Rational Design of Recombinant Papain-Like Cysteine Protease: Optimal Domain Structure and Expression Conditions for Wheat-Derived Enzyme Triticain-α
Abstract
:1. Introduction
2. Results and Discussion
2.1. Expression of Full-Length Triticain-α in E. coli
2.2. Expression of Catalytic Domain of Triticain-α in E. coli
2.3. Co-Expression of Catalytic Domain of Triticain-α in E. coli with Folding Chaperones
2.4. Expression of Two-Domain Triticain-α Constructs in E. coli
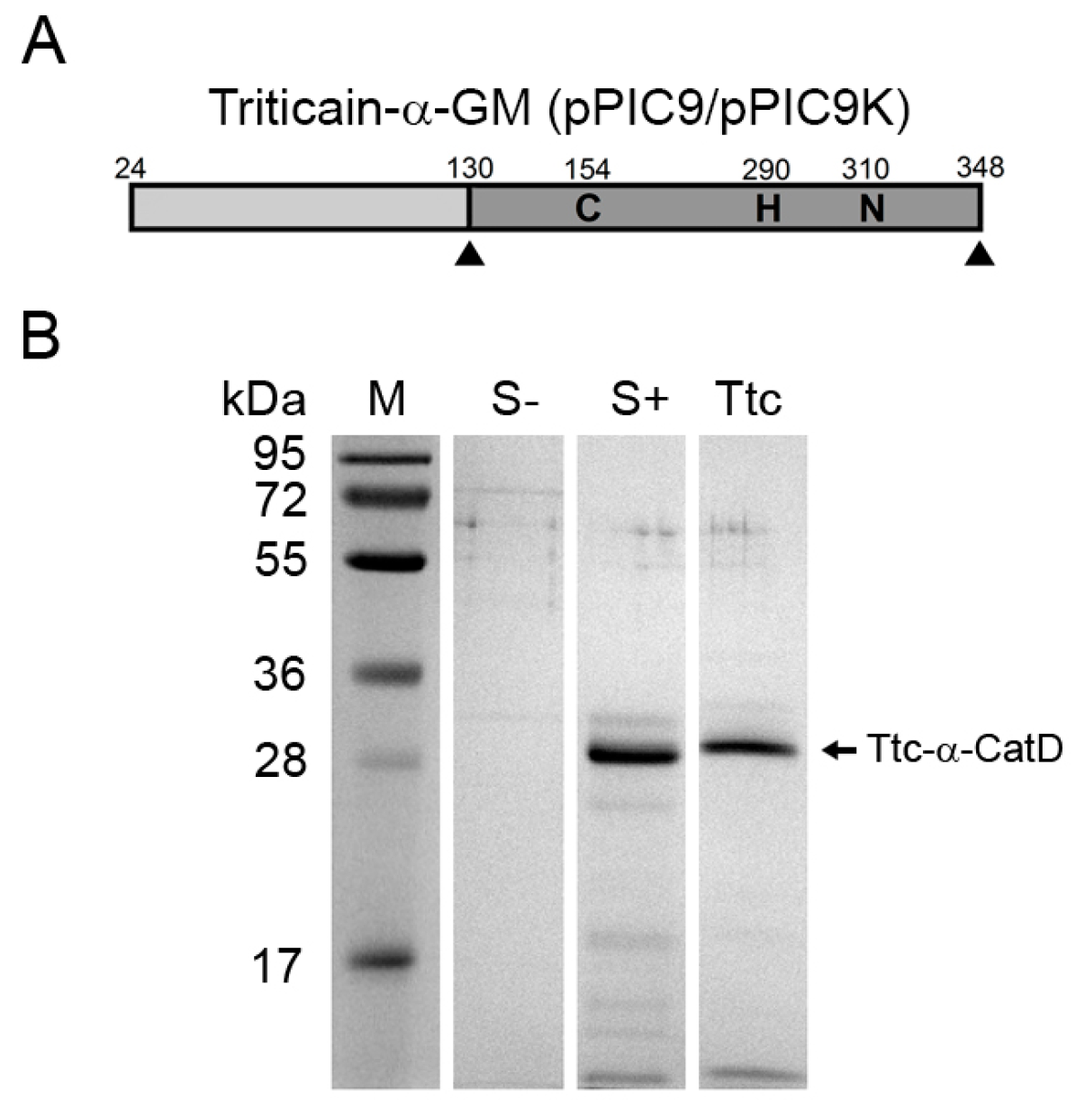
2.5. Expression of Triticain-α Constructs in Yeast
2.6. Selection of Bacterial Strain for Expression of Triticain-α Constructs in Soluble Form
3. Materials and Methods
3.1. Materials
3.2. Genetic Constructs for Expression in E. coli
3.3. Genetic Constructs for Expression in P. pastoris
3.4. Expression and Co-Expression of Recombinant Proteins in E. coli
3.5. Expression of Recombinant Proteins in P. pastoris
3.6. Purification of Recombinant Proteins
3.7. Fluorescent Protease Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ac-PLVQ-AMC | acetyl-(PLVQ)-7-amino-4-methylcoumarin |
| CD | celiac disease also known as celiac sprue |
| extP | 16-mer folding peptide (NYEEVIKKYRGEENF) from Plasmodium falciparum cysteine protease falcipain-2 |
| GST | glutathione S-transferase |
| ORF | open reading frame |
| SDS-PAGE | sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| PB | sodium phosphate buffer |
| pelB | N-terminal bacterial periplasmic signal sequence |
| Triticain-α-CatD | Triticain-α catalytic domain |
| Triticain-α-GM | Triticain-α lacking signal peptide and granulin domain |
| Triticain-α-ProD | Triticain-α prodomain |
References
- Craik, C.S.; Page, M.J.; Madison, E.L. Proteases as therapeutics. Biochem. J. 2011, 435, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kurada, S.; Yadav, A.; Leffler, D.A. Current and novel therapeutic strategies in celiac disease. Exp. Rev. Clin. Pharmacol. 2016, 9, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Catassi, C. Clinical practice. Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Savvateeva, L.V.; Erdes, S.I.; Antishin, A.S.; Zamyatnin, A.A., Jr. Overview of celiac disease in russia: Regional data and estimated prevalence. J. Immunol. Res. 2017, 2017, 2314813. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of gluten intolerance: Gluten structure, evolution, pathogenicity and detoxification capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Molberg, O.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Savvateeva, L.V.; Zamyatnin, A.A. Prospects of developing medicinal therapeutic strategies and pharmaceutical design for effective gluten intolerance treatment. Curr. Pharm. Des. 2016, 22, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Lahdeaho, M.L.; Kaukinen, K.; Laurila, K.; Vuotikka, P.; Koivurova, O.P.; Karja-Lahdensuu, T.; Marcantonio, A.; Adelman, D.C.; Maki, M. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 2014, 146, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Kiyosaki, T.; Asakura, T.; Matsumoto, I.; Tamura, T.; Terauchi, K.; Funaki, J.; Kuroda, M.; Misaka, T.; Abe, K. Wheat cysteine proteases triticain alpha, beta and gamma exhibit mutually distinct responses to gibberellin in germinating seeds. J. Plant Physiol. 2009, 166, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Savvateeva, L.V.; Gorokhovets, N.V.; Makarov, V.A.; Serebryakova, M.V.; Solovyev, A.G.; Morozov, S.Y.; Reddy, V.P.; Zernii, E.Y.; Zamyatnin, A.A., Jr.; Aliev, G. Glutenase and collagenase activities of wheat cysteine protease triticain-alpha: Feasibility for enzymatic therapy assays. Int. J. Biochem. Cell Boil. 2015, 62, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bromme, D.; Nallaseth, F.S.; Turk, B. Production and activation of recombinant papain-like cysteine proteases. Methods 2004, 32, 199–206. [Google Scholar] [CrossRef]
- Richau, K.H.; Kaschani, F.; Verdoes, M.; Pansuriya, T.C.; Niessen, S.; Stuber, K.; Colby, T.; Overkleeft, H.S.; Bogyo, M.; van der Hoorn, R.A. Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics. Plant Physiol. 2012, 158, 1583–1599. [Google Scholar] [CrossRef] [PubMed]
- Niemer, M.; Mehofer, U.; Verdianz, M.; Porodko, A.; Schahs, P.; Kracher, D.; Lenarcic, B.; Novinec, M.; Mach, L. Nicotiana benthamiana cathepsin B displays distinct enzymatic features which differ from its human relative and aleurain-like protease. Biochimie 2016, 122, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nature Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Oppezzo, P. Overcoming the solubility problem in E. coli: Available approaches for recombinant protein production. Methods Mol. Biol. 2015, 1258, 27–44. [Google Scholar] [PubMed]
- Pandey, K.C.; Sijwali, P.S.; Singh, A.; Na, B.K.; Rosenthal, P.J. Independent intramolecular mediators of folding, activity, and inhibition for the plasmodium falciparum cysteine protease falcipain-2. J. Boil. Chem. 2004, 279, 3484–3491. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, I.I.; Senin, I.I.; Tikhomirova, N.K.; Komolov, K.E.; Permyakov, S.E.; Zernii, E.Y.; Koch, K.W.; Philippov, P.P. Synergetic effect of recoverin and calmodulin on regulation of rhodopsin kinase. Front. Mol. Neurosci. 2012, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, V.A.; Gorokhovets, N.V.; Savvateeva, L.V.; Severin, S.E. Characterization of recombinant human hsp70’s domains functions and interdomain interactions. Mol. Biol. 2011, 45, 903–913. [Google Scholar] [CrossRef]
- Gu, C.; Shabab, M.; Strasser, R.; Wolters, P.J.; Shindo, T.; Niemer, M.; Kaschani, F.; Mach, L.; van der Hoorn, R.A. Post-translational regulation and trafficking of the granulin-containing protease RD21 of Arabidopsis thaliana. PLoS ONE 2012, 7, e32422. [Google Scholar] [CrossRef] [PubMed]
- Paireder, M.; Mehofer, U.; Tholen, S.; Porodko, A.; Schahs, P.; Maresch, D.; Biniossek, M.L.; van der Hoorn, R.A.; Lenarcic, B.; Novinec, M.; et al. The death enzyme CP14 is a unique papain-like cysteine proteinase with a pronounced S2 subsite selectivity. Arch. Biochem. Biophys. 2016, 603, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Matsushima, R.; Nishimura, M.; Hara-Nishimura, I. A slow maturation of a cysteine protease with a granulin domain in the vacuoles of senescing Arabidopsis leaves. Plant Physiol. 2001, 127, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Paireder, M.; Tholen, S.; Porodko, A.; Biniossek, M.L.; Mayer, B.; Novinec, M.; Schilling, O.; Mach, L. The papain-like cysteine proteinases NbCysP6 and NbCysP7 are highly processive enzymes with substrate specificities complementary to Nicotiana benthamiana cathepsin B. Biochim. Biophys. Acta 2017, 1865, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Watabe, S.; Kageyama, T.; Takahashi, S.Y. Proregion of Bombyx mori cysteine proteinase functions as an intramolecular chaperone to promote proper folding of the mature enzyme. Arch. Insect Biochem. Physiol. 1999, 42, 167–178. [Google Scholar] [CrossRef]
- Boock, J.T.; Waraho-Zhmayev, D.; Mizrachi, D.; DeLisa, M.P. Beyond the cytoplasm of Escherichia coli: Localizing recombinant proteins where you want them. Methods Mol. Boil. 2015, 1258, 79–97. [Google Scholar]
- Seto, M.; Ogawa, T.; Kodama, K.; Muramoto, K.; Kanayama, Y.; Sakai, Y.; Chijiwa, T.; Ohno, M. A novel recombinant system for functional expression of myonecrotic snake phospholipase A2 in Escherichia coli using a new fusion affinity tag. Protein Expres. Purif. 2008, 58, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Novinec, M.; Pavsic, M.; Lenarcic, B. A simple and efficient protocol for the production of recombinant cathepsin V and other cysteine cathepsins in soluble form in Escherichia coli. Protein Expr. Purif. 2012, 82, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Drenth, J.; Jansonius, J.N.; Koekoek, R.; Swen, H.M.; Wolthers, B.G. Structure of papain. Nature 1968, 218, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, I.G.; Kalk, K.H.; Swarte, M.B.A.; Drenth, J. Structure of papain refined at 1.65 a resolution. J. Mol. Biol. 1984, 179, 233–256. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Savvateeva, L.V.; Schwartz, A.M.; Gorshkova, L.B.; Gorokhovets, N.V.; Makarov, V.A.; Reddy, V.P.; Aliev, G.; Zamyatnin, A.A., Jr. Prophylactic admission of an in vitro reconstructed complexes of human recombinant heat shock proteins and melanoma antigenic peptides activates anti-melanoma responses in mice. Curr. Mol. Med. 2015, 15, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 1989. [Google Scholar]
- Ke, S.H.; Madison, E.L. Rapid and efficient site-directed mutagenesis by single-tube ‘megaprimer’ PCR method. Nucleic Acids Res. 1997, 25, 3371–3372. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
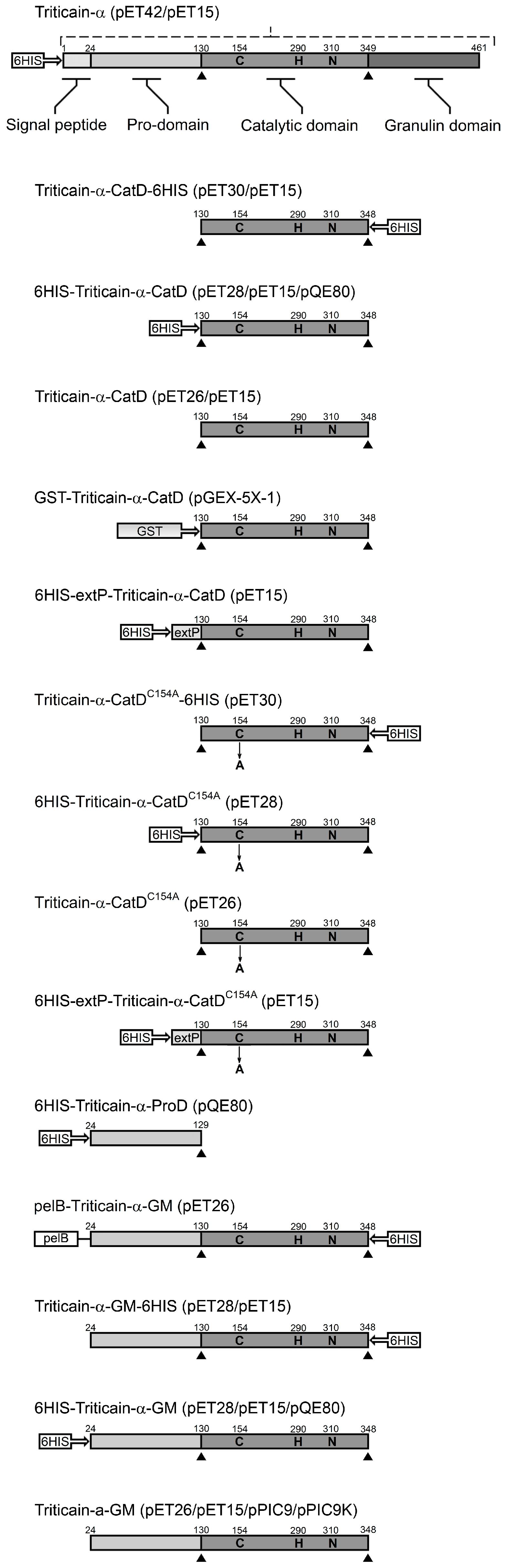


| Recombinant Protein | 6HIS-Tag Position | AA a | Strain | Expression Vector, Phenotype | Localization of the Product | Estimated Yield b (mg/L) | Specific Productivity c (µg/OD) |
|---|---|---|---|---|---|---|---|
| Escherichia coli | |||||||
| 6HIS-Triticain-α | N-terminal | 469 | BL21(DE3) | pET42-6HIS-Triticain-α | inclusion bodies | 23.2 ± 1.88 | 11.1 ± 0.9 |
| 469 | Rosetta gami B (DE3) | pET15-6HIS-Triticain-α | inclusion bodies | 17.2± 6.64 | 12.66 ± 4.34 | ||
| pelB-Triticain-α-GM-6HIS | C-terminal | 359 | BL21(DE3) | pET26-Triticain-α-GM-6HIS | inclusion bodies | 26.7 ± 2.76 | 18.4 ± 1.9 |
| 6HIS-Triticain-α-GM | N-terminal | 345 | BL21(DE3) | pET28-6HIS-Triticain-α-GM | inclusion bodies | 61 ± 5.3 | 23 ± 2.0 |
| 345 | Rosetta gami B (DE3) | pET15-6HIS-Triticain-α-GM | inclusion bodies | 21.4 ± 2.88 | 13.4 ± 1.8 | ||
| soluble fraction | 17.3 ± 2.24 | 10.8 ± 1.4 | |||||
| 335 | JM109 | pQE-6HIS-Triticain-α-GM | inclusion bodies | 34.2 ± 5.13 | 15.13 ± 2.27 | ||
| Triticain-α-GM-6HIS | C-terminal | 336 | BL21(DE3) | pET28-Triticain-α-GM-6HIS | inclusion bodies | 26.8 ± 0.91 | 11.8 ± 0.4 |
| 334 | Rosetta gami B (DE3) | pET15-Triticain-α-GM-6HIS | inclusion bodies | 0 | 0 | ||
| soluble fraction | 5.15 ± 0.5 | 3.55 ± 0.35 | |||||
| Triticain-α-GM | none | 325 | BL21(DE3) | pET26-Triticain-α-GM | inclusion bodies | 32.8 ± 1.6 | 16.4 ± 0.8 |
| 325 | Rosetta gami B (DE3) | pET15-Triticain-α-GM | inclusion bodies | 29.6 ± 1.1 | 12.05 ± 0.45 | ||
| 6HIS-Triticain-α-CatD | N-terminal | 240 | BL21(DE3) | pET28-6HIS-Triticain-α-CatD | inclusion bodies | 174.6 ± 4.5 | 58.2 ± 1.5 |
| 240 | Rosetta gami B (DE3) | pET15-6HIS-Triticain-α-CatD | inclusion bodies | 43.8 ± 0.26 | 25.85 ± 0.15 | ||
| 231 | JM109 | pQE-6HIS-Triticain-α-CatD | inclusion bodies | 72.2 ± 6.37 | 41.96 ± 3.54 | ||
| Triticain-α-CatD-6HIS | C-terminal | 228 | BL21(DE3) | pET30-Triticain-α-CatD-6HIS | inclusion bodies | 23.5 ± 5.36 | 11.05 ± 2.55 |
| 227 | Rosetta gami B (DE3) | pET15-Triticain-α-CatD-6HIS | no expression | – | – | ||
| Triticain-α-CatD | none | 220 | BL21(DE3) | pET26-Triticain-α-CatD | inclusion bodies | 12.35 ± 5.14 | 5.8 ± 2.4 |
| 220 | Rosetta gami B (DE3) | pET15-Triticain-α-CatD | inclusion bodies | 5.4 ± 0.79 | 2.45 ± 0.35 | ||
| GST-Triticain-α-CatD | none | 446 | BL21 | pGEX-GST-Triticain-α-CatD | inclusion bodies | 150 ± 13.3 | 37.28 ± 3.32 |
| 6HIS-expP-Triticain-α-CatD | N-terminal | 257 | BL21(DE3) | pET15-6HIS-expP-Triticain-α-CatD | no expression | – | − |
| Rosetta gami B (DE3) | pET15-6HIS-expP-Triticain-α-CatD | inclusion bodies | 19.24 ± 0.54 | 8.87 ± 0.25 | |||
| Pichia pastoris | |||||||
| Triticain-α-GM | none | 332 | GS115 | pPIC9-Triticain-α-GM, Mut+ | growth medium | 92 ± 12 | − |
| GS115 | pPIC9-Triticain-α-GM, Muts | growth medium | 164 ± 8 | − | |||
| GS115 | pPIC9(pPIC9K)-Triticain-α-GM, Mut+ | growth medium | 178 ± 6 | − | |||
| GS115 | pPIC9(pPIC9K)-Triticain-α-GM, Muts | growth medium | 276 ± 28 | − | |||
| Recombinant Protein/Chaperone | 6HIS-Tag Position in Catalytic Domain | Co-Expressing Vectors | Localization of the Product | ||
|---|---|---|---|---|---|
| Cell Growth Conditions | |||||
| 37 °C, 3 h | 28 °C, 15 h | 18 °C, 20 h | |||
| BL21(DE3) | |||||
| 6HIS-Triticain-α-CatD/6HIS-Triticain-α ProD | N-terminal | pET28-6HIS-Triticain-α-CatD | inclusion bodies | − | − |
| pQE80-6HIS-Triticain-α-ProD | inclusion bodies, partially soluble | ||||
| Triticain-α-CatD-6HIS/6HIS-Triticain-α ProD | C-terminal | pET30-Triticain-α-CatD-6HIS | inclusion bodies | − | − |
| pQE80-6HIS-Triticain-α-ProD | inclusion bodies, partially soluble | ||||
| Triticain-α-CatD/6HIS-Triticain-α ProD | none | pET26-Triticain-α-CatD | inclusion bodies | − | − |
| pQE80-6HIS-Triticain-α-ProD | inclusion bodies, partially soluble | ||||
| 6HIS-Triticain-α-CatD C154A/6HIS-Triticain-α ProD | N-terminal | pET28-6HIS-Triticain-α-CatD C154A | inclusion bodies | inclusion bodies | − |
| pQE80-6HIS-Triticain-α-ProD | inclusion bodies, partially soluble | inclusion bodies, partially soluble | |||
| Triticain-α-CatD C154A-6HIS/6HIS-Triticain-α ProD | C-terminal | pET30-Triticain-α-CatD C154A-6HIS | inclusion bodies | inclusion bodies | − |
| pQE80-6HIS-Triticain-α-proD | inclusion bodies, partially soluble | inclusion bodies, partially soluble | |||
| Triticain-α-CatD C154A/6HIS-Triticain-α ProD | none | pET26-Triticain-α-CatDC154A | inclusion bodies | inclusion bodies | − |
| pQE80-6HIS-Triticain-α-proD | inclusion bodies, partially soluble | inclusion bodies, partially soluble | |||
| 6HIS-Triticain-α-CatD C154A/6HIS-HSP70A1B | N-terminal | pET28-6HIS-Triticain-α-CatDC154A | − | inclusion bodies | − |
| pQE80-6HIS-HSP70A1B | soluble, partially inclusion bodies | ||||
| Triticain-α-CatD C154A-6HIS/6HIS-HSP70A1B | C-terminal | pET30-Triticain-α-CatD C154A-6HIS | − | no expression | − |
| pQE80-6HIS-HSP70A1B | soluble | ||||
| Rosetta gami B (DE3) | |||||
| 6HIS-Triticain-α-CatD/6HIS-Triticain-α ProD | N-terminal | pET15[6HIS-Triticain-α-CatD+ 6HIS-Triticain-α-ProD] | − | − | inclusion bodies |
| soluble | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorokhovets, N.V.; Makarov, V.A.; Petushkova, A.I.; Prokopets, O.S.; Rubtsov, M.A.; Savvateeva, L.V.; Zernii, E.Y.; Zamyatnin Jr., A.A. Rational Design of Recombinant Papain-Like Cysteine Protease: Optimal Domain Structure and Expression Conditions for Wheat-Derived Enzyme Triticain-α. Int. J. Mol. Sci. 2017, 18, 1395. https://doi.org/10.3390/ijms18071395
Gorokhovets NV, Makarov VA, Petushkova AI, Prokopets OS, Rubtsov MA, Savvateeva LV, Zernii EY, Zamyatnin Jr. AA. Rational Design of Recombinant Papain-Like Cysteine Protease: Optimal Domain Structure and Expression Conditions for Wheat-Derived Enzyme Triticain-α. International Journal of Molecular Sciences. 2017; 18(7):1395. https://doi.org/10.3390/ijms18071395
Chicago/Turabian StyleGorokhovets, Neonila V., Vladimir A. Makarov, Anastasiia I. Petushkova, Olga S. Prokopets, Mikhail A. Rubtsov, Lyudmila V. Savvateeva, Evgeni Yu. Zernii, and Andrey A. Zamyatnin Jr. 2017. "Rational Design of Recombinant Papain-Like Cysteine Protease: Optimal Domain Structure and Expression Conditions for Wheat-Derived Enzyme Triticain-α" International Journal of Molecular Sciences 18, no. 7: 1395. https://doi.org/10.3390/ijms18071395






