Predictive Structure and Topology of Peroxisomal ATP-Binding Cassette (ABC) Transporters
Abstract
:1. Introduction
2. Results
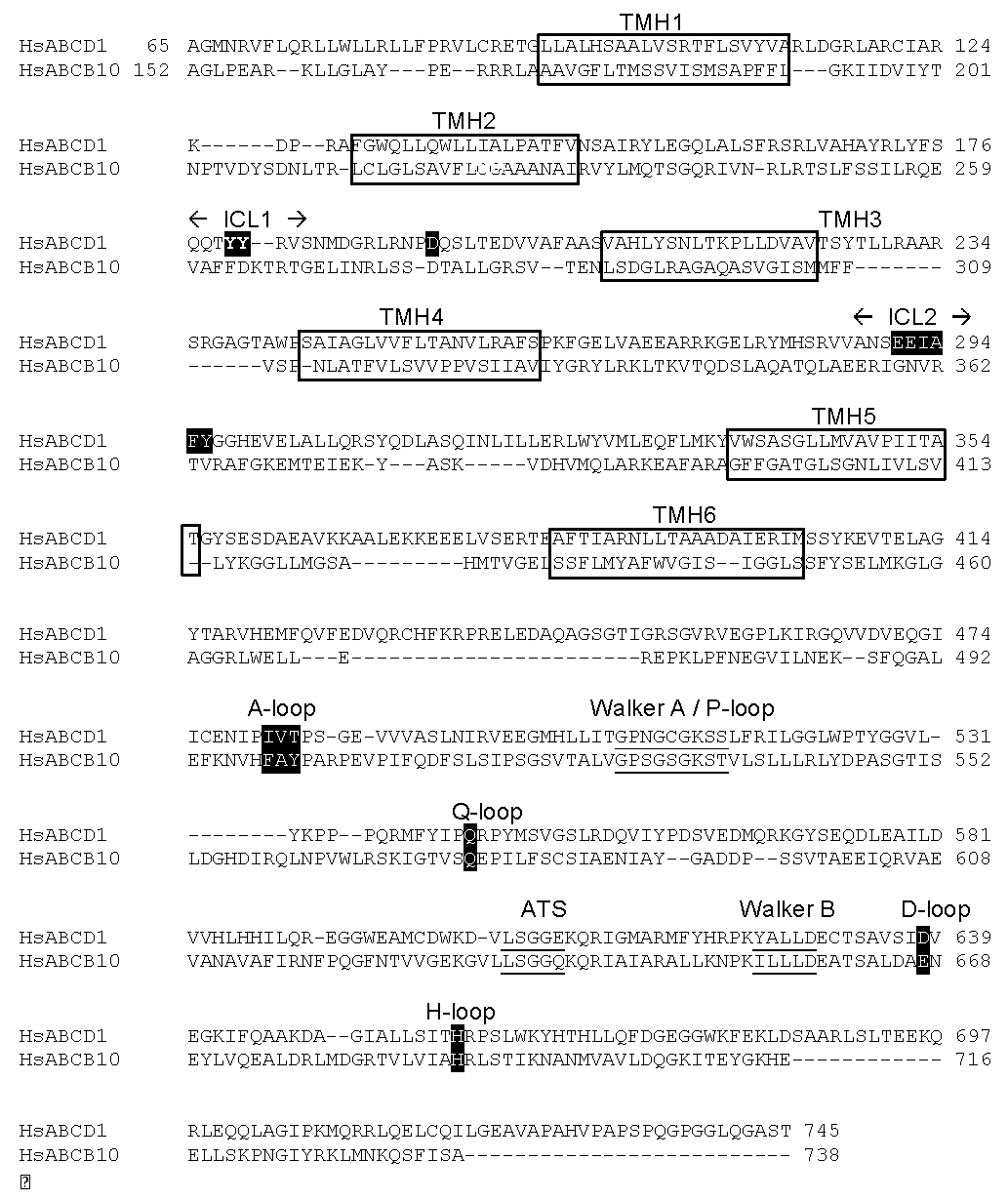
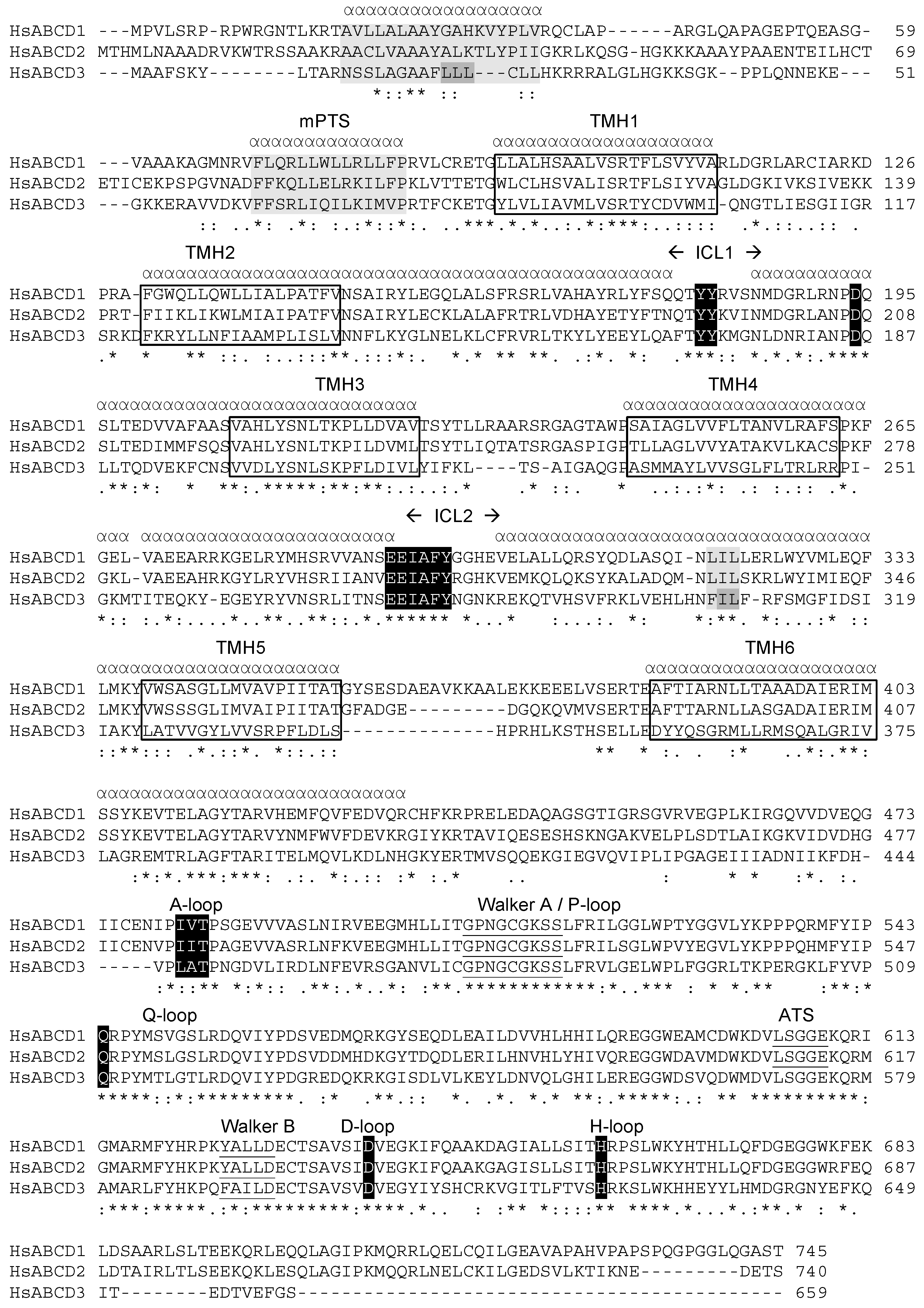
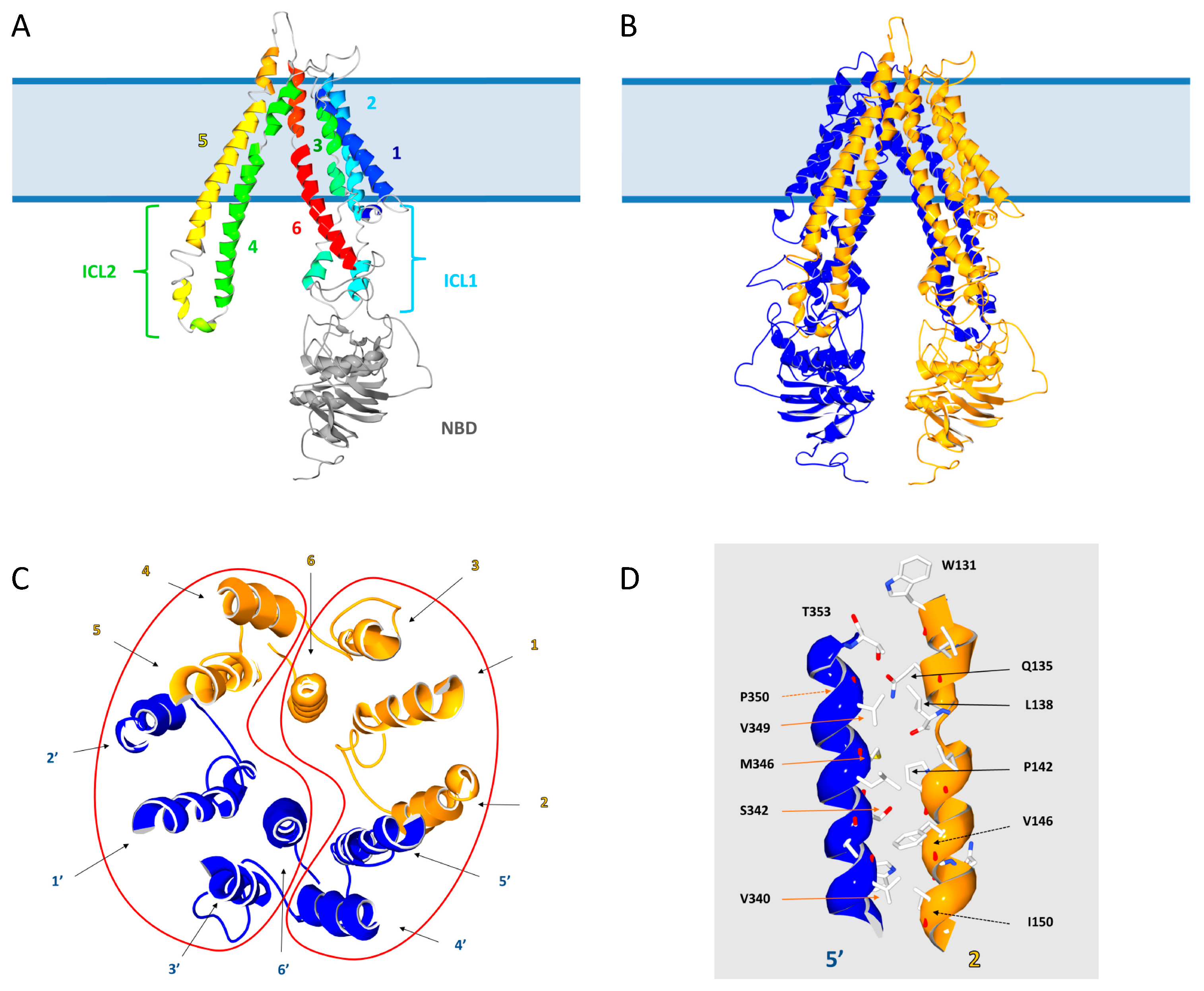
2.1. Position of Transmembrane α Helices
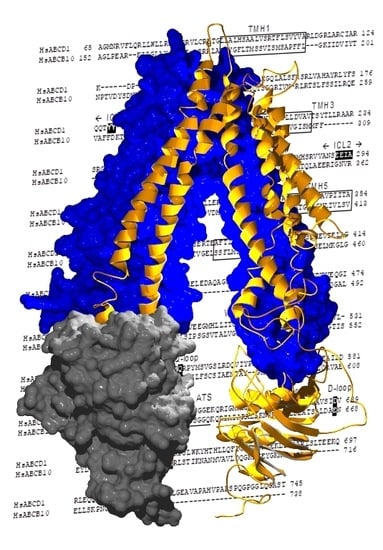
2.2. Structural Model of ABCD1 Based on ABCB10 Homology
3. Discussion
3.1. The Membrane Peroxisome Targeting Signal and the PEX19 Binding Site
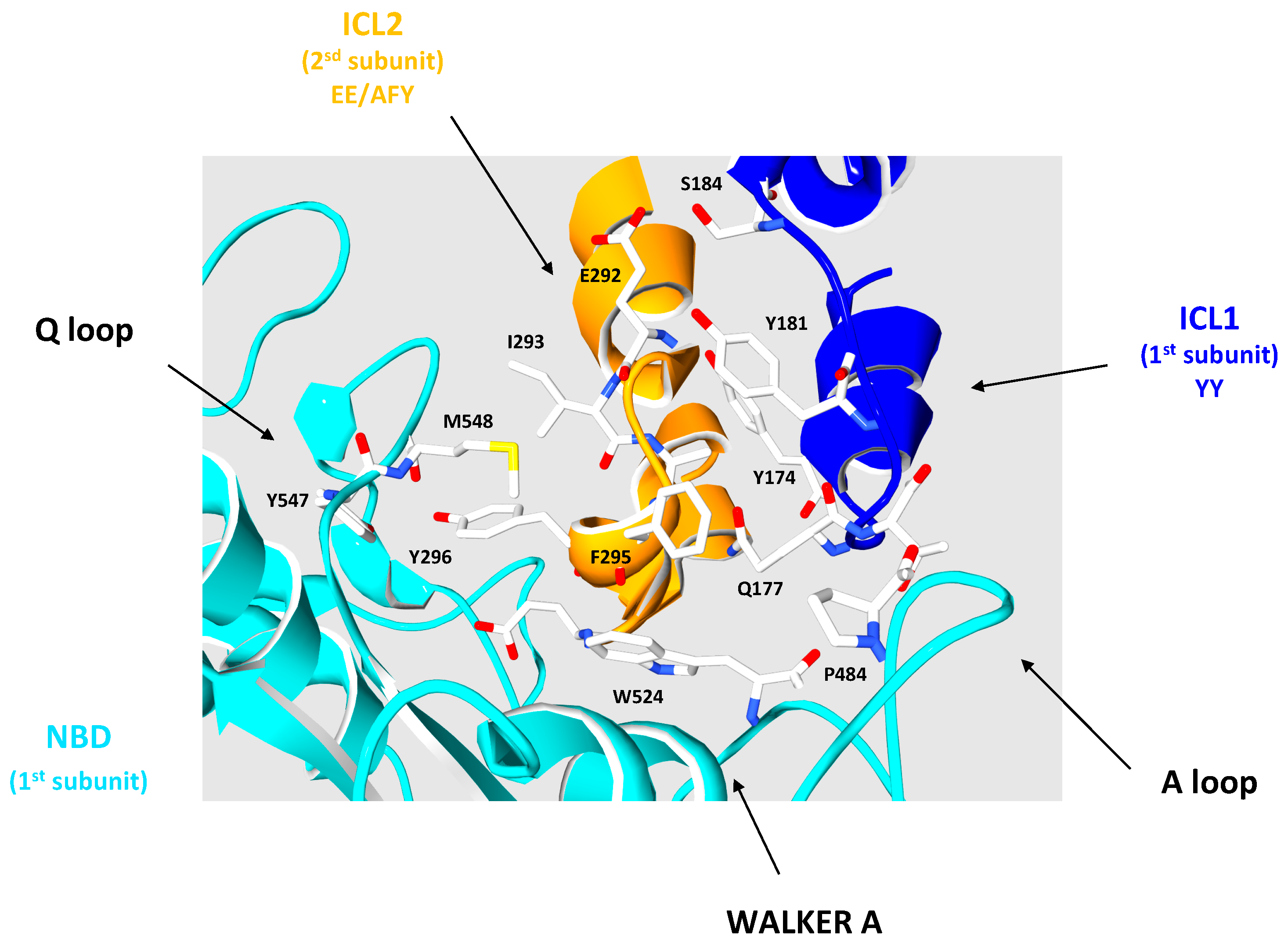
3.2. The Intracellular Loops
3.3. Motifs Putatively Involved in Substrate Specificity
3.4. Motifs Putatively Involved in Dimerization
4. Methods
4.1. Prediction of Transmembrane Helices
4.2. Alignment
4.3. Homology Modeling
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABC | ATP-binding Cassette |
| ATS | ABC Transporter Signature |
| ICL | IntraCellular Loop |
| FA | Fatty Acid |
| MSA | Multiple Sequence Alignment |
| NBD | Nucleotide-binding Domain |
| TMD | TransMembrane Domain |
| TMH | TransMembrane Helix |
| VLCFA | Very Long-Chain Fatty Acid |
| X-ALD | X-linked Adrenoleukodystrophy |
Appendix A
References
- Dean, M.; Annilo, T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genom. Hum. Genet. 2005, 6, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Kos, V.; Ford, R.C. The ATP-binding cassette family: A structural perspective. Cell. Mol. Life Sci. 2009, 66, 3111–3126. [Google Scholar] [CrossRef] [PubMed]
- Biemans-Oldehinkel, E.; Doeven, M.K.; Poolman, B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 2006, 580, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Mosser, J.; Douar, A.M.; Sarde, C.O.; Kioschis, P.; Feil, R.; Moser, H.; Poustka, A.M.; Mandel, J.L.; Aubourg, P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993, 361, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Lombard-Platet, G.; Savary, S.; Sarde, C.O.; Mandel, J.L.; Chimini, G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. USA 1996, 93, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, K.; Taketani, S.; Yokota, S.; Osumi, T.; Hashimoto, T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J. Biol. Chem. 1990, 265, 4534–4540. [Google Scholar] [PubMed]
- Kim, J.C.; Lee, N.C.; Hwu, P.W.; Chien, Y.H.; Fahiminiya, S.; Majewski, J.; Watkins, D.; Rosenblatt, D.S. Late onset of symptoms in an atypical patient with the cblJ inborn error of vitamin B12 metabolism: Diagnosis and novel mutation revealed by exome sequencing. Mol. Genet. Metab. 2012, 107, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.; Kim, J.C.; Miousse, I.R.; Fung, S.; Du Moulin, M.; Buers, I.; Suormala, T.; Burda, P.; Frapolli, M.; Stucki, M.; et al. Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat. Genet. 2012, 44, 1152–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwayama, Y.; Seki, M.; Yasui, A.; Murasaki, Y.; Morita, M.; Yamashita, Y.; Sakaguchi, M.; Tanaka, Y.; Imanaka, T. 70-kDa peroxisomal membrane protein related protein (P70R/ABCD4) localizes to endoplasmic reticulum not peroxisomes, and NH2-terminal hydrophobic property determines the subcellular localization of ABC subfamily D proteins. Exp. Cell Res. 2009, 315, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Geillon, F.; Gondcaille, C.; Charbonnier, S.; van Roermund, C.W.; Lopez, T.E.; Dias, A.M.; Pais de Barros, J.P.; Arnould, C.; Wanders, R.J.; Trompier, D.; et al. Structure-Function Analysis of Peroxisomal ATP-binding Cassette Transporters Using Chimeric Dimers. J. Biol. Chem. 2014, 289, 24511–24520. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.C.; Geillon, F.; Gondcaille, C.; Athias, A.; Gambert, P.; Trompier, D.; Savary, S. Substrate specificity overlap and interaction between adrenoleukodystrophy protein (ALDP/ABCD1) and adrenoleukodystrophy-related protein (ALDRP/ABCD2). J. Biol. Chem. 2011, 286, 8075–8085. [Google Scholar] [CrossRef] [PubMed]
- Van Roermund, C.W.; Visser, W.F.; Ijlst, L.; van Cruchten, A.; Boek, M.; Kulik, W.; Waterham, H.R.; Wanders, R.J. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008, 22, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.P.; Domingues, P.; Aubourg, P.; Fouquet, F.; Pujol, A.; Jimenez-Sanchez, G.; Sa-Miranda, C.; Azevedo, J.E. Mouse liver PMP70 and ALDP: Homomeric interactions prevail in vivo. Biochim. Biophys. Acta-Mol. Basis Dis. 2004, 1689, 235–423. [Google Scholar] [CrossRef] [PubMed]
- Geillon, F.; Gondcaille, C.; Raas, Q.; Dias, A.M.M.; Pecqueur, D.; Truntzer, C.; Lucchi, G.; Ducoroy, P.; Falson, P.; Savary, S.; et al. Peroxisomal ATP-binding cassette transporters form mainly tetramers. J. Biol. Chem. 2017, 292, 6965–6977. [Google Scholar] [CrossRef] [PubMed]
- Trompier, D.; Savary, S. X-Linked Adrenoleukodystrophy, 1st ed.; Morgan and Claypool Life Sciences Publishers: San Rafael, CA, USA, 2013; ISBN 9781615045549. [Google Scholar]
- Ward, A.B.; Szewczyk, P.; Grimard, V.; Lee, C.W.; Martinez, L.; Doshi, R.; Caya, A.; Villaluz, M.; Pardon, E.; Cregger, C.; et al. Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc. Natl. Acad. Sci. USA 2013, 110, 13386–13391. [Google Scholar] [CrossRef] [PubMed]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Z.; Csanady, L.; Gadsby, D.C.; Chen, J. Molecular structure of the human CFTR Ion channel. Cell 2017, 169, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Carrier, D.J.; Schaedler, T.; Waterham, H.R.; van Roermund, C.W.; Theodoulou, F.L. Peroxisomal ABC transporters: Functions and mechanism. Biochem. Soc. Trans. 2015, 43, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Van Roermund, C.W.; Ijlst, L.; Wagemans, T.; Wanders, R.J.; Waterham, H.R. A role for the human peroxisomal half-transporter ABCD3 in the oxidation of dicarboxylic acids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1841, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Van Roermund, C.W.; Visser, W.F.; Ijlst, L.; Waterham, H.R.; Wanders, R.J. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid beta-oxidation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1811, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, S.; Liu, X.; Brown, J.A.; Newman, K.E.; Sunkara, M.; Morris, A.J.; Bhatnagar, S.; Li, X.; Pujol, A.; et al. The absence of ABCD2 sensitizes mice to disruptions in lipid metabolism by dietary erucic acid. J. Lipid Res. 2012, 53, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, S.; Ruiz, M.; Camps, C.; Schluter, A.; Houten, S.M.; Mooyer, P.A.; Pampols, T.; Dacremont, G.; Wanders, R.J.; Giros, M.; et al. A key role for the peroxisomal ABCD2 transporter in fatty acid homeostasis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E211–E221. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Ferrer, I.; Camps, C.; Metzger, E.; Hindelang, C.; Callizot, N.; Ruiz, M.; Pampols, T.; Giros, M.; Mandel, J.L. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: A therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 2004, 13, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Hindelang, C.; Callizot, N.; Bartsch, U.; Schachner, M.; Mandel, J.L. Late onset neurological phenotype of the X-ALD gene inactivation in mice: A mouse model for adrenomyeloneuropathy. Hum. Mol. Genet. 2002, 11, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Jimenez-Sanchez, G.; Koster, J.; Denis, S.; van Roermund, C.W.; Silva-Zolezzi, I.; Moser, A.B.; Visser, W.F.; Gulluoglu, M.; Durmaz, O.; et al. A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum. Mol. Genet. 2015, 24, 361–730. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.P.; Sa-Miranda, C.; Azevedo, J.E. Probing substrate-induced conformational alterations in adrenoleukodystrophy protein by proteolysis. J. Hum. Genet. 2005, 50, 99–105. [Google Scholar] [CrossRef] [PubMed]
- De Marcos Lousa, C.; van Roermund, C.W.; Postis, V.L.; Dietrich, D.; Kerr, I.D.; Wanders, R.J.; Baldwin, S.A.; Baker, A.; Theodoulou, F.L. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. USA 2013, 110, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L.; Carrier, D.J.; Schaedler, T.A.; Baldwin, S.A.; Baker, A. How to move an amphipathic molecule across a lipid bilayer: different mechanisms for different ABC transporters? Biochem. Soc. Trans. 2016, 44, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.A.; Ellis, J.M. Peroxisomal acyl-CoA synthetases. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Casteels, M.; Schepers, L.; van Veldhoven, P.P.; Eyssen, H.J.; Mannaerts, G.P. Separate peroxisomal oxidases for fatty acyl-CoAs and trihydroxycoprostanoyl-CoA in human liver. J. Lipid Res. 1990, 31, 1865–1872. [Google Scholar] [PubMed]
- Baumgart, E.; vanhooren, J.C.; Fransen, M.; Marynen, P.; Puype, M.; vandekerckhove, J.; Leunissen, J.A.; Fahimi, H.D.; Mannaerts, G.P.; van Veldhoven, P.P. Molecular characterization of the human peroxisomal branched-chain acyl-CoA oxidase: cDNA cloning, chromosomal assignment, tissue distribution, and evidence for the absence of the protein in Zellweger syndrome. Proc. Natl. Acad. Sci. USA 1996, 93, 13748–13753. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Pujol, A.; Waterham, H.R.; van Geel, B.M.; Boehm, C.D.; Raymond, G.V.; Cutting, G.R.; Wanders, R.J.; Moser, H.W. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: Role in diagnosis and clinical correlations. Hum. Mutat. 2001, 18, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Morita, M.; Maeda, T.; Harayama, Y.; Shimozawa, N.; Suzuki, Y.; Furuya, H.; Sato, R.; Kashiwayama, Y.; Imanaka, T. Adrenoleukodystrophy: subcellular localization and degradation of adrenoleukodystrophy protein (ALDP/ABCD1) with naturally occurring missense mutations. J. Neurochem. 2007, 101, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Kashiwayama, Y.; Asahina, K.; Morita, M.; Imanaka, T. Hydrophobic regions adjacent to transmembrane domains 1 and 5 are important for the targeting of the 70-kDa peroxisomal membrane protein. J. Biol. Chem. 2007, 282, 33831–33844. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, P.; Mayerhofer, P.U.; Polanetz, R.; Roscher, A.A.; Holzinger, A. Targeting of the human adrenoleukodystrophy protein to the peroxisomal membrane by an internal region containing a highly conserved motif. Eur. J. Cell Biol. 2003, 82, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Shani, N.; Sapag, A.; Valle, D. Characterization and analysis of conserved motifs in a peroxisomal ATP-binding cassette transporter. J. Biol. Chem. 1996, 271, 8725–8730. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.; Croning, M.D.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, J.M.; Doyle, D.A.; Sansom, M.S. Transmembrane helix prediction: A comparative evaluation and analysis. Protein Eng. Des. Sel. 2005, 18, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 1994, 33, 3038–3049. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Porollo, A.; Adamczak, R.; Jarrell, M.; Meller, J. Enhanced recognition of protein transmembrane domains with prediction-based structural profiles. Bioinformatics 2006, 22, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Shen, H.B. Prediction enhancement of residue real-value relative accessible surface area in transmembrane helical proteins by solving the output preference problem of machine learning-based predictors. J. Chem. Inf. Model. 2015, 55, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Lemesle-Varloot, L.; Henrissat, B.; Gaboriaud, C.; Bissery, V.; Morgat, A.; Mornon, J.P. Hydrophobic cluster analysis: Procedures to derive structural and functional information from 2-D-representation of protein sequences. Biochimie 1990, 72, 555–574. [Google Scholar] [CrossRef]
- Callebaut, I.; Labesse, G.; Durand, P.; Poupon, A.; Canard, L.; Chomilier, J.; Henrissat, B.; Mornon, J.P. Deciphering protein sequence information through hydrophobic cluster analysis (HCA): Current status and perspectives. Cell. Mol. Life Sci. 1997, 53, 621–645. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jaimes, K.F.; Aller, S.G. Refined structures of mouse P-glycoprotein. Protein Sci. 2014, 23, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Shintre, C.A.; Pike, A.C.; Li, Q.; Kim, J.I.; Barr, A.J.; Goubin, S.; Shrestha, L.; Yang, J.; Berridge, G.; Ross, J.; et al. Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states. Proc. Natl. Acad. Sci. USA 2013, 110, 9710–9715. [Google Scholar] [CrossRef] [PubMed]
- Biermanns, M.; Gartner, J. Targeting elements in the amino-terminal part direct the human 70-kDa peroxisomal integral membrane protein (PMP70) to peroxisomes. Biochem. Biophys. Res. Commun. 2001, 285, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, S.; Tsuchida, M.; Tsukuda, M.; Yamashita, Y.; Emi, Y.; Kida, Y.; Komori, M.; Kashiwayama, Y.; Imanaka, T.; Sakaguchi, M. Multiple organelle-targeting signals in the N-terminal portion of peroxisomal membrane protein PMP70. J. Biochem. 2009, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Gloeckner, C.J.; Mayerhofer, P.U.; Landgraf, P.; Muntau, A.C.; Holzinger, A.; Gerber, J.K.; Kammerer, S.; Adamski, J.; Roscher, A.A. Human adrenoleukodystrophy protein and related peroxisomal ABC transporters interact with the peroxisomal assembly protein PEX19p. Biochem. Biophys. Res. Commun. 2000, 271, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kashiwayama, Y.; Asahina, K.; Shibata, H.; Morita, M.; Muntau, A.C.; Roscher, A.A.; Wanders, R.J.; Shimozawa, N.; Sakaguchi, M.; Kato, H.; et al. Role of Pex19p in the targeting of PMP70 to peroxisome. Biochim. Biophys. Acta-Mol. Cell Res. 2005, 1746, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Morrell, J.C.; Gould, S.J. PEX19 is a predominantly cytosolic chaperone and import receptor for class 1 peroxisomal membrane proteins. J. Cell Biol. 2004, 164, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Halbach, A.; Lorenzen, S.; Landgraf, C.; Volkmer-Engert, R.; Erdmann, R.; Rottensteiner, H. Function of the PEX19-binding site of human adrenoleukodystrophy protein as targeting motif in man and yeast. PMP targeting is evolutionarily conserved. J. Biol. Chem. 2005, 280, 21176–21182. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.C.; Tajkhorshid, E. Conformational coupling of the nucleotide-binding and the transmembrane domains in ABC transporters. Biophys. J. 2011, 101, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Saurin, W.; Köster, W.; Dassa, E. Bacterial binding protein-dependent permeases: Characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol. Microbiol. 1994, 12, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Seigneuret, M.; Garnier-Suillerot, A. A structural model for the open conformation of the mdr1 P-glycoprotein based on the MsbA crystal structure. J. Biol. Chem. 2003, 278, 30115–30124. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.J.; Locher, K.P. Structure of a bacterial multidrug ABC transporter. Nature 2006, 443, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Human P-glycoprotein is active when the two halves are clamped together in the closed conformation. Biochem. Biophys. Res. Commun. 2010, 395, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, G.; Molzer, B.; Forss-Petter, S.; Berger, J. Co-expression of mutated and normal adrenoleukodystrophy protein reduces protein function: Implications for gene therapy of X-linked adrenoleukodystrophy. Hum. Mol. Genet. 2000, 9, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Ter Beek, J.; Guskov, A.; Slotboom, D.J. Structural diversity of ABC transporters. J. Gen. Physiol. 2014, 143, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Ravna, A.W.; Sylte, I.; Sager, G. Binding site of ABC transporter homology models confirmed by ABCB1 crystal structure. Theor. Biol. Med. Model. 2009, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.Y.; Chen, L.Y.; Fu, R.H.; Chen, S.M.; Ho, M.H.; Huang, J.M.; Hsu, C.C.; Wang, C.C.; Chen, M.S.; Tsai, R.T. Involvement of the carboxyl-terminal region of the yeast peroxisomal half ABC transporter Pxa2p in its interaction with Pxa1p and in transporter function. PLoS ONE 2014, 9, e104892. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Janvier, K.; Berteaux-Lecellier, V.; Cartier, N.; Benarous, R.; Aubourg, P. Homo- and heterodimerization of peroxisomal ATP-binding cassette half-transporters. J. Biol. Chem. 1999, 274, 32738–32743. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
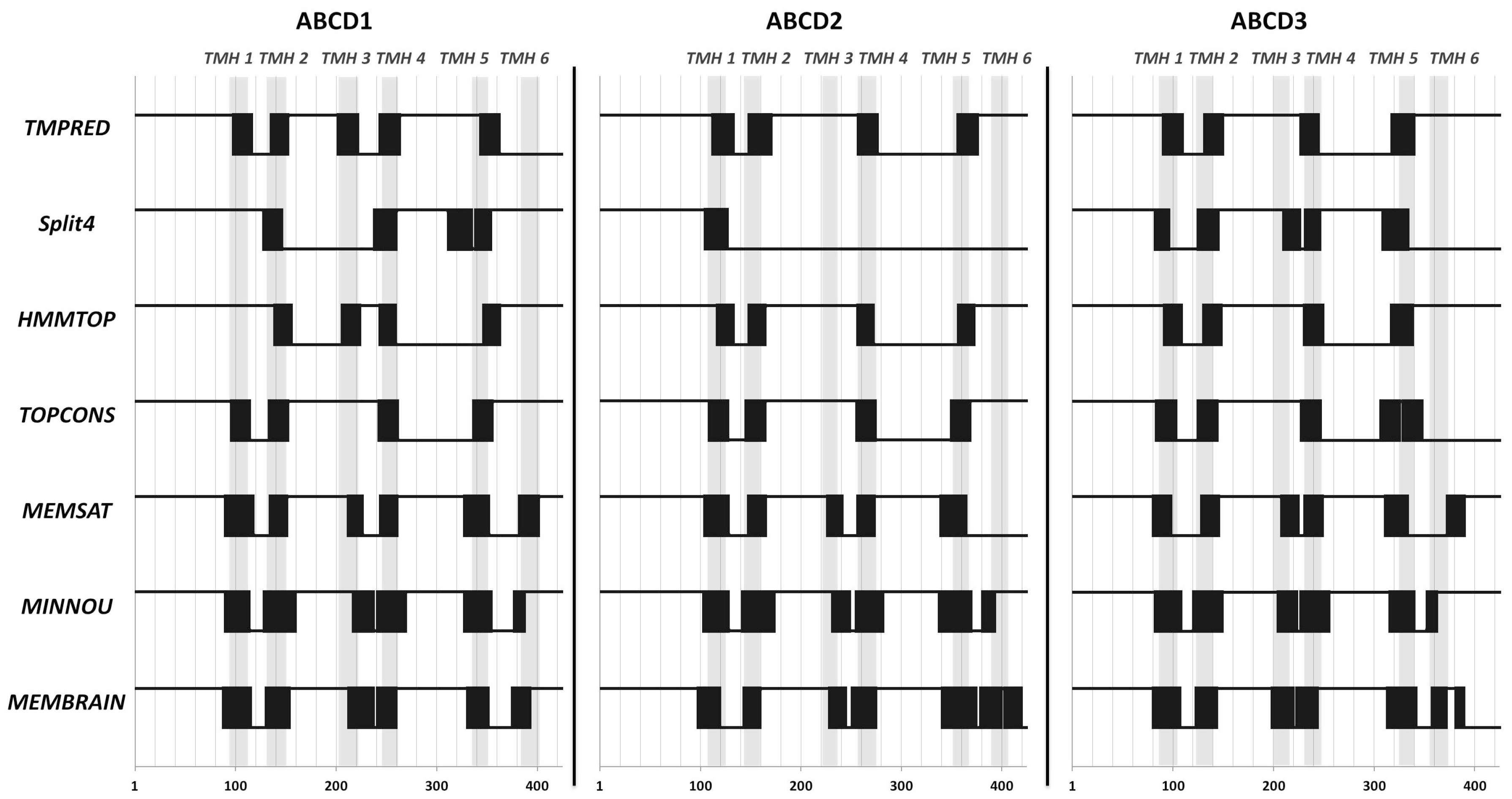
| Transporter | TMH 1 | TMH 2 | TMH 3 | TMH 4 | TMH 5 | TMH 6 |
|---|---|---|---|---|---|---|
| ABCD1 | 93–112 | 130–147 | 208–224 | 244–262 | 338–355 | 384–403 |
| ABCD2 | 106–125 | 143–160 | 221–237 | 257–274 | 351–368 | 388–407 |
| ABCD3 | 85–104 | 122–139 | 200–216 | 231–248 | 324–341 | 356–375 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreoletti, P.; Raas, Q.; Gondcaille, C.; Cherkaoui-Malki, M.; Trompier, D.; Savary, S. Predictive Structure and Topology of Peroxisomal ATP-Binding Cassette (ABC) Transporters. Int. J. Mol. Sci. 2017, 18, 1593. https://doi.org/10.3390/ijms18071593
Andreoletti P, Raas Q, Gondcaille C, Cherkaoui-Malki M, Trompier D, Savary S. Predictive Structure and Topology of Peroxisomal ATP-Binding Cassette (ABC) Transporters. International Journal of Molecular Sciences. 2017; 18(7):1593. https://doi.org/10.3390/ijms18071593
Chicago/Turabian StyleAndreoletti, Pierre, Quentin Raas, Catherine Gondcaille, Mustapha Cherkaoui-Malki, Doriane Trompier, and Stéphane Savary. 2017. "Predictive Structure and Topology of Peroxisomal ATP-Binding Cassette (ABC) Transporters" International Journal of Molecular Sciences 18, no. 7: 1593. https://doi.org/10.3390/ijms18071593