Fungi in Bronchiectasis: A Concise Review
Abstract
1. Introduction
2. Pathogeny of Bronchiectasis
3. Microbiology of Bronchiectasis
4. Prevalence of Fungal Infection and Risk Factors
5. Yeasts
6. Filamentous Fungi
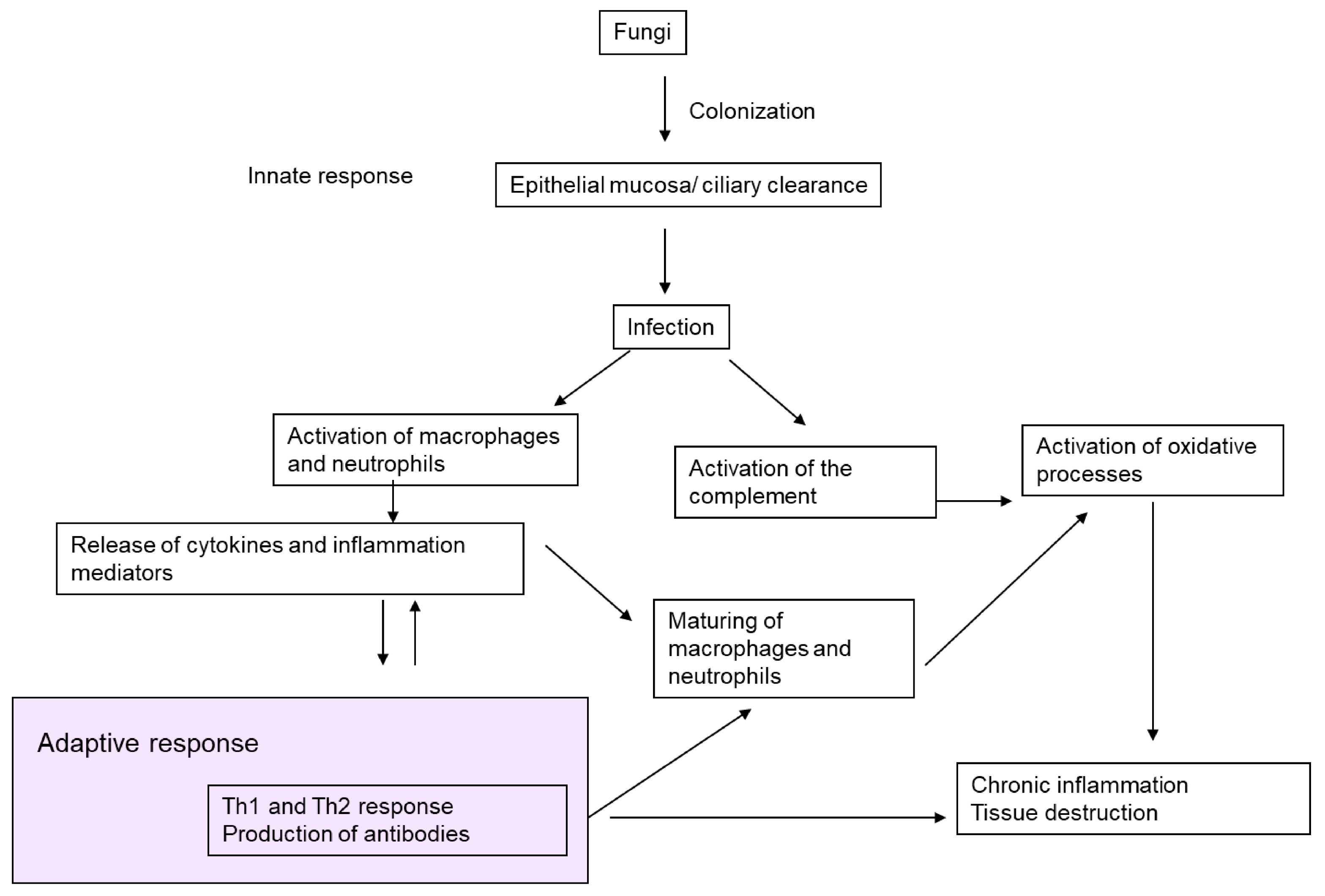
7. Pathogenic Mechanisms
8. Clinical Significance and Association with Other Microorganisms
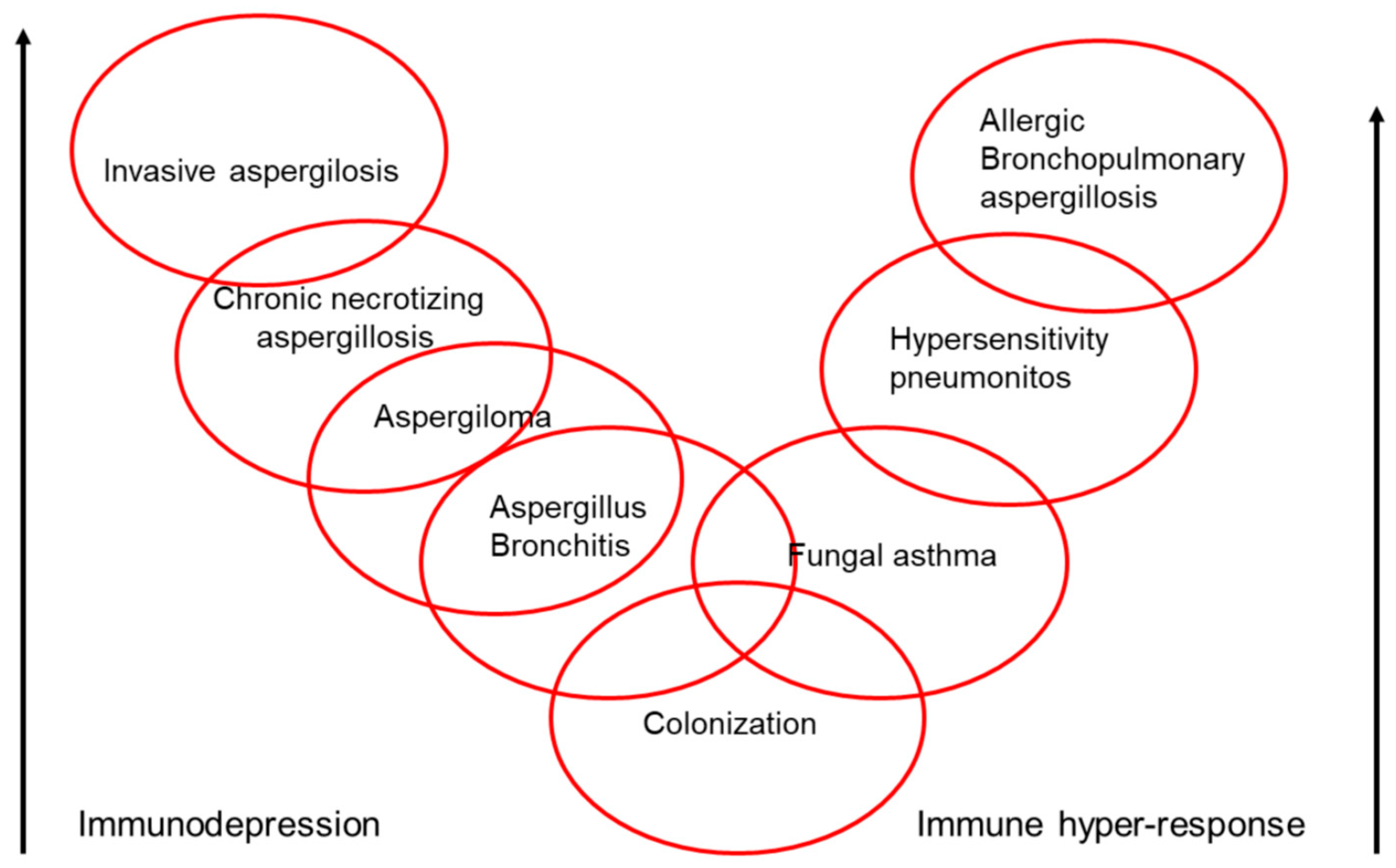
9. Clinical Spectrum of Aspergillus
10. Allergic Bronchopulmonary Mycosis
11. Mycobiome
12. Future Research
- Studies on bronchiectasis patients in a stable phase and in exacerbations, to evaluate how they are affected by fungi.
- Studies on the microbiome (including bacteria and potentially pathogenic fungi) with detailed data on phenotypical studies.
- Studies on the prevalence of fungi.
- Studies on fungi (both alone and in joint infection with other pathogens) in bronchiectasis patients in a stable phase and in exacerbations, and on the impact of fungi on the severity and evolution of the disease in these patients.
- Studies to evaluate whether chronic antibiotics are a predisposing risk factor for fungal respiratory diseases.
13. Conclusions
Author Contributions
Conflicts of Interest
References
- Martínez-García, M.A.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Normativa sobre la valoración y el diagnóstico de las bronquiectasias en el adulto. Arch. Bronconeumol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Chotirmall, S.H.; Al-Alawi, M.; Mirkovic, B.; Lavelle, G.; Logan, P.M.; Greene, C.M.; McElvaney, N.G. Aspergillus-associated airway disease, inflammation, and the innate immune response. Biomed. Res. Int. 2013, 2013, 723129. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Martínez, E.; Ruiz-Gaitán, A.; Pemán-García, J. Update on the diagnosis of invasive fungal infection. Rev. Esp. Quimioter. 2017, 30 (Suppl. S1), 16–21. [Google Scholar] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B. Fungi in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin. Respir. Crit. Care Med. 2015, 36, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.J. Inflammation: A two-edged sword—The model of bronchiectasis. Eur. J. Respir. Dis. Suppl. 1986, 147, 6–15. [Google Scholar] [PubMed]
- McDonnell, M.J.; Jary, H.R.; Perry, A.; MacFarlane, J.G.; Hester, K.L.; Small, T.; Molyneux, C.; Perry, J.D.; Walton, K.E.; De Soyza, A. Non cystic fibrosis bronchiectasis: A longitudinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respir. Med. 2015, 109, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, M.; Ozerovitch, L.J.; Davies, G.; Wodehouse, T.; Chadwick, M.V.; Abdallah, S.; Shah, P.; Wilson, R. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax 2005, 60, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Máiz, L.; Girón, R.; Olveira, C.; Vendrell, M.; Nieto, R.; Martínez-García, M.A. Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: A multicenter observational study. BMC Infect. Dis. 2016, 16, 437. [Google Scholar] [CrossRef] [PubMed]
- Máiz, L.; Girón, R.; Olveira, C.; Vendrell, M.; Nieto, R.; Martínez-García, M.A. Prevalence and factors associated with isolation of Aspergillus and Candida from sputum in patients with non-cystic fibrosis bronchiectasis. Respiration 2015, 89, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; van der Gast, C.J.; Cuthbertson, L.; Thomson, S.K.; Bruce, K.D.; Martin, M.L.; Serisier, D.J. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax 2013, 68, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Modha, D.E.; Gaillard, E.A. What is the clinical significance of filamentous fungi positive sputum cultures in patients with cystic fibrosis? J. Cyst. Fibros. 2013, 12, 187–193. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Holdsworth, S.R.; Freezer, N.J.; Villanueva, E.; Holmes, P.W. Microbiologic follow-up study in adult bronchiectasis. Respir. Med. 2007, 101, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Máiz, L.; Cuevas, M.; Lamas, A.; Sousa, A.; Quirce, S.; Suárez, L. Aspergillus fumigatus and Candida albicans in cystic fibrosis: Clinical significance and specific immune response involving serum immunoglobulins G, A, and M. Arch. Bronconeumol. 2008, 44, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Pashley, C.H. Fungal culture and sensitisation in asthma, cystic fibrosis and chronic obstructive pulmonary disorder: What does it tell us? Mycopathologia 2014, 178, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Dupuis, A.; Aaron, S.D.; Ratjen, F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 2010, 137, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Sudfeld, C.R.; Dasenbrook, E.C.; Merz, W.G.; Carroll, K.C.; Boyle, M.P. Prevalence and risk factors for recovery of filamentous fungi in individuals with cystic fibrosis. J. Cyst. Fibros. 2010, 9, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chotirmall, S.H.; O’Donoghue, E.; Bennett, K.; Gunaratnam, C.; O’Neill, S.J.; McElvaney, N.G. Sputum Candida albicans presages FEV1 decline and hospital-treated exacerbations in cystic fibrosis. Chest 2010, 138, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.L.; Van Dalfsen, J.M.; Shawar, R.M.; Otto, K.L.; Garber, R.L.; Quan, J.M.; Montgomery, A.B.; Albers, G.M.; Ramsey, B.W.; Smith, A.L. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J. Infect. Dis. 1999, 179, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Angrill, J.; Agustí, C.; de Celis, R.; Rañó, A.; Gonzalez, J.; Solé, T.; Xaubet, A.; Rodriguez-Roisin, R.; Torres, A. Bacterial colonisation in patients with bronchiectasis: Microbiological pattern and risk factors. Thorax 2002, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, M.B.; Rivera, M.; Dale, A.M.; Shepherd, R.; Carter, R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest 1995, 108, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Mukaino, T.; Koga, T.; Oshita, Y.; Narita, Y.; Obata, S.; Aizawa, H. Exophiala dermatitidis infection in non-cystic fibrosis bronchiectasis. Respir. Med. 2006, 100, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.L.; Johansen, H.K.; Fuursted, K.; Knudsen, J.D.; Gahrn-Hansen, B.; Jensen, R.H.; Howard, S.J.; Arendrup, M.C. A prospective survey of Aspergillus spp. in respiratory tract samples: Prevalence, clinical impact and antifungal susceptibility. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Takayanagi, N.; Kagiyama, N.; Shimizu, Y.; Yanagisawa, T.; Sugita, Y. Clinical characteristics of biopsy-proven allergic bronchopulmonary mycosis: Variety in causative fungi and laboratory findings. Intern. Med. 2014, 53, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Milla, C.E.; Wielinski, C.L.; Regelmann, W.E. Clinical significance of the recovery of Aspergillus species from the respiratory secretions of cystic fibrosis patients. Pediatr. Pulmonol. 1996, 21, 6–10. [Google Scholar] [CrossRef]
- Jubin, V.; Ranque, S.; Stremler Le Bel, N.; Sarles, J.; Dubus, J.C. Risk factors for Aspergillus colonization and allergic bronchopulmonary aspergillosis in children with cystic fibrosis. Pediatr. Pulmonol. 2010, 45, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Bargon, J.; Dauletbaev, N.; Köhler, B.; Wolf, M.; Posselt, H.G.; Wagner, T.O. Prophylactic antibiotic therapy is associated with an increased prevalence of Aspergillus colonization in adult cystic fibrosis patients. Respir. Med. 1999, 93, 835–838. [Google Scholar] [CrossRef]
- El-Dahr, J.M.; Fink, R.; Selden, R.; Arruda, L.K.; Platts-Mills, T.A.; Heymann, P.W. Development of immune responses to Aspergillus at an early age in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1994, 150, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.G.; Moore, C.B.; Jones, A.M.; Webb, A.K.; Denning, D.W. IgE-mediated immune responses and airway detection of Aspergillus and Candida in adult cystic fibrosis. Chest 2013, 143, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Elborn, J.S.; Millar, B.C.; Walker, J.M.; Goldsmith, C.E.; Rendall, J.; Moore, J.E. Comparison of techniques to examine the diversity of fungi in adult patients with cystic fibrosis. Med. Mycol. 2010, 48, 166.e1–176.e1. [Google Scholar] [CrossRef] [PubMed]
- Chabi, M.L.; Goracci, A.; Roche, N.; Paugam, A.; Lupo, A.; Revel, M.P. Pulmonary aspergillosis. Diagn. Interv. Imaging 2015, 96, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Ohn, M.; Robinson, P.; Selvadurai, H.; Fitzgerald, D.A. Question 11: How should Allergic Bronchopulmonary Aspergillosis [ABPA] be managed in Cystic Fibrosis? Paediatr. Respir. Rev. 2017, 24, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, H.; Jin, J.; Zhan, Q. Is Bulpa criteria suitable for the diagnosis of probable invasive pulmonary Aspergillosis in critically ill patients with chronic obstructive pulmonary disease? A comparative study with EORTC/ MSG and ICU criteria. BMC Infect. Dis. 2017, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Bush, R.K.; Demain, J.G.; Denning, D.W.; Dixit, A.; Fairs, A.; Greenberger, P.A.; Kariuki, B.; Kita, H.; Kurup, V.P.; et al. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012, 129, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.; Ferreira, J.A.; Moss, R.B.; Valente, J.; Veríssimo, C.; Carolino, E.; Clemons, K.V.; Everson, C.; Banaei, N.; Penner, J.; et al. Molecular epidemiology of Aspergillus collected from cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Delhaes, L.; Monchy, S.; Fréalle, E.; Hubans, C.; Salleron, J.; Leroy, S.; Prevotat, A.; Wallet, F.; Wallaert, B.; Dei-Cas, E.; et al. The airway microbiota in cystic fibrosis: A complex fungal and bacterial community--implications for therapeutic management. PLoS ONE 2012, 7, e36313. [Google Scholar] [CrossRef] [PubMed]
- Chotirmall, S.H.; Martin-Gomez, M.T. Aspergillus species in bronchiectasis: Challenges in the cystic fibrosis and non-cystic fibrosis airways. Mycopathologia 2017. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.; McDonnell, M.J.; Abo-Leyah, H.; Aliberti, S.; Chalmers, J.D. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann. Am. Thorac. Soc. 2015, 12, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pashley, C.; Hartl, D.; Wardlaw, A.; Godet, C.; Del Giacco, S.; Delhaes, L.; Sergejeva, S. Fungal allergy in asthma-state of the art and research needs. Clin. Transl. Allergy 2014, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Zain, N.M.; Bruce, K.D.; Burr, L.D.; Chen, A.C.; Rivett, D.W.; McGuckin, M.A.; Serisier, D.J. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann. Am. Thorac. Soc. 2014, 11, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Moutaouakil, M.; Latge, J.P.; Davies, J. Secretion of a potential virulence factor, a fungal ribonucleotoxin, during human aspergillosis infections. Mol. Microbiol. 1991, 5, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.; Longbottom, J.L. Characterization of a second major antigen Ag 13 (antigen C) of Aspergillus fumigatus and investigation of its immunological reactivity. Clin. Exp. Immunol. 1987, 70, 247–254. [Google Scholar] [PubMed]
- Frosco, M.; Chase, T., Jr.; Macmillan, J.D. Purification and properties of the elastase from Aspergillus fumigatus. Infect. Immun. 1992, 60, 728–734. [Google Scholar] [PubMed]
- Chotirmall, S.H.; Mirkovic, B.; Lavelle, G.M.; McElvaney, N.G. Immunoevasive Aspergillus virulence factors. Mycopathologia 2014, 178, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, J.L.; Gomez, P.; Ruan, J.; Hackett, T.L.; Moore, M.M.; Knight, D.A.; Tebbutt, S.J. Dual organism transcriptomics of airway epithelial cells interacting with conidia of Aspergillus fumigatus. PLoS ONE 2011, 6, e20527. [Google Scholar] [CrossRef] [PubMed]
- Porter, P.C.; Yang, T.; Luong, A.; Delclos, G.L.; Abramson, S.L.; Kheradmand, F.; Corry, D.B. Proteinases as molecular adjuvants in allergic airway disease. Biochim. Biophys. Acta 2011, 1810, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Porter, P.; Polikepahad, S.; Qian, Y.; Knight, J.M.; Lu, W.; Tai, W.M.; Roberts, L.; Ongeri, V.; Yang, T.; Seryshev, A.; et al. Respiratory tract allergic disease and atopy: Experimental evidence for a fungal infectious etiology. Med. Mycol. 2011, 49 (Suppl. 1), S158–S163. [Google Scholar] [CrossRef] [PubMed]
- Amvar, S.; Warn, P.; Farnell, E.; Bromley, M.; Fraczek, M.; Bowyer, P.; Herrick, S. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin. Exp. Allergy 2015, 45, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Diamond, R.D. Mechanisms of resistance of Aspergillus fumigatus conidia to killing by neutrophils in vitro. J. Infect. Dis. 1985, 152, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kurup, V.P. Interaction of Aspergillus fumigatus spores and pulmonary alveolar macrophages of rabbits. Immunobiology 1984, 166, 53–61. [Google Scholar] [CrossRef]
- Levitz, S.M.; Selsted, M.E.; Ganz, T.; Lehrer, R.I.; Diamond, R.D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J. Infect. Dis. 1986, 154, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Iliev, I.D. The mycobiota: Interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; Gresnigt, M.S.; Smeekens, S.P.; Jacobs, C.W.; Magis-Escurra, C.; Jaegerm, M.; Wang, X.; Lubbers, R.; Oosting, M.; Joosten, L.A.; et al. Pattern recognition pathways leading to a Th2 cytokine bias in allergic bronchopulmonary aspergillosis patients. Clin. Exp. Allergy 2015, 45, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chakrabarti, A.; Shah, A.; Gupta, D.; Meis, J.F.; Guleria, R.; Moss, R.; Denning, D.W. ABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy 2013, 43, 850–873. [Google Scholar] [CrossRef] [PubMed]
- Kunst, H.; Wickremasinghe, M.; Wells, A.; Wilson, R. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur. Respir. J. 2006, 28, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Grisaru-Soen, G.; Lerner-Geva, L.; Kerem, E.; Blau, H.; Bentur, L.; Aviram, M.; Rivlin, J.; Picard, E.; Lavy, A.; et al. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg. Infect. Dis. 2008, 14, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Mussaffi, H.; Rivlin, J.; Shalit, I.; Ephros, M.; Blau, H. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur. Respir. J. 2005, 25, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.H. Atypical mycobacterial infection in the lung: CT appearance. Radiology 1993, 187, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Agarwal, K.; Kathuria, S.; Gaur, S.N.; Randhawa, H.S.; Meis, J.F. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: A global overview. Crit. Rev. Microbiol. 2014, 40, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Vincken, W.; Schandevul, W.; Roels, P. Allergic bronchopulmonary aspergillosis caused by Aspergillus terreus. Am. Rev. Respir. Dis. 1983, 127, 388–389. [Google Scholar] [PubMed]
- Bahous, J.; Malo, J.L.; Paquin, R.; Cartier, A.; Vyas, P.; Longbottom, J.L. Allergic bronchopulmonary aspergillosis and sensitization to Aspergillus fumigatus in chronic bronchiectasis in adults. Clin. Allergy 1985, 15, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Laham, M.N.; Allen, R.C.; Greene, J.C. Allergic bronchopulmonary aspergillosis (ABPA) caused by Aspergillus terreus: Specific lymphocyte sensitization and antigen-directed serum opsonic activity. Ann. Allergy 1981, 46, 74–80. [Google Scholar] [PubMed]
- Nakahara, Y.; Katoh, O.; Yamada, H.; Sumida, I.; Hanada, M. Allergic bronchopulmonary aspergillosis caused by Aspergillus terreus presenting lobar collapse. Intern. Med. 1992, 31, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Tillie-Leblond, I.; Tonnel, A.B. Allergic bronchopulmonary aspergillosis. Allergy 2005, 60, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Olveira, C.; Padilla, A.; Martínez-García, M.Á.; de la Rosa, D.; Girón, R.M.; Vendrell, M.; Máiz, L.; Borderías, L.; Polverino, E.; Martínez-Moragón, E.; et al. Etiology of bronchiectasis in a cohort of 2047 patients. An analysis of the Spanish Historical Bronchiectasis Registry. Arch. Bronconeumol. 2017, 53, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Routh, C.; Welham, S. National BTS bronchiectasis audit 2012: Is the quality standard being adhered to in adult secondary care? Thorax 2014, 69, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.; Deloséa, N.; Ballinari, P.; Gallati, S.; Crameri, R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, P.A.; Miller, T.P.; Roberts, M.; Smith, L.L. Allergic bronchopulmonary aspergillosis in patients with and without evidence of bronchiectasis. Ann. Allergy 1993, 70, 333–338. [Google Scholar] [PubMed]
- Viviani, L.; Harrison, M.J.; Zolin, A.; Haworth, C.S.; Floto, R.A. Epidemiology of nontuberculous mycobacteria (NTM) amongst individuals with cystic fibrosis (CF). J. Cyst. Fibros. 2016, 15, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Huffnagle, G.B. The Lung Microbiome: New principles for respiratory bacteriology in health and disease. PLoS Pathog. 2015, 11, e1004923. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.D.; Viscogliosi, E.; Delhaes, L. The lung mycobiome: An emerging field of the human respiratory microbiome. Front. Microbiol. 2015, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Willger, S.D.; Grim, S.L.; Dolben, E.L.; Shipunova, A.; Hampton, T.H.; Morrison, H.G.; Filkins, L.M.; O’Toole, G.A.; Moulton, L.A.; Ashare, A.; et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome 2014, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Sauer-Heilborn, A.; Welte, T.; Jauregui, R.; Brettar, I.; Guzman, C.A.; Höfle, M.G. High individuality of respiratory bacterial communities in a large cohort of adult cystic fibrosis patients under continuous antibiotic treatment. PLoS ONE 2015, 10, e0117436. [Google Scholar] [CrossRef] [PubMed]
- De Dios Caballero, J.; Vida, R.; Cobo, M.; Máiz, L.; Suárez, L.; Galeano, J.; Baquero, F.; Cantón, R.; Del Campo, R. Individual patterns of complexity in cystic fibrosis lung microbiota, including predator bacteria, over a 1-Year Period. MBio 2017, 8, e00959-17. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, R.; Garriga, M.; Pérez-Aragón, A.; Guallarte, P.; Lamas, A.; Máiz, L.; Bayón, C.; Roy, G.; Cantón, R.; Zamora, J.; et al. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: A double blind prospective study. J. Cyst. Fibros. 2014, 13, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.M.; Einarsson, G.G.; Wei, L.; Drain, M.; Klem, E.R.; Cardwell, C.; Ennis, M.; Boucher, R.C.; Wolfgang, M.C.; Elborn, J.S. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am. J. Respir. Crit. Care Med. 2013, 187, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Tipton, L.; Ghedin, E.; Morris, A. The lung mycobiome in the next-generation sequencing era. Virulence 2017, 8, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.; Masefield, S.; Polverino, E.; De Soyza, A.; Loebinger, M.R.; Menendez, R.; Ringshausen, F.C.; Vendrell, M.; Powell, P.; Chalmers, J.D.; et al. Research priorities in bronchiectasis: A consensus statement from the EMBARC Clinical Research Collaboration. Eur. Respir. J. 2016, 48, 632–647. [Google Scholar] [CrossRef] [PubMed]

| Yeasts | Filamentous Fungi |
|---|---|
| Candida albicans | Aspergllus fumigatus |
| Candida glabrata | Aspergillus niger |
| Candida parapsilosis | Aspergullus terreus |
| Saccharomyces cerevisiae | Scedosporium apiospermum |
| Trichosporon beigellii | Penicillium spp. |
| Exophiala dermatitidis | Fusarium spp. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Máiz, L.; Nieto, R.; Cantón, R.; Gómez G. de la Pedrosa, E.; Martinez-García, M.Á. Fungi in Bronchiectasis: A Concise Review. Int. J. Mol. Sci. 2018, 19, 142. https://doi.org/10.3390/ijms19010142
Máiz L, Nieto R, Cantón R, Gómez G. de la Pedrosa E, Martinez-García MÁ. Fungi in Bronchiectasis: A Concise Review. International Journal of Molecular Sciences. 2018; 19(1):142. https://doi.org/10.3390/ijms19010142
Chicago/Turabian StyleMáiz, Luis, Rosa Nieto, Rafael Cantón, Elia Gómez G. de la Pedrosa, and Miguel Ángel Martinez-García. 2018. "Fungi in Bronchiectasis: A Concise Review" International Journal of Molecular Sciences 19, no. 1: 142. https://doi.org/10.3390/ijms19010142
APA StyleMáiz, L., Nieto, R., Cantón, R., Gómez G. de la Pedrosa, E., & Martinez-García, M. Á. (2018). Fungi in Bronchiectasis: A Concise Review. International Journal of Molecular Sciences, 19(1), 142. https://doi.org/10.3390/ijms19010142