Variability in DNA Repair Capacity Levels among Molecular Breast Cancer Subtypes: Triple Negative Breast Cancer Shows Lowest Repair
Abstract
:1. Introduction
2. Results
2.1. Description of Study Group
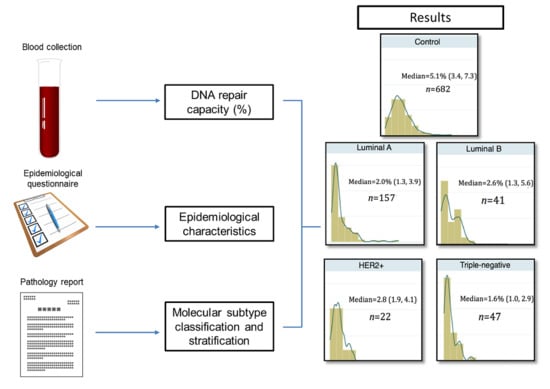
2.2. Differential Distribution of DRC
2.3. Molecular Subtypes of Breast Cancer by Different Characteristics
2.4. Pathological Characteristics of Breast Tumors
2.5. Magnitude of the Association
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Use of Human Subjects
4.3. Blood Collection and Isolation of Lymphocytes
4.4. DNA Repair Capacity Measurements
4.5. Hormone Receptor Status
4.6. Classification of Tumors Based on IHC Receptor Status Information
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| DRC | DNA repair capacity |
| NER | Nucleotide excision repair |
| IHC | Immunohistochemistry |
| TNBC | Triple-negative breast cancer |
| BMI | Body mass index |
| HRT | Hormone replacement therapy |
| TN | Triple-negative |
| SEM | Standard error of the mean |
| OR | Odds ratio |
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11; International Agency for Research on Cancer: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 18 June 2017).
- American Cancer Society (ACS). Cancer Facts & Figures 2017; American Cancer Society: Atlanta, GA, USA, 2017; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html (accessed on 18 June 2017).
- PRCCC: Puerto Rico Comprehensive Cancer Control Plan: 2015–2020. In Puerto Rico Cancer Control Coalition and Puerto Rico Comprehensive Control Program. 2014. Available online: http://mindoven.rocks/ccpdf/ (accessed on 15 June 2017).
- Gaudet, M.M.; Press, M.F.; Haile, R.W.; Lynch, C.F.; Glaser, S.L.; Schildkraut, J.; Gammon, M.D.; Thompson, W.D.; Bernstein, J.L. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 2011, 130, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.N.; Schwab, R.B.; Martinez, M.E. Reproductive risk factors and breast cancer subtypes: A review of the literature. Breast Cancer Res. Treat. 2014, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Banegas, M.P.; Tao, L.; Altekruse, S.; Anderson, W.F.; John, E.M.; Clarke, C.A.; Gomez, S.L. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res. Treat. 2014, 144, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ding, Y.; Zhang, L.Y.; Song, D.; Gong, Y.; Adams, S.; Ross, D.S.; Wang, J.H.; Grover, S.; Doval, D.C.; et al. Distinct breast cancer subtypes in women with early-onset disease across races. Am. J. Cancer Res. 2014, 4, 337–352. [Google Scholar] [PubMed]
- Perou, C.M. Molecular stratification of triple-negative breast cancers. Oncologist 2011, 16, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.; He, X.; Perou, C. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.E.; Caldeira, J.R.; Felipes, J.; Bertonha, F.B.; Quevedo, F.C.; Domingues, M.A.; Moraes Neto, F.A.; Rogatto, S.R. Evaluation of estrogen receptor α and β and progesterone receptor expression and correlation with clinicopathologic factors and proliferative marker Ki-67 in breast cancers. Hum. Pathol. 2008, 39, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Rumble, R.B.; Carey, L.A.; Come, S.E.; Davidson, N.E.; di Leo, A.; Gralow, J.; Hortobagyi, G.N.; Moy, B.; Yee, D.; et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Echenique, M.; Negron, E.; Morales, L.; Vargas, W.; Gaetan, F.S.; Lizardi, E.R.; Torres, A.; Rosado, J.O.; Bolanos, G.; et al. The association of DNA Repair with breast cancer risk in women. A comparative observational study. BMC Cancer 2012, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.L.; Villa, J.L.; Ramos, J.M.; Sanchez, J.; Chompre, G.; Ruiz, A.; Grossman, L. DNA repair and nonmelanoma skin cancer in Puerto Rican populations. J. Am. Acad. Dermatol. 2003, 49, 433–439. [Google Scholar] [CrossRef]
- Wei, Q.; Matanoski, G.M.; Farmer, E.R.; Hedayati, M.A.; Grossman, L. DNA repair and aging in basal cell carcinoma: A molecular epidemiology study. Proc. Natl. Acad. Sci. USA 1993, 90, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.; Ruiz, A.; Colen, R.; Lopez, I.D.; Grossman, L.; Matta, J.L. DNA repair and breast carcinoma susceptibility in women. Cancer 2004, 100, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.J.; Johnson, J.M.; Kelly, C.M.; Miles, T.D.; Beaudry-Rodgers, K.A.; Lalanne, N.A.; Vogel, V.G.; Kanbour-Shakir, A.; Kelley, J.L.; Johnson, R.R.; et al. Nucleotide excision repair deficiency is intrinsic in sporadic stage I breast cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 21725–21730. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Morales, L.; Ortiz, C.; Adams, D.; Vargas, W.; Casbas, P.; Dutil, J.; Echenique, M.; Suarez, E. Estrogen receptor expression is associated with DNA repair capacity in breast cancer. PLoS ONE 2016, 11, e0152422. [Google Scholar] [CrossRef] [PubMed]
- Voduc, K.D.; Cheang, M.C.U.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.; DeRouen, M.C.; Press, D.J.; Kurian, A.W.; Clarke, C.A. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012, 14, R55. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.P.; Frias, O.; Perez, J.; Cabanillas, F.; Martinez, L.; Sanchez, C.; Capo-Ramos, D.E.; Gonzalez-Keelan, C.; Mora, E.; Suarez, E. Breast cancer molecular subtypes and survival in a hospital-based sample in Puerto Rico. Cancer Med. 2013, 2, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ovcaricek, T.; Frkovic, S.G.; Matos, E.; Mozina, B.; Borstnar, S. Triple negative breast cancer—Prognostic factors and survival. Radiol. Oncol. 2011, 45, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Lüchtenborg, M.; Davies, E.A.; Jack, R.H. The treatment and survival of patients with triple negative breast cancer in a London population. SpringerPlus 2014, 3, 553. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Ganzinelli, M.; Andreis, D.; Bertoni, R.; Giardini, R.; Fox, S.B.; Broggini, M.; Bottini, A.; Zanoni, V.; Bazzola, L.; et al. Triple negative breast cancers have a reduced expression of DNA repair genes. PLoS ONE 2013, 8, e66243. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Sprott, K.; Farrow, D.A.; Wang, X.; D‘Andrea, A.D.; Schnitt, S.J.; Collins, L.C.; Weaver, D.T.; Garber, J.E. DNA repair protein biomarkers associated with time to recurrence in triple-negative breast cancer. Clin. Cancer Res. 2010, 16, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Bekele, R.T.; Venkatraman, G.; Liu, R.Z.; Tang, X.; Mi, S.; Benesch, M.G.; Mackey, J.R.; Godbout, R.; Curtis, J.M.; McMullen, T.P.; et al. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: Implications for tamoxifen therapy and resistance. Sci. Rep. 2016, 6, 21164. [Google Scholar] [CrossRef] [PubMed]
- Leprat, F.; Alapetite, C.; Rosselli, F.; Ridet, A.; Schlumberger, M.; Sarasin, A.; Suarez, H.G.; Moustacchi, E. Impaired DNA repair as assessed by the “comet“ assay in patients with thyroid tumors after a history of radiation therapy: A preliminary study. Int. J. Radiat Oncol. Biol. Phys. 1998, 40, 1019–1026. [Google Scholar] [CrossRef]
- Bosken, C.H.; Wei, Q.; Amos, C.I.; Spitz, M.R. An analysis of DNA repair as a determinant of survival in patients with non-small-cell lung cancer. J. Natl. Cancer Inst. 2002, 94, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, A.A.; Timmerman, A.J.; van der Schans, G.P.; Lohman, P.H.; Baan, R.A. Different repair kinetics of radiation-induced DNA lesions in human and murine white blood cells. Carcinogenesis 1992, 13, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Mendez, P.; Taron, M.; Moran, T.; Fernandez, M.A.; Requena, G.; Rosell, R. A modified host-cell reactivation assay to quantify DNA repair capacity in cryopreserved peripheral lymphocytes. DNA Repair. 2011, 10, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Athas, W.F.; Hedayati, M.A.; Matanoski, G.M.; Farmer, E.R.; Grossman, L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991, 51, 5786–5793. [Google Scholar] [PubMed]
- Morales, L.; Alvarez-Garriga, C.; Matta, J.; Ortiz, C.; Vergne, Y.; Vargas, W.; Acosta, H.; Ramirez, J.; Perez-Mayoral, J.; Bayona, M. Factors associated with breast cancer in Puerto Rican women. J. Epidemiol. Glob. Health 2013, 3, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Vergne, Y.; Matta, J.; Morales, L.; Vargas, W.; Alvarez-Garriga, C.; Bayona, M. Breast cancer and DNA repair capacity: Association with use of multivitamin and calcium supplements. Integr. Med. 2013, 12, 38–46. [Google Scholar]
- Wang, L.; Wei, Q.; Shi, Q.; Guo, Z.; Qiao, Y.; Spitz, M.R. A modified host-cell reactivation assay to measure repair of alkylating DNA damage for assessing risk of lung adenocarcinoma. Carcinogenesis 2007, 28, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Wolff, A.C.; Mangu, P.B.; Temin, S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Oncol. Pract. 2010, 6, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef] [PubMed]

| Socio-demographic Characteristics | Controls n (%) | Luminal A n (%) | Luminal B n (%) | HER2+ n (%) | TN n (%) | p-Value a |
|---|---|---|---|---|---|---|
| n = 949 | 682 (71.9) | 157 (16.5) | 41 (4.3) | 22 (2.3) | 47 (4.9) | |
| DRC | <0.001 | |||||
| <4.3 | 271 (39.7) | 122 (77.7) | 26 (63.4) | 17 (77.3) | 39 (83.0) | |
| ≥4.3 | 411 (60.3) | 35 (22.3) | 15 (36.6) | 5 (22.7) | 8 (17.0) | |
| Family History of BC | 0.082 | |||||
| No | 457 (67.0) | 118 (75.2) | 33 (80.5) | 18 (81.8) | 32 (68.1) | |
| Yes | 225 (33.0) | 39 (24.8) | 8 (19.5) | 4 (18.2) | 15 (31.9) | |
| Age at Diagnosis | 0.040 | |||||
| <45 | 193 (28.3) | 26 (16.6) | 6 (14.6) | 3 (13.6) | 9 (19.1) | |
| 45–55 | 228 (33.4) | 46 (29.3) | 15 (36.6) | 6 (27.3) | 16 (34.0) | |
| 56–65 | 142 (20.8) | 43 (27.4) | 15 (36.6) | 7 (31.8) | 10 (21.3) | |
| 66–75 | 95 (13.9) | 33 (21.0) | 4 (9.8) | 5 (22.7) | 9 (19.1) | |
| >75 | 24 (3.5) | 9 (5.7) | 1 (2.4) | 1 (4.6) | 3 (6.4) | |
| Median age b | 51 (43, 61) | 58 (47, 66) | 55 (50, 62) | 54 (46, 66) | 60 (52, 69) | |
| Menopausal Status | 0.004 | |||||
| No | 258 (37.8) | 39 (25.0) | 8 (19.5) | 6 (27.3) | 13 (27.7) | |
| Yes | 424 (62.2) | 117 (75.0) | 33 (80.5) | 16 (72.7) | 34 (72.3) | |
| Not specified | 0 | 1 | 0 | 0 | 0 | |
| Age at Menarche | >0.1 | |||||
| <13 | 292 (42.9) | 74 (48.1) | 19 (46.3) | 13 (59.1) | 22 (46.8) | |
| ≥13 | 389 (57.1) | 80 (51.9) | 22 (53.7) | 9 (40.9) | 25 (53.2) | |
| Not specified | 1 | 3 | 0 | 0 | 0 | |
| BMI | >0.1 | |||||
| <25 | 236 (34.6) | 43 (27.4) | 14 (34.2) | 10 (45.5) | 13 (27.7) | |
| 25–30 | 258 (37.8) | 52 (33.1) | 18 (43.9) | 5 (22.7) | 20 (42.6) | |
| >30 | 188 (27.6) | 62 (39.5) | 9 (21.9) | 7 (31.8) | 14 (29.8) | |
| Alcohol Intake | 0.033 | |||||
| Never | 555 (82.6) | 138 (89.0) | 39 (95.1) | 17 (77.3) | 43 (91.5) | |
| Ever | 117 (17.4) | 17 (11.0) | 2 (4.9) | 5 (22.7) | 4 (8.5) | |
| Not specified | 10 | 2 | 0 | 0 | 0 | |
| Smoking Habit | >0.1 | |||||
| Never | 614 (91.1) | 137 (87.8) | 39 (95.1) | 20 (90.9) | 43 (91.5) | |
| Ever | 60 (8.9) | 19 (12.2) | 2 (4.9) | 2 (9.1) | 4 (8.5) | |
| Not specified | 8 | 1 | 0 | 0 | 0 | |
| Oral Contraceptives (Pre-Menopausal Women Only) | 0.054 | |||||
| Never | 303 (45.2) | 89 (58.2) | 22 (53.7) | 12 (54.5) | 23 (48.9) | |
| Ever | 367 (54.8) | 64 (41.8) | 19 (46.3) | 10 (45.5) | 24 (51.1) | |
| Not specified | 12 | 4 | 0 | 0 | 0 | |
| Vitamin Intake (Multivitamin or Calcium) | <0.001 | |||||
| No | 333 (48.8) | 96 (61.1) | 28 (68.3) | 14 (63.6) | 37 (78.7) | |
| Yes | 349 (51.2) | 61 (38.9) | 13 (31.7) | 8 (36.4) | 10 (21.3 ) | |
| HRT (Post-Menopausal Women Only) | 0.009 | |||||
| Never | 205 (48.3) | 68 (58.1) | 22 (66.7) | 12 (75.0) | 23 (67.6) | |
| Ever | 219 (51.7) | 49 (41.9) | 11 (33.3) | 4 (25.0) | 11 (32.4) | |
| Pathological Characteristics | Luminal A n (%) | Luminal B n (%) | HER2+ n (%) | TN n (%) | p-Value a |
|---|---|---|---|---|---|
| Type of Breast Cancer | >0.1 | ||||
| Carcinoma in situ | 7 (4.6) | 3 (7.3) | 4 (18.2) | 1 (2.2) | |
| Invasive | 130 (85.0) | 34 (82.9) | 17 (77.3) | 42 (93.3) | |
| Mixed invasive | 16 (10.4) | 4 (9.8) | 1 (4.5) | 2 (4.4) | |
| Not specified | 2 | 0 | 0 | 2 | |
| Grade | <0.001 | ||||
| I | 28 (20.3) | 2 (5.0) | 0 (0.0) | 0 (0.0) | |
| II | 81 (58.7) | 22 (55.0) | 9 (42.9) | 14 (31.8) | |
| III | 29 (21.0) | 16 (40.0) | 12 (57.1) | 30 (68.2) | |
| Not specified | 19 | 1 | 1 | 3 |
| Outcome | DRC<4.3% | DRC ≥ 4.3% (Reference) | Crude OR (95% CI) | Adjusted OR (95% CI) a |
|---|---|---|---|---|
| Controls (reference) | 271 | 411 | 1.0 | 1.0 |
| Luminal A | 122 | 35 | 5.3 (3.5, 7.9) | 5.4 (3.5, 2.8) |
| Luminal B | 26 | 15 | 2.6 (1.4, 5.1) | 2.5 (1.3, 4.9) |
| HER2+ | 17 | 5 | 5.2 (1.9, 14.4) | 5.2 (1.9, 14.3) |
| Triple-negative | 39 | 8 | 7.4 (3.4, 16.1) | 7.2 (3.3, 15.7) |
| TOTALS | 475 | 474 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matta, J.; Ortiz, C.; Encarnación, J.; Dutil, J.; Suárez, E. Variability in DNA Repair Capacity Levels among Molecular Breast Cancer Subtypes: Triple Negative Breast Cancer Shows Lowest Repair. Int. J. Mol. Sci. 2017, 18, 1505. https://doi.org/10.3390/ijms18071505
Matta J, Ortiz C, Encarnación J, Dutil J, Suárez E. Variability in DNA Repair Capacity Levels among Molecular Breast Cancer Subtypes: Triple Negative Breast Cancer Shows Lowest Repair. International Journal of Molecular Sciences. 2017; 18(7):1505. https://doi.org/10.3390/ijms18071505
Chicago/Turabian StyleMatta, Jaime, Carmen Ortiz, Jarline Encarnación, Julie Dutil, and Erick Suárez. 2017. "Variability in DNA Repair Capacity Levels among Molecular Breast Cancer Subtypes: Triple Negative Breast Cancer Shows Lowest Repair" International Journal of Molecular Sciences 18, no. 7: 1505. https://doi.org/10.3390/ijms18071505