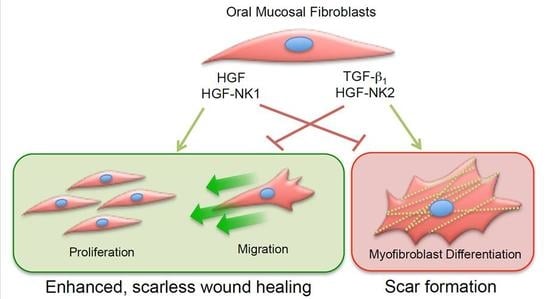
Hepatocyte Growth Factor Mediates Enhanced Wound Healing Responses and Resistance to Transforming Growth Factor-β1-Driven Myofibroblast Differentiation in Oral Mucosal Fibroblasts
Abstract
:1. Introduction
2. Results
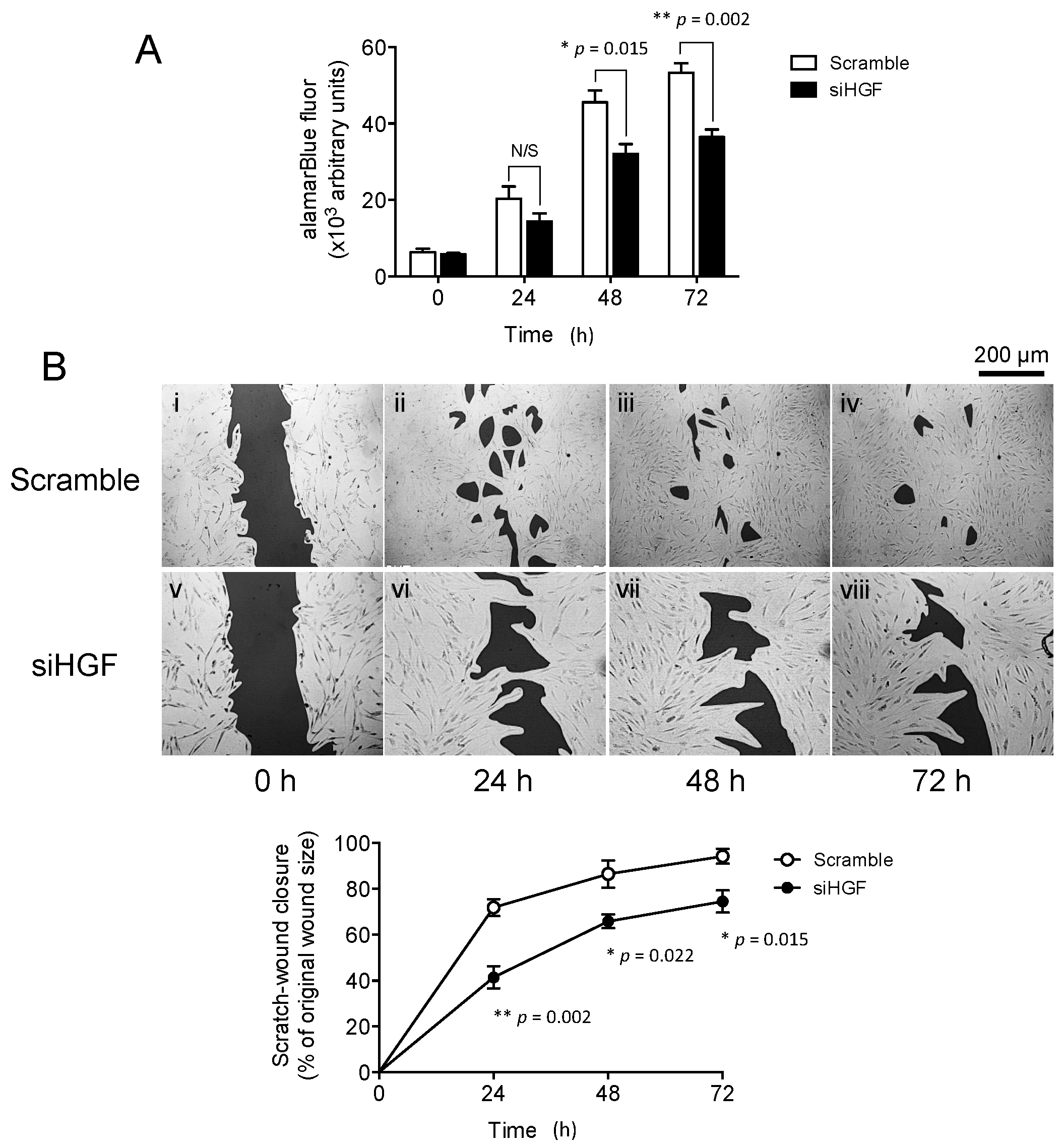
2.1. Hepatocyte Growth Factor (HGF) Knockdown Inhibits Oral Mucosal Fibroblast Proliferation and In Vitro Scratch Wound Repopulation
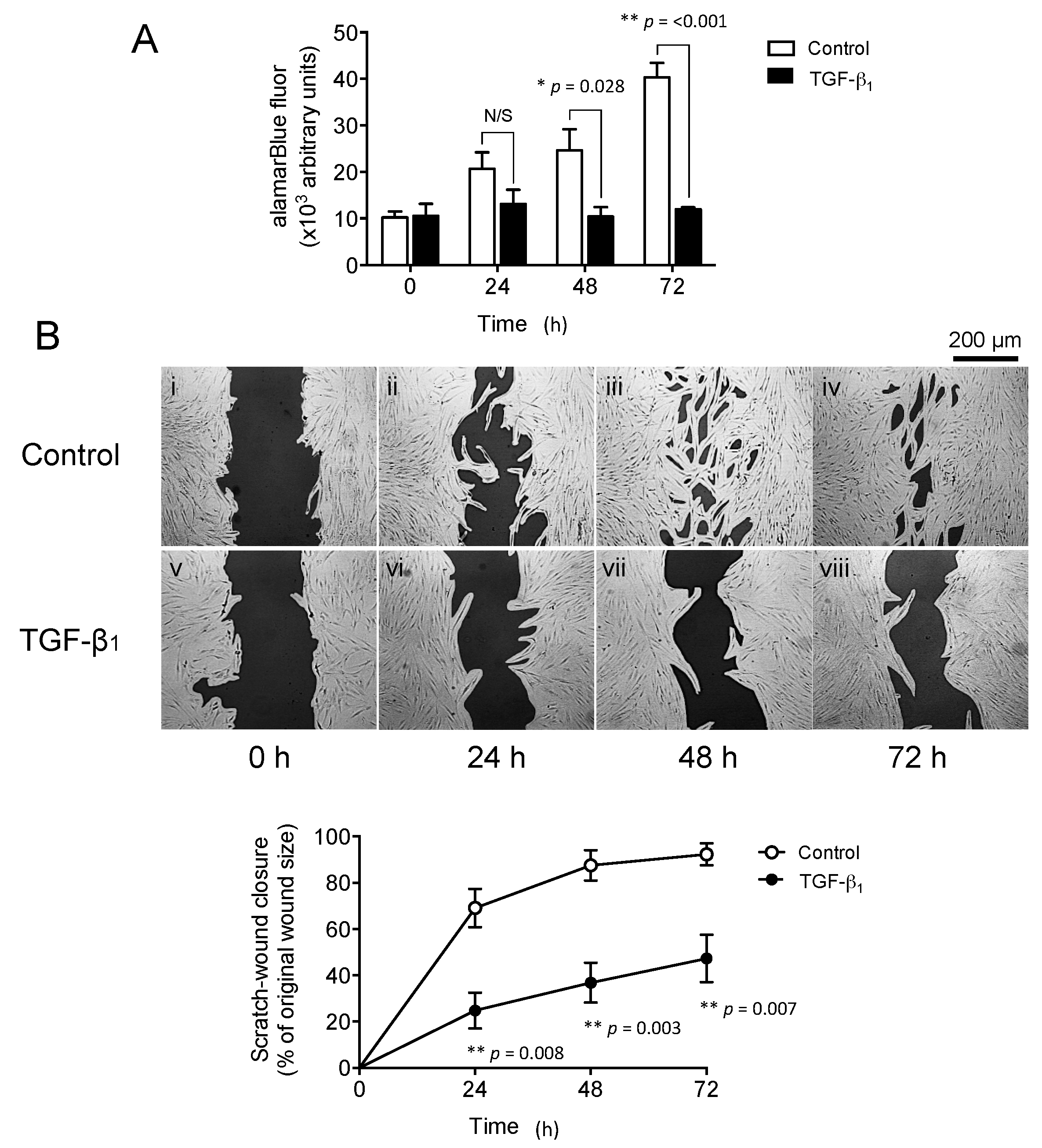
2.2. Exogenous Transforming Growth Factor-β1 (TGF-β1) Supplementation Inhibits Oral Mucosal Fibroblast Proliferation and In Vitro Scratch Wound Repopulation
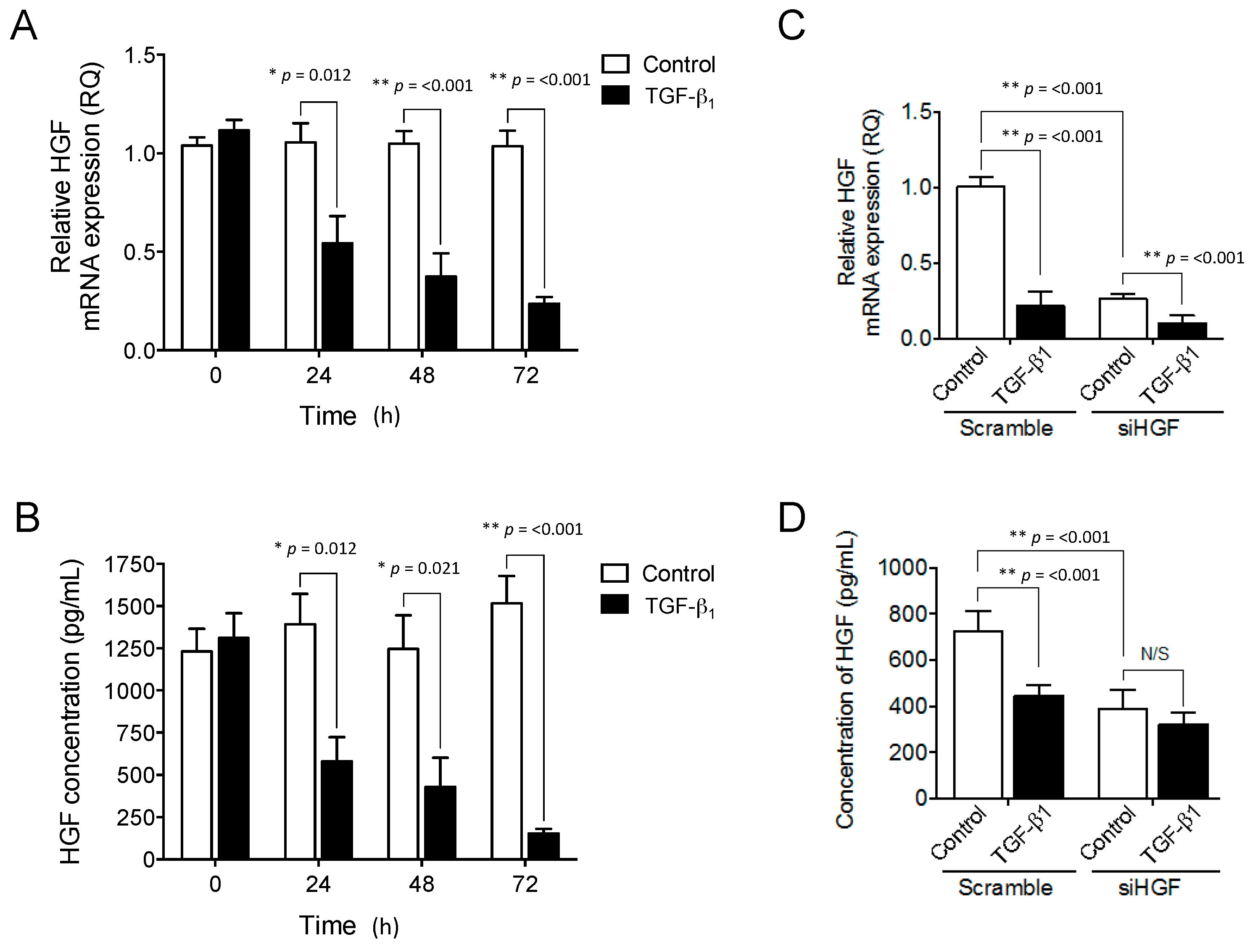
2.3. Exogenous TGF-β1 Supplementation Suppresses HGF Expression in a Similar Manner to Targeted HGF Knockdown
2.4. HGF Knockdown Impairs Oral Mucosal Fibroblast Resistance to TGF-β1-Driven, Myofibroblast Differentiation
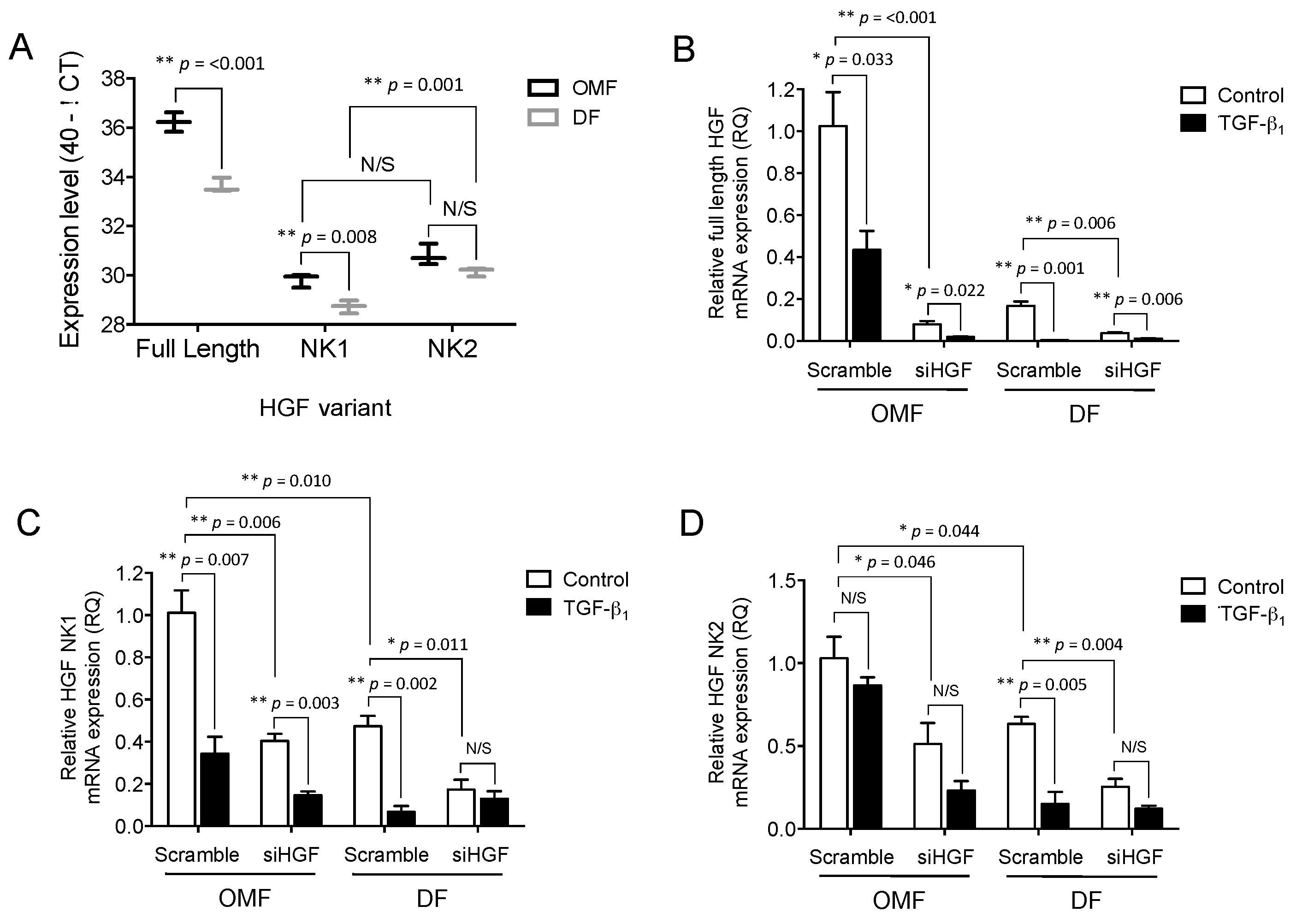
2.5. Full-Length HGF and Truncated HGF isoforms (HGF-NK), NK1 and NK2, Expression Levels Differ between Oral and Dermal Fibroblasts and Are Differentially Influenced by TGF-β1
3. Discussion
4. Material and Methods
4.1. Reagents
4.2. Oral Mucosal and Patient-Matched, Dermal Fibroblast Cultures
4.3. Short Interfering RNA (siRNA) Transfection
4.4. TGF-β1 Supplementation
4.5. Proliferation Assay
4.6. In Vitro Scratch Wound Repopulation
4.7. Reverse Transcription PCR (RT-PCR) and Real Time Quantitative PCR (RT-qPCR)
4.8. HGF Enzyme-Linked Immunosorbant Assay (ELISA)
4.9. Immunocytochemistry
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dang, C.; Ting, K.; Soo, C.; Longaker, M.T.; Lorenz, H.P. Foetal wound healing: Current perspectives. Clin. Plast. Surg. 2003, 30, 13–23. [Google Scholar] [CrossRef]
- Enoch, S.; Moseley, R.; Stephens, P.; Thomas, D.W. The oral mucosa: A model of wound healing with reduced scarring. Oral Surg. 2008, 1, 11–21. [Google Scholar] [CrossRef]
- Glim, J.E.; van Egmond, M.; Niessen, F.B.; Everts, V.; Beelen, R.H. Detrimental dermal wound healing: What can we learn from the oral mucosa? Wound Rep. Regen. 2013, 21, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Eun, H.C. Differences between fibroblasts cultured from oral mucosa and normal skin: Implication to wound healing. J. Dermatol. Sci. 1999, 21, 176–182. [Google Scholar] [CrossRef]
- Enoch, S.; Wall, I.; Peake, M.; Davies, L.C.; Farrier, J.; Giles, P.; Baird, D.; Kipling, D.; Price, P.; Moseley, R.; et al. Increased oral fibroblast lifespan is telomerase-independent. J. Dent. Res. 2009, 88, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Wall, I.; Peake, M.; Davies, L.C.; Farrier, J.; Giles, P.; Kipling, D.; Price, P.; Moseley, R.; Thomas, D.; et al. “Young” oral fibroblasts are geno/phenotypically distinct. J. Dent. Res. 2010, 89, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Davies, K.J.; al-Khateeb, T.; Shepherd, J.P.; Thomas, D.W. A comparison of the ability of intra-oral and extra-oral fibroblasts to stimulate extracellular matrix reorganisation in a model of wound contraction. J. Dent. Res. 1996, 7, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Davies, K.J.; Occleston, N.; Pleass, R.D.; Kon, C.; Daniels, J.; Khaw, P.T.; Thomas, D.W. Skin and oral fibroblasts exhibit phenotypic differences in extracellular matrix reorganization and matrix metalloproteinase activity. Br. J. Dermatol. 2001, 144, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Shannon, D.B.; McKeown, S.T.; Lundy, F.T.; Irwin, C.R. Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound. Rep. Regen. 2006, 14, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Lygoe, K.A.; Wall, I.; Stephens, P.; Lewis, M.P. Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation. Biol. Cell 2007, 99, 601–614. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.T.; Barnes, J.J.; Hyland, P.L.; Lundy, F.T.; Fray, M.J.; Irwin, C.R. Matrix metalloproteinase-3 differences in oral and skin fibroblasts. J. Dent. Res. 2007, 86, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Karbiener, M.; Darnhofer, B.; Frisch, M.T.; Rinner, B.; Birner-Gruenberger, R.; Gugatschka, M. Comparative proteomics of paired vocal fold and oral mucosa fibroblasts. J. Proteom. 2017, 155, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Meran, S.; Thomas, D.W.; Stephens, P.; Martin, J.; Bowen, T.; Phillips, A.O.; Steadman, R. Involvement of hyaluronan in regulation of fibroblast phenotype. J. Biol. Chem. 2007, 282, 25687–25697. [Google Scholar] [CrossRef] [PubMed]
- Schrementi, M.E.; Ferreira, A.M.; Zender, C.; DiPietro, L.A. Site-specific production of TGF-β in oral mucosal and cutaneous wounds. Wound Rep. Regen. 2008, 16, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Meran, S.; Thomas, D.W.; Stephens, P.; Enoch, S.; Martin, J.; Steadman, R.; Phillips, A.O. Hyaluronan facilitates transforming growth factor-β1-mediated fibroblast proliferation. J. Biol. Chem. 2008, 283, 6530–6545. [Google Scholar] [CrossRef] [PubMed]
- Peake, M.A.; Caley, M.; Giles, P.J.; Wall, I.; Enoch, S.; Davies, L.C.; Kipling, D.; Thomas, D.W.; Stephens, P. Identification of a transcriptional signature for the wound healing continuum. Wound Rep. Regen. 2014, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Horiguchi, T.; Tanimura, A.; Hagita, H.; Noma, T. Gene signature of human oral mucosa fibroblasts: Comparison with dermal fibroblasts and induced pluripotent stem cells. Biomed. Res. Int. 2015, 2015, 121575. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Hiscox, S.; Cook, H.; Jiang, W.G.; Zhiquiang, W.; Thomas, D.W. Phenotypic variation in the production of bioactive hepatocyte growth factor/scatter factor by oral mucosal and skin fibroblasts. Wound Rep. Regen. 2001, 9, 34–43. [Google Scholar] [CrossRef]
- Grøn, B.; Stoltze, K.; Andersson, A.; Dabelsteen, E. Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS 2002, 110, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Yoshimura, K.; Uchida, G.; Harii, K. Elevated expression of hepatocyte and keratinocyte growth factor in cultured buccal mucosa-derived fibroblasts compared with normal skin-derived fibroblasts. J. Dermatol. Sci. 2002, 30, 108–115. [Google Scholar] [CrossRef]
- Conway, K.; Price, P.; Harding, K.G.; Jiang, W.G. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Rep. Regen. 2006, 14, 2–10. [Google Scholar] [CrossRef]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte growth factor 20 years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011, 26, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, S.; Villalobos-Hernandez, A.; Bobbala, D.; Ramanathan, S. The hepatocyte growth factor (HGF)-MET receptor tyrosine kinase signaling pathway: Diverse roles in modulating immune cell functions. Cytokine 2016, 82, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Cao, B.; Law, S.; Xie, Y.; Lee, P.Y.; Cheung, L.; Chen, Y.; Huang, X.; Chan, H.M.; Zhao, P.; et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: A prognostic marker of human esophageal squamous cell carcinomas. Clin. Cancer Res. 2005, 11, 6190–6197. [Google Scholar] [CrossRef] [PubMed]
- Matusmoto, K.; Umitsu, M.; de Silva, D.N.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Kitamura, A.; Naka, D.; Kitamura, N. An alternatively processed mRNA generated from human hepatocyte growth factor gene. Eur. J. Biochem. 1991, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Cioce, V.; Csaky, K.G.; Chan, A.M.; Bottaro, D.P.; Taylor, W.G.; Jensen, R.; Aaronson, S.A.; Rubin, J.S. Hepatocyte growth factor (HGF)/NK1 is a naturally occurring HGF/scatter factor variant with partial agonist/antagonist activity. J. Biol. Chem. 1996, 271, 13110–13115. [Google Scholar] [CrossRef] [PubMed]
- Mungunsukh, O.; Lee, Y.H.; Bottaro, D.P.; Day, R.M. The hepatocyte growth factor isoform NK2 activates motogenesis and survival but not proliferation due to lack of Akt activation. Cell Signal. 2016, 28, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Tsubouchi, H.; Naka, D.; Takahashi, K.; Okigaki, M.; Arakaki, N.; Nakayama, H.; Hirono, S.; Sakiyama, O.; Takahashi, K.; et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem. Biophys. Res. Commun. 1989, 163, 967–973. [Google Scholar] [CrossRef]
- Kirchhofer, D.; Yao, X.; Peek, M.; Eigenbrot, C.; Lipari, M.T.; Billeci, K.L.; Maun, H.R.; Moran, P.; Santell, L.; Wiesmann, C.; et al. Structural and functional basis of the serine protease-like hepatocyte growth factor β-chain in Met binding and signalling. J. Biol. Chem. 2004, 279, 39915–39924. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.; Lazarus, R.A.; Yao, X.; Kirchhofer, D.; Wiesmann, C. Crystal structure of the HGF β-chain in complex with the Sema domain of the Met receptor. EMBO J. 2004, 23, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Cioce, V.; Breckenridge, D.; Castagnino, P.; Bottaro, D.P. Differential signaling by alternative HGF isoforms through c-Met: Activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene 1999, 18, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGF-β signaling in growth control, cancer, and heritable disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef]
- Matsumoto, K.; Tajima, H.; Okazaki, H.; Nakamura, T. Negative regulation of HGF gene expression in human lung fibroblasts and leukemic cells by TGF-β1 and glucocorticoids. J. Biol. Chem. 1992, 267, 24917–24920. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Bradley, L.; Bomford, A. Mechanism of regulation of HGF/SF gene expression in fibroblasts by TGF-β1. Biochem. Biophys. Res. Commun. 2000, 271, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Imado, T.; Kitano, S.; Sano, H. Hepatocyte growth factor ameliorates dermal sclerosis in the tight-skin mouse model of scleroderma. Arthritis Res. Ther. 2006, 8, R161. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Li, X.; Zhou, Y.; Ren, S.; Wan, W.; Feng, G.; Jiang, X. Hepatocyte growth factor regulates the TGF-β1-induced proliferation, differentiation and secretory function of cardiac fibroblasts. Int. J. Mol. Med. 2014, 34, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Kaneda, Y.; Tsutsui, H.; Nakanishi, K.; Sawa, Y.; Morishita, R.; Matsumoto, K.; Nakamura, T.; Takahashi, H.; Okamoto, E.; et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat. Med. 1999, 5, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Okada, H.; Kobayashi, T.; Watanabe, Y.; Kanno, Y.; Kopp, J.B.; Nishida, T.; Takigawa, M.; Ueno, M.; Nakamura, T.; et al. Hepatocyte growth factor counteracts transforming growth factor-beta1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. FASEB J. 2003, 17, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Hepatocyte growth factor in kidney fibrosis: Therapeutic potential and mechanisms of action. Am. J. Physiol. Ren. Physiol. 2004, 287, F7–F16. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, J.; Kojima, N.; Sato, H.; Higashi, S.; Kikuchi, K.; Sakai, K.; Matsumoto, K.; Miyazaki, K. Inhibition of transforming growth factor-β signaling potentiates tumor cell invasion into collagen matrix induced by fibroblast-derived hepatocyte growth factor. Exp. Cell Res. 2014, 326, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Szpaderska, A.M.; Zuckerman, J.D.; DiPietro, L.A. Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res. 2003, 82, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.; Manji, A.; Gallant-Behm, C.; Wiebe, C.; Hart, D.A.; Larjava, H.; Häkkinen, L. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J. Dermatol. Sci. 2009, 56, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Dai, C. Hepatocyte growth factor antagonizes the profibrotic action of TGF-β1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J. Am. Soc. Nephrol. 2004, 15, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Zhang, X.; Yang, J.; Li, Y.; Liu, Y. Molecular basis for the cell type specific induction of SnoN expression by hepatocyte growth factor. Am. Soc. Nephrol. 2007, 18, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Higashi, K.; Kushida, M.; Hong, Y.Y.; Nakao, S.; Higashiyama, R.; Moro, T.; Itoh, J.; Mikami, T.; Kimura, T.; et al. Hepatocyte growth factor suppresses profibrogenic signal transduction via nuclear export of Smad3 with galectin-7. Gastroenterology 2008, 134, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad pathways in TGF-β signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; French, L.C.; Hirano, S.; Ossoff, R.H.; Rousseau, B. Effect of hepatocyte growth factor on gene expression of extracellular matrix during wound healing of the injured rat vocal fold. Ann. Otol. Rhinol. Laryngol. 2008, 117, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Hirano, S.; Suehiro, A.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J. Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts. Ann. Otol. Rhinol. Laryngol. 2009, 118, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Otsuka, T.; Yamazaki, Y.; Kosone, T.; Sohara, N.; Ichikawa, T.; Sato, K.; Kakizaki, S.; Takagi, H.; Mori, M. Overexpression of NK2 promotes liver fibrosis in carbon tetrachloride-induced chronic liver injury. Liver Int. 2008, 28, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, W.D.; Daugherty-Holtrop, J.; Gherardi, E.; Vande Woude, G.; Xu, H.E. Structural basis for agonism and antagonism of hepatocyte growth factor. Proc. Natl. Acad. Sci. USA 2010, 107, 13264–13269. [Google Scholar] [CrossRef] [PubMed]
- Mungunsukh, O.; Day, R.M. Transforming growth factor-β1 selectively inhibits hepatocyte growth factor expression via a micro-RNA-199-dependent posttranscriptional mechanism. Mol. Biol. Cell 2013, 24, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ebina, M.; Orson, F.M.; Nakamura, A.; Kubota, K.; Koinuma, D.; Akiyama, K.; Maemondo, M.; Okouchi, S.; Tahara, M.; et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Mol. Ther. 2005, 12, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, J.Y.; Wang, J.Y.; Du, S.L.; Lu, W.Y.; Liu, M.; Xie, C.; Shi, J.Y. Effect of hepatocyte growth factor encapsulated in targeted liposomes on liver cirrhosis. J. Control. Release 2008, 131, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Yaekashiwa, M.; Nakayama, S.; Ohnuma, K.; Sakai, T.; Abe, T.; Satoh, K.; Matsumoto, K.; Nakamura, T.; Takahashi, T.; Nukiwa, T. Simultaneous or delayed administration of hepatocyte growth factor (HGF) equally repress the fibrotic change in murine lung injury induced by bleomycin: A morphologic study. Am. J. Respir. Crit. Care Med. 1997, 156, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Kurosawa, T.; Matsumoto, K.; Mizuno-Horikawa, Y.; Okamoto, M.; Nakamura, T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J. Clin. Investig. 1998, 101, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Bevan, D.; Gherardi, E.; Fan, T.P.; Edwards, D.; Warn, R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004, 203, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Ono, I.; Yamashita, T.; Hida, T.; Jin, H.Y.; Ito, Y.; Hamada, H.; Akasaka, Y.; Ishii, T.; Jimbow, K. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J. Surg. Res. 2004, 120, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.R.; Ahn, H.M.; Choi, I.K.; Yun, C.O.; Rah, D.K.; Lew, D.H.; Lee, W.J. Hepatocyte growth factor-expressing adenovirus upregulates matrix metalloproteinase-1 expression in keloid fibroblasts. Int. J. Dermatol. 2016, 55, 356–361. [Google Scholar] [CrossRef] [PubMed]

| Target | Primer Sequence (5′–3′) |
|---|---|
| HGF | Forward: GGACGCAGCAAGGGAACAGT |
| Reverse: CCCGATAGCTGTGTTCGTGTGGT | |
| Full-length HGF | Forward: ACTGCCGAAATCCAGATGGG |
| Reverse: TTGGGAGCAGTAGCCAACTC | |
| Truncated HGF (NK1) | Forward: TGCCATGTGGGCCATTCTAT |
| Reverse: TAGTTGCATTTGCACGAACAACA | |
| Truncated HGF (NK2) | Forward: ATGGGCTCTCAACTGATGGTG |
| Reverse: AGCGAGAGAGGTAGGGATCA | |
| GAPDH | Forward: CCTCTGACTTCAACAGCGACAC |
| Reverse: TGTCATACCAGGAAATGAGCTTGA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dally, J.; Khan, J.S.; Voisey, A.; Charalambous, C.; John, H.L.; Woods, E.L.; Steadman, R.; Moseley, R.; Midgley, A.C. Hepatocyte Growth Factor Mediates Enhanced Wound Healing Responses and Resistance to Transforming Growth Factor-β1-Driven Myofibroblast Differentiation in Oral Mucosal Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1843. https://doi.org/10.3390/ijms18091843
Dally J, Khan JS, Voisey A, Charalambous C, John HL, Woods EL, Steadman R, Moseley R, Midgley AC. Hepatocyte Growth Factor Mediates Enhanced Wound Healing Responses and Resistance to Transforming Growth Factor-β1-Driven Myofibroblast Differentiation in Oral Mucosal Fibroblasts. International Journal of Molecular Sciences. 2017; 18(9):1843. https://doi.org/10.3390/ijms18091843
Chicago/Turabian StyleDally, Jordanna, Jabur S. Khan, Alex Voisey, Chrisandrea Charalambous, Hannah L. John, Emma L. Woods, Robert Steadman, Ryan Moseley, and Adam C. Midgley. 2017. "Hepatocyte Growth Factor Mediates Enhanced Wound Healing Responses and Resistance to Transforming Growth Factor-β1-Driven Myofibroblast Differentiation in Oral Mucosal Fibroblasts" International Journal of Molecular Sciences 18, no. 9: 1843. https://doi.org/10.3390/ijms18091843