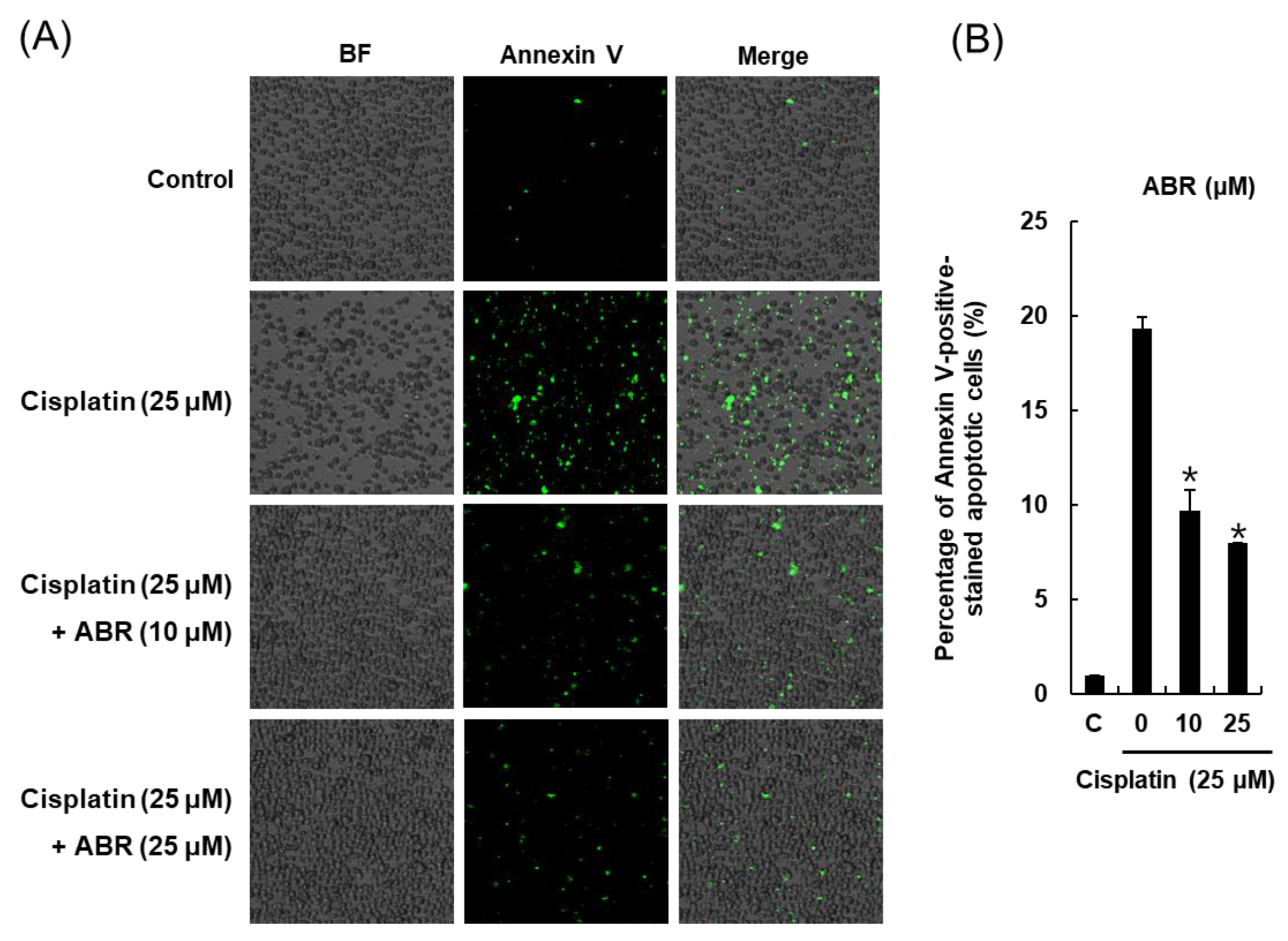
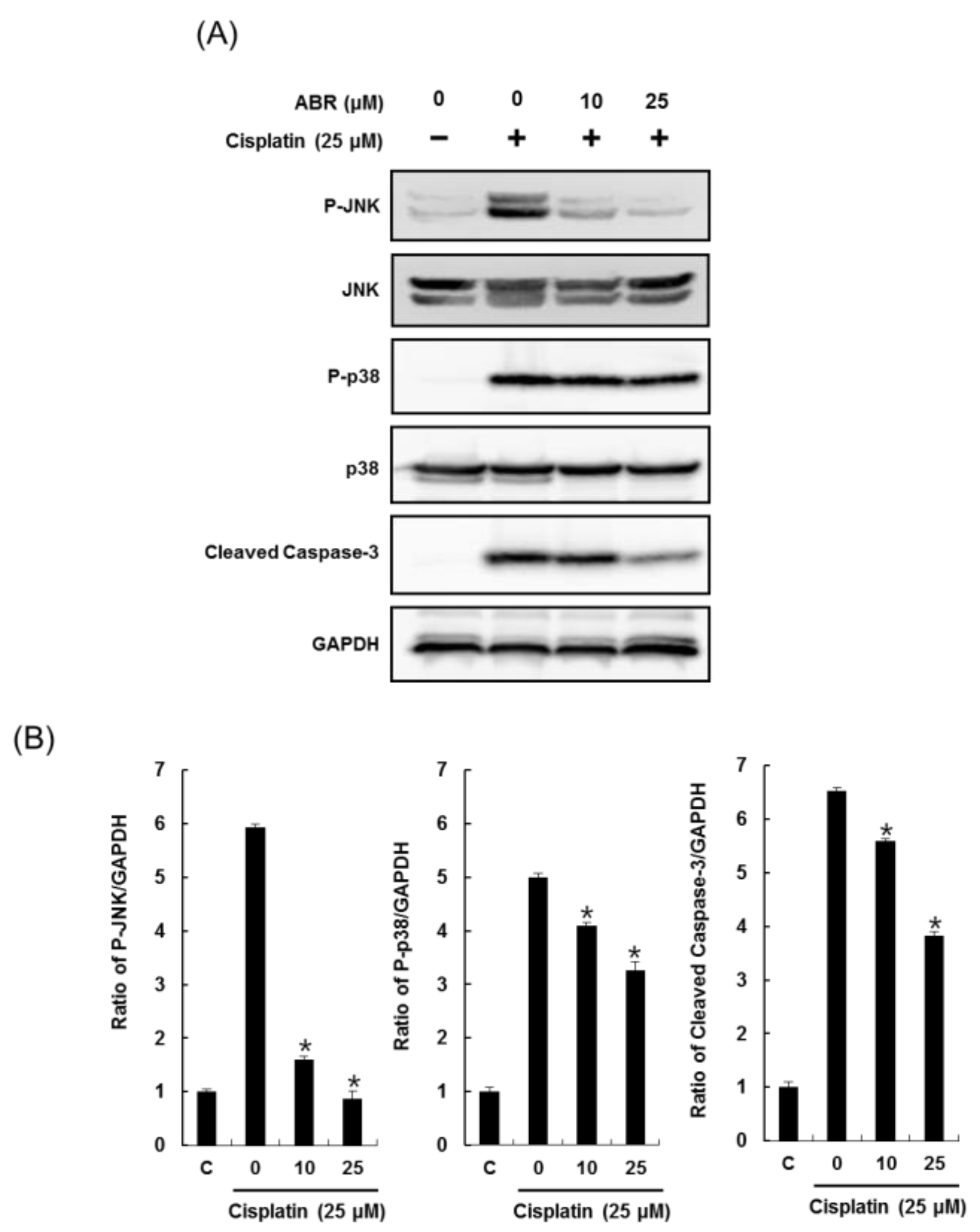
2.3. Effects of ABR on Apoptosis in LLC-PK1 Cells Exposed to Cisplatin
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Extraction and Isolation
4.3. Cell Culture and MTT Cell Viability Assay
4.4. Image-Based Cytometric Assay
4.5. Western Blotting Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gomez-Ruiz, S.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. On the discovery, biological effects, and use of cisplatin and metallocenes in anticancer chemotherapy. Bioinorg. Chem. Appl. 2012, 2012, 140284. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.N.; Liu, Z.; Wang, Z.; Ren, S.; Tang, S.; Wang, Y.P.; Xiao, S.Y.; Chen, C.; Li, W. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-κB signaling pathways. Food Chem. Toxicol. 2017, 110, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S.; Schnellmann, R.G. Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and -independent pathways. J. Pharmacol. Exp. Ther. 2002, 302, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Kim, H.-J.; Shen, A.H.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.-S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kidera, Y.; Kawakami, H.; Sakiyama, T.; Okamoto, K.; Tanaka, K.; Takeda, M.; Kaneda, H.; Nishina, S.; Tsurutani, J.; Fujiwara, K.; et al. Risk factors for cisplatin-induced nephrotoxicity and potential of magnesium supplementation for renal Protection. PLoS ONE 2014, 9, e101902. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Lee, D.; Eom, H.J.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Identification and mechanism of action of renoprotective constituents from peat moss Sphagnum palustre in cisplatin-induced nephrotoxicity. J. Funct. Foods 2016, 20, 358–368. [Google Scholar] [CrossRef]
- Hanigan, M.H.; Devarajan, P. Cisplatin nephrotoxicity: Molecular mechanisms. Cancer Ther. 2003, 1, 47–61. [Google Scholar] [PubMed]
- Rodríguez-García, M.E.; Quiroga, A.G.; Castro, J.; Ortiz, A.; Aller, P.; Mata, F. Inhibition of p38-MAPK potentiates cisplatin-induced apoptosis via GSH depletion and increases intracellular drug accumulation in growth-arrested kidney tubular epithelial cells. Toxicol. Sci. 2009, 111, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Sapiro, J.M.; Monks, T.J.; Lau, S.S. All-trans-retinoic acid-mediated cytoprotection in LLC-PK1 renal epithelial cells is coupled to p-ERK activation in a ROS-independent manner. Am. J. Physiol. Renal. Physiol. 2017, 313, F1200–F1208. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ramadhar, T.R.; Beemelmanns, C.; Cao, S.; Poulsen, M.; Currie, C.R.; Clardy, J. Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci. 2014, 5, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Carr, G.; Poulsen, M.; Klassen, J.L.; Hou, Y.; Wyche, T.P.; Bugni, T.S.; Currie, C.R.; Clardy, J. Microtermolides A and B from termite-associated Streptomyces sp. and structural revision of vinylamycin. Org. Lett. 2012, 14, 2822–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beemelmanns, C.; Ramadhar, T.R.; Kim, K.H.; Klassen, J.L.; Cao, S.; Wyche, T.P.; Hou, Y.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; et al. Macrotermycins A-D, glycosylated macrolactams from a termite-associated Amycolatopsis sp. M39. Org. Lett. 2017, 19, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Lee, D.; Benndorf, R.; Jung, W.H.; Beemelmanns, C.; Kang, K.S.; Kim, K.H. Termisoflavones A–C, isoflavonoid glycosides from termite-associated Streptomyces sp. RB1. J. Nat. Prod. 2016, 79, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Ben Ameur Mehdi, R.; Shaaban, K.A.; Rebai, I.K.; Smaoui, S.; Bejar, S.; Mellouli, L. Five naturally bioactive molecules including two rhamnopyranoside derivatives isolated from the Streptomyces sp. strain TN58. Nat. Prod. Res. 2009, 23, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Papastavrou, I.; Zeeck, A. Studies of precursor-directed biosynthesis with Streptomyces: Structural diversity of 1-O-acyl α-l-rhamnopyranosides by precursor-directed biosynthesis with Streptomyces griseoviridis. Eur. J. Org. Chem. 2000, 2000, 1875–1881. [Google Scholar] [CrossRef]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol. Renal. Physiol. 2005, 289, F166–F174. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.Y.; Liu, J.; Nikolic-Paterson, D.J. The role of stress-activated protein kinase signaling in renal pathophysiology. Braz. J. Med. Biol. Res. 2009, 42, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, H.X.; Xing, S.H.; Pei, D.S.; Guan, Q.H. SP600125, a selective JNK inhibitor, protects ischemic renal injury via suppressing the extrinsic pathways of apoptosis. Life Sci. 2007, 80, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Cullen, S.P.; Martin, S.J. Caspase activation pathways: Some recent progress. Cell Death Differ. 2009, 16, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kalvala, A.K.; Koneru, M.; Mahesh Kumar, J.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS ONE 2014, 9, e105070. [Google Scholar] [CrossRef] [PubMed]
- Alhoshani, A.R.; Hafez, M.M.; Husain, S.; Al-Sheikh, A.M.; Alotaibi, M.R.; Al Rejaie, S.S.; Alshammari, M.A.; Almutairi, M.M.; Al-Shabanah, O.A. Protective effect of rutin supplementation against cisplatin-induced nephrotoxicity in rats. BMC Nephrol. 2017, 18, 194. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E.; Othman, M.S.; Aref, A.M. Azadirachta indica attenuates cisplatin-induced nephrotoxicity and oxidative stress. Biomed. Res. Int. 2014, 2014, 647131. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kumar, J.M.; Sistla, R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS ONE 2015, 10, e0134139. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.J.; Jeong, J.Y.; Chang, Y.K.; Na, K.R.; Lee, K.W.; Shin, Y.T.; Choi, D.E. C-phycocyanin attenuates cisplatin-induced nephrotoxicity in mice. Ren. Fail. 2012, 34, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Aminuddin, A.; Susanti, D.; Aminudin, N.I.; On, S.; Ahmad, F.; Hamidon, H. Cytotoxic, anti-inflammatory and adipogenic effects of inophyllum D, calanone, isocordato-oblongic acid, and morelloflavone on cell lines. Nat. Prod. Sci. 2016, 22, 122–128. [Google Scholar] [CrossRef]
- Peng, Y.; Zhong, Y.; Li, G. Tubeimoside-1 suppresses breast cancer metastasis through downregulation of CXCR4 chemokine receptor expression. BMB Rep. 2016, 49, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Baek, J.; Park, H.B.; Moon, E.; Kim, S.Y.; Choi, S.U.; Kim, K.H. A new rearranged eudesmane sesquiterpene and bioactive sesquiterpenes from the twigs of Lindera glauca (Sieb. et Zucc.) Blume. Arch. Pharm. Res. 2016, 39, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Kopalli, S.R.; Cha, K.M.; Jeong, M.S.; Lee, S.H.; Sung, J.H.; Seo, S.K.; Kim, S.K. Pectinase-treated Panax ginseng ameliorates hydrogen peroxide-induced oxidative stress in GC-2 sperm cells and modulates testicular gene expression in aged rats. J. Ginseng Res. 2016, 40, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Uifălean, A.; Schneider, S.; Gierok, P.; Ionescu, C.; Iuga, C.A.; Lalk, M. The impact of soy isoflavones on MCF-7 and MDA-MB-231 breast cancer cells using a global metabolomic approach. Int. J. Mol. Sci. 2016, 17, 1443. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kang, K.S.; Lee, H.-J.; Kim, K.H. Chemical Characterization of a Renoprotective Metabolite from Termite-Associated Streptomyces sp. RB1 against Cisplatin-Induced Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 174. https://doi.org/10.3390/ijms19010174
Lee D, Kang KS, Lee H-J, Kim KH. Chemical Characterization of a Renoprotective Metabolite from Termite-Associated Streptomyces sp. RB1 against Cisplatin-Induced Cytotoxicity. International Journal of Molecular Sciences. 2018; 19(1):174. https://doi.org/10.3390/ijms19010174
Chicago/Turabian StyleLee, Dahae, Ki Sung Kang, Hae-Jeung Lee, and Ki Hyun Kim. 2018. "Chemical Characterization of a Renoprotective Metabolite from Termite-Associated Streptomyces sp. RB1 against Cisplatin-Induced Cytotoxicity" International Journal of Molecular Sciences 19, no. 1: 174. https://doi.org/10.3390/ijms19010174
APA StyleLee, D., Kang, K. S., Lee, H.-J., & Kim, K. H. (2018). Chemical Characterization of a Renoprotective Metabolite from Termite-Associated Streptomyces sp. RB1 against Cisplatin-Induced Cytotoxicity. International Journal of Molecular Sciences, 19(1), 174. https://doi.org/10.3390/ijms19010174







