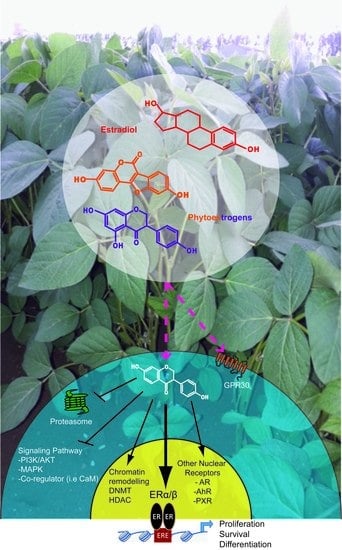
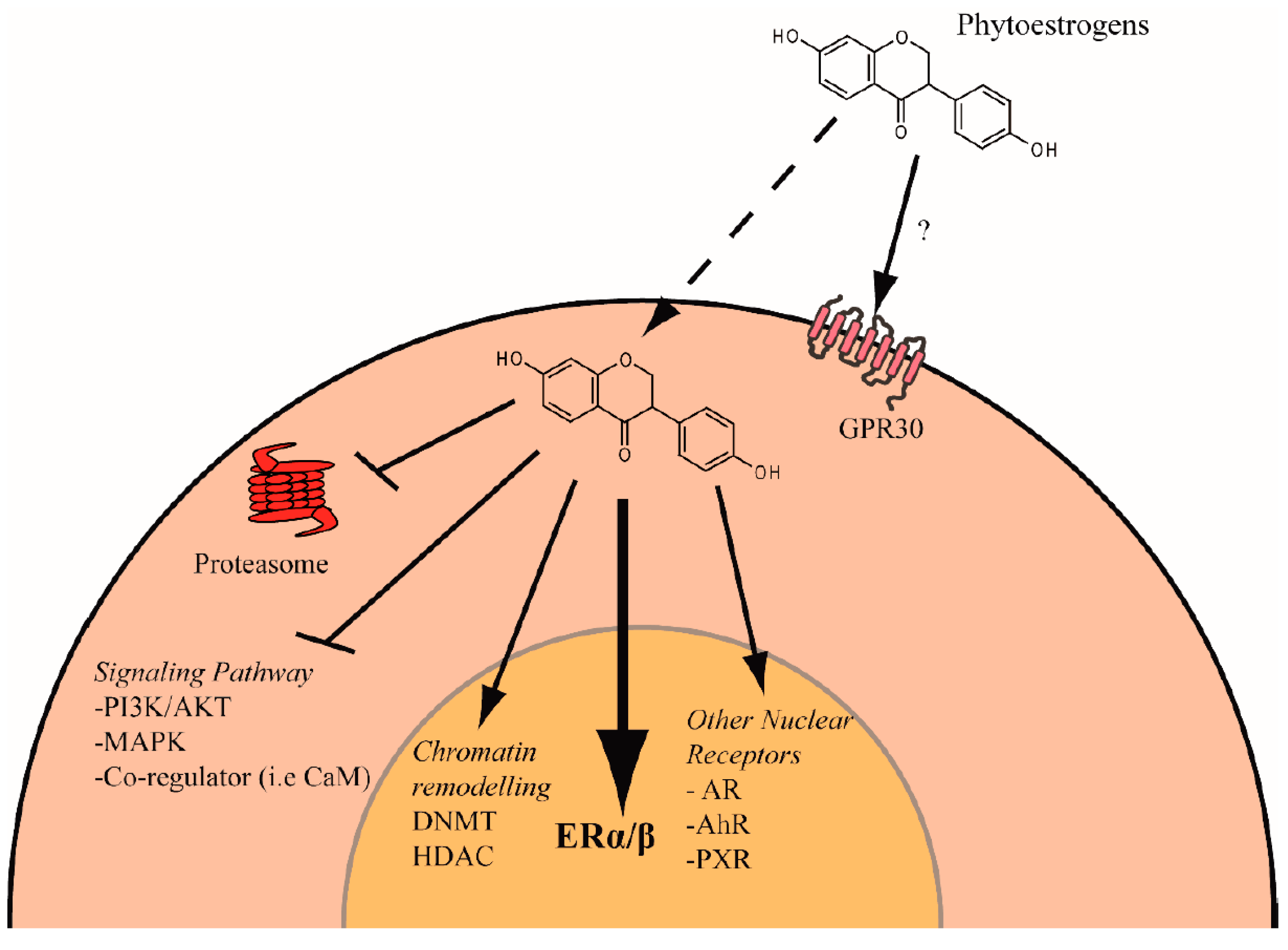
Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects?
Abstract
:1. Introduction
2. Structure and Sources of the Major Dietary Phytoestrogens
2.1. Flavonoids
2.2. Pterocarpans
2.3. Coumestans
2.4. Stilbenes
2.5. Lignans
2.6. Mycoestrogens
3. In Vitro Effects of Phytoestrogens
4. In Vivo Effects of Phytoestrogens
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Couse, J.F.; Korach, K.S. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr. Rev. 1999, 20, 358–417. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.-A. What pharmacologists can learn from recent advances in estrogen signaling. Trends Pharmacol. Sci. 2003, 24, 479–485. [Google Scholar] [CrossRef]
- Germain, P.; Staels, B.; Dacquet, C.; Spedding, M.; Laudet, V. Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 2006, 58, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Kim, K.; Kim, K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J. Mol. Endocrinol. 2008, 41, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.S.; Meyer, C.A.; Song, J.; Li, W.; Geistlinger, T.R.; Eeckhoute, J.; Brodsky, A.S.; Keeton, E.K.; Fertuck, K.C.; Hall, G.F.; et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006, 38, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Le Dily, F.; Beato, M. TADs as modular and dynamic units for gene regulation by hormones. FEBS Lett. 2015, 589, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Le Dily, F.; Baù, D.; Pohl, A.; Vicent, G.P.; Serra, F.; Soronellas, D.; Castellano, G.; Wright, R.H.G.; Ballare, C.; Filion, G.; et al. Distinct structural transitions of chromatin topological domains correlate with coordinated hormone-induced gene regulation. Genes Dev. 2014, 28, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [PubMed]
- La Rosa, P.; Pesiri, V.; Leclercq, G.; Marino, M.; Acconcia, F. Palmitoylation regulates 17β-estradiol-induced estrogen receptor-α degradation and transcriptional activity. Mol. Endocrinol. 2012, 26, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Aitkenhead, M.; Hughes, C.C.W.; Levin, E.R. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J. Biol. Chem. 2002, 277, 50768–50775. [Google Scholar] [CrossRef] [PubMed]
- Vicent, G.P.; Nacht, A.S.; Zaurín, R.; Ballaré, C.; Clausell, J.; Beato, M. Minireview: Role of kinases and chromatin remodeling in progesterone signaling to chromatin. Mol. Endocrinol. 2010, 24, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Extranuclear estrogen receptor’s roles in physiology: Lessons from mouse models. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E133–E140. [Google Scholar] [CrossRef] [PubMed]
- Gosden, J.R.; Middleton, P.G.; Rout, D. Localization of the human oestrogen receptor gene to chromosome 6q24----q27 by in situ hybridization. Cytogenet. Cell Genet. 1986, 43, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Enmark, E.; Pelto-Huikko, M.; Grandien, K.; Lagercrantz, S.; Lagercrantz, J.; Fried, G.; Nordenskjöld, M.; Gustafsson, J.A. Human estrogen receptor β-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 1997, 82, 4258–4265. [Google Scholar] [CrossRef] [PubMed]
- Kerdivel, G.; Flouriot, G.; Pakdel, F. Modulation of estrogen receptor α activity and expression during breast cancer progression. Vitam. Horm. 2013, 93, 135–160. [Google Scholar] [PubMed]
- Wang, Z.; Zhang, X.; Shen, P.; Loggie, B.W.; Chang, Y.; Deuel, T.F. Identification, cloning, and expression of human estrogen receptor-α36, a novel variant of human estrogen receptor-α66. Biochem. Biophys. Res. Commun. 2005, 336, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T. Oestrogen receptor β (ER β). Rev. Reprod. 1998, 3, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Delbès, G.; Levacher, C.; Duquenne, C.; Racine, C.; Pakarinen, P.; Habert, R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor α. Endocrinology 2005, 146, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Staub, C.; Rauch, M.; Ferrière, F.; Trépos, M.; Dorval-Coiffec, I.; Saunders, P.T.; Cobellis, G.; Flouriot, G.; Saligaut, C.; Jégou, B. Expression of Estrogen Receptor ESR1 and Its 46-kDa Variant in the Gubernaculum Testis. Biol. Reprod. 2005, 73, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Westberry, J.M.; Trout, A.L. Estrogen receptor-α gene expression in the cortex: Sex differences during development and in adulthood. Horm. Behav. 2011, 59, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Kim, H.-J.; Warner, M.; Gustafsson, J.-A. Estrogen receptor β is essential for sprouting of nociceptive primary afferents and for morphogenesis and maintenance of the dorsal horn interneurons. Proc. Natl. Acad. Sci. USA 2007, 104, 13696–13701. [Google Scholar] [CrossRef] [PubMed]
- Kerdivel, G.; Habauzit, D.; Pakdel, F. Assessment and molecular actions of endocrine-disrupting chemicals that interfere with estrogen receptor pathways. Int. J. Endocrinol. 2013, 2013, 501851. [Google Scholar] [CrossRef] [PubMed]
- Sohoni, P.; Sumpter, J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, C.; Soto, A.M. An updated review of environmental estrogen and androgen mimics and antagonists. J. Steroid Biochem. Mol. Biol. 1998, 65, 143–150. [Google Scholar] [CrossRef]
- Rasier, G.; Toppari, J.; Parent, A.-S.; Bourguignon, J.-P. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: A review of rodent and human data. Mol. Cell. Endocrinol. 2006, 254–255, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Toppari, J.; Virtanen, H.; Skakkebaek, N.E.; Main, K.M. Environmental effects on hormonal regulation of testicular descent. J. Steroid Biochem. Mol. Biol. 2006, 102, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Coumoul, X.; Fernandez-Salguero, P.M. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007, 581, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kanjo, Y.; Mizutani, S. A review of phytoestrogens: Their occurrence and fate in the environment. Water Res. 2010, 44, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medecine-MeSH National Library of Medecine-MeSH, 2016. Available online: https://www.nlm.nih.gov/cgi/mesh/2016/MB_cgi (accessed on 12 November 2016).
- Van de Schans, M.G. M.; Vincken, J.-P.; Bovee, T.F. H.; Cervantes, A.D.; Logtenberg, M.J.; Gruppen, H. Structural changes of 6a-hydroxy-pterocarpans upon heating modulate their estrogenicity. J. Agric. Food Chem. 2014, 62, 10475–10484. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.C.; Tilghman, S.L.; Boué, S.M.; Salvo, V.A.; Elliott, S.; Williams, K.Y.; Skripnikova, E.V.; Ashe, H.; Payton-Stewart, F.; Vanhoy-Rhodes, L.; et al. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J. Pharmacol. Exp. Ther. 2010, 332, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ayers, A.R.; Ebel, J.; Finelli, F.; Berger, N.; Albersheim, P. Host-pathogen interactions: IX. Quantitative assays of elicitor activity and characterization of the elicitor present in the extracellular medium of cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976, 57, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Nikov, G.N.; Hopkins, N.E.; Boue, S.; Alworth, W.L. Interactions of dietary estrogens with human estrogen receptors and the effect on estrogen receptor-estrogen response element complex formation. Environ. Health Perspect. 2000, 108, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Tuskaev, V.A. Synthesis and biological activity of coumestan derivatives. Pharm. Chem. J. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Nehybová, T.; Šmarda, J.; Beneš, P. Plant coumestans: Recent advances and future perspectives in cancer therapy. Anticancer Agents Med. Chem. 2014, 14, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Bickoff, E.M.; Booth, A.N.; Lyman, R.L.; Livingston, A.L.; Thompson, C.R.; Deeds, F. Coumestrol, a new estrogen isolated from forage crops. Science 1957, 126, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007, 101, 449–457. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, L.; Chalabi, N.; Delort, L.; Bignon, Y.-J.; Bernard-Gallon, D.J. Resveratrol and breast cancer chemoprevention: Molecular mechanisms. Mol. Nutr. Food Res. 2005, 49, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Rosmalena, A.; Prasasty, V.D.; Hanafi, M.; Budianto, E.; Elya, B. Lignan derivatives potential as Plasmodium falciparum lactate dehydrogenase inhibitors: Molecular docking approach of antiplasmodial drug design. Int. J. Pharm. Pharm. Sci. 2015, 7, 394–398. [Google Scholar]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001, 39, 1435–1438. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain Scientific. Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar]
- Lin, P.; Chen, F.; Sun, J.; Zhou, J.; Wang, X.; Wang, N.; Li, X.; Zhang, Z.; Wang, A.; Jin, Y. Mycotoxin zearalenone induces apoptosis in mouse Leydig cells via an endoplasmic reticulum stress-dependent signalling pathway. Reprod. Toxicol. 2015, 52, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Le Guevel, R.; Pakdel, F. Assessment of oestrogenic potency of chemicals used as growth promoter by in vitro methods. Hum. Reprod. 2001, 16, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Frasor, J.; Danes, J.M.; Komm, B.; Chang, K.C.N.; Lyttle, C.R.; Katzenellenbogen, B.S. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: Insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 2003, 144, 4562–4574. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.C.; Frasor, J.; Komm, B.; Katzenellenbogen, B.S. Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology 2006, 147, 4831–4842. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.C.; Charn, T.H.; Park, S.-H.; Helferich, W.G.; Komm, B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen Receptors α and β as Determinants of Gene Expression: Influence of Ligand, Dose, and Chromatin Binding. Mol. Endocrinol. 2008, 22, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mao, Z.; Brinton, R.D. A select combination of clinically relevant phytoestrogens enhances estrogen receptor β-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology 2009, 150, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Shanle, E.K.; Hawse, J.R.; Xu, W. Generation of stable reporter breast cancer cell lines for the identification of ER subtype selective ligands. Biochem. Pharmacol. 2011, 82, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C.K.; et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Nadal-Serrano, M.; Blanquer-Rossello, M.M.; Sastre-Serra, J.; Oliver, J.; Roca, P. Genistein Modulates Proliferation and Mitochondrial Functionality in Breast Cancer Cells Depending on ERα/ERβ Ratio. J. Cell. Biochem. 2014, 115, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Sotoca, A.M.; Ratman, D.; van der Saag, P.; Ström, A.; Gustafsson, J.A.; Vervoort, J.; Rietjens, I.M.C.M.; Murk, A.J. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERα/ERβ ratio. J. Steroid Biochem. Mol. Biol. 2008, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding genistein in cancer: The good and the bad effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.M. Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells. Cancer Lett. 1996, 110, 41–48. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Park, M.-A.; Kang, N.-H.; Yi, B.-R.; Hyun, S.-H.; Jeung, E.-B.; Choi, K.-C. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 β-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor α and insulin-like growth factor-1 receptor signaling pathways. Toxicol. Appl. Pharmacol. 2013, 272, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Prietsch, R.F.; Monte, L.G.; da Silva, F.A.; Beira, F.T.; Del Pino, F.A.B.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sarkar, F.H. Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002, 8, 2369–2377. [Google Scholar] [PubMed]
- Gong, L.; Li, Y.; Nedeljkovic-Kurepa, A.; Sarkar, F.H. Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 2003, 22, 4702–4709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Kang, Y.; Li, X.; Roife, D.; Zhang, R.; Fleming, J.B. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014, 34, 4685–4692. [Google Scholar] [PubMed]
- Li, C.; Teng, R.-H.; Tsai, Y.-C.; Ke, H.-S.; Huang, J.-Y.; Chen, C.-C.; Kao, Y.-L.; Kuo, C.-C.; Bell, W.R.; Shieh, B. H-Ras oncogene counteracts the growth-inhibitory effect of genistein in T24 bladder carcinoma cells. Br. J. Cancer 2005, 92, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Padhye, S. Lesson learned from nature for the development of novel anti-cancer agents: Implication of isoflavone, curcumin, and their synthetic analogs. Curr. Pharm. Des. 2010, 16, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, S.; Kyo, S.; Banerjee, P.P. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006, 66, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.J.; Casper, R.F.; Rogers, I.M. Epigenetic changes to human umbilical cord blood cells cultured with three proteins indicate partial reprogramming to a pluripotent state. Exp. Cell Res. 2010, 316, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H. Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp. Biol. Med. 2011, 236, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Gregorieff, A.; Clevers, H. Wnt signaling in the intestinal epithelium: From endoderm to cancer. Genes Dev. 2005, 19, 877–890. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhu, Y.-Q.; Luo, J.; Tao, W.-H. Hypermethylation and expression regulation of secreted frizzled-related protein genes in colorectal tumor. World J. Gastroenterol. 2006, 12, 7113–7117. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Q.; Chen, H. Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PLoS ONE 2012, 7, e40955. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.B.; Manno, M.; Mrkonjic, M.; Daftary, D.; Dicks, E.; Buchanan, D.D.; Younghusband, H.B.; Parfrey, P.S.; Young, J.P.; Pollett, A.; et al. Promoter methylation of Wnt antagonists DKK1 and SFRP1 is associated with opposing tumor subtypes in two large populations of colorectal cancer patients. Carcinogenesis 2011, 32, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Fraga, M.F.; Ballestar, E.; Paz, M.F.; Herranz, M.; Espada, J.; García, J.M.; Muñoz, A.; Esteller, M.; González-Sancho, J.M. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 2006, 25, 4116–4121. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Nakajima, K.; Kawamoto, K.; Kikuno, N.; Ueno, K.; Yamamura, S.; Zaman, M.S.; Khatri, G.; Chen, Y.; et al. Wnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinoma. Int. J. Cancer 2011, 128, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Muthugounder, S.; Presser, N.; Viswanathan, S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv. Exp. Med. Biol. 2004, 546, 121–165. [Google Scholar] [PubMed]
- Mahmoud, A.M.; Al-Alem, U.; Ali, M.M.; Bosland, M.C. Genistein increases estrogen receptor β expression in prostate cancer via reducing its promoter methylation. J. Steroid Biochem. Mol. Biol. 2015, 152, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Anastasius, N.; Boston, S.; Lacey, M.; Storing, N.; Whitehead, S.A. Evidence that low-dose, long-term genistein treatment inhibits oestradiol-stimulated growth in MCF-7 cells by down-regulation of the PI3-kinase/Akt signalling pathway. J. Steroid Biochem. Mol. Biol. 2009, 116, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, K.; Crane, S.R.; Nowers, J.L.; Lacey, M.; Whitehead, S.A. Long-term genistein treatment of MCF-7 cells decreases acetylated histone 3 expression and alters growth responses to mitogens and histone deacetylase inhibitors. J. Steroid Biochem. Mol. Biol. 2010, 120, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Whitten, P.L.; Patisaul, H.B. Cross-species and interassay comparisons of phytoestrogen action. Environ. Health Perspect. 2001. [Google Scholar] [CrossRef]
- Soukup, S.T.; Al-Maharik, N.; Botting, N.; Kulling, S.E. Quantification of soy isoflavones and their conjugative metabolites in plasma and urine: An automated and validated UHPLC-MS/MS method for use in large-scale studies. Anal. Bioanal. Chem. 2014, 406, 6007–6020. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-A.; Shu, X.-O.; Li, H.; Yang, G.; Cai, H.; Wen, W.; Ji, B.-T.; Gao, J.; Gao, Y.-T.; Zheng, W. Adolescent and adult soy food intake and breast cancer risk: Results from the shanghai women’s health study. Am. J. Clin. Nutr. 2009, 89, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. The chemopreventive properties of soy isoflavonoids in animal models of breast cancer. Breast Cancer Res. Treat. 1997, 46, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Reeves, J.M.; Rebello, S.A.; Thomas, W.; Slaton, J.W.; Kurzer, M.S. Isoflavone-rich soy protein isolate suppresses androgen receptor expression without altering estrogen receptor-β expression or serum hormonal profiles in men at high risk of prostate cancer. J. Nutr. 2007, 137, 1769–1775. [Google Scholar] [PubMed]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, M.A.; Van Veldhuizen, P.J.; Thrasher, J.B. Effects of soy phytoestrogens on the prostate. Prostate Cancer Prostatic Dis. 2007, 10, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F.; Chen, C.; Newton, K.M.; Potter, J.D.; Lampe, J.W. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2004, 13, 644–648. [Google Scholar]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Harwood, M.; Hlywka, J.J.; Stephen, A.M.; Doull, J.; Flamm, W.G.; Adlercreutz, H. Soy isoflavones: A safety review. Nutr. Rev. 2003, 61, 1–33. [Google Scholar] [CrossRef] [PubMed]
- He, F.-J.; Chen, J.-Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Wellness 2013, 2, 146–161. [Google Scholar] [CrossRef]
- Ju, Y.H.; Carlson, K.E.; Sun, J.; Pathak, D.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A.; Helferich, W.G. Estrogenic effects of extracts from cabbage, fermented cabbage, and acidified brussels sprouts on growth and gene expression of estrogen-dependent human breast cancer (MCF-7) cells. J. Agric. Food Chem. 2000, 48, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.; Hossaini, A.; Svendsen, G.W.; Brouwer, C.; Nielsen, E. Estrogenic activity of flavonoids in mice. The importance of estrogen receptor distribution, metabolism and bioavailability. Food Chem. Toxicol. 2000, 38, 555–564. [Google Scholar] [CrossRef]
- Owens, W.; Ashby, J.; Odum, J.; Onyon, L. The OECD program to validate the rat uterotrophic bioassay. Phase 2: Dietary phytoestrogen analyses. Environ. Health Perspect. 2003, 111, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Phrakonkham, P.; Chevalier, J.; Desmetz, C.; Pinnert, M.-F.; Bergès, R.; Jover, E.; Davicco, M.-J.; Bennetau-Pelissero, C.; Coxam, V.; Artur, Y.; et al. Isoflavonoid-based bone-sparing treatments exert a low activity on reproductive organs and on hepatic metabolism of estradiol in ovariectomized rats. Toxicol. Appl. Pharmacol. 2007, 224, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Padilla-Banks, E.; Clark, G.; Newbold, R.R. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 179–189. [Google Scholar] [CrossRef]
- Inudo, M.; Ishibashi, H.; Matsumura, N.; Matsuoka, M.; Mori, T.; Taniyama, S.; Kadokami, K.; Koga, M.; Shinohara, R.; Hutchinson, T.H.; et al. Effect of estrogenic activity, and phytoestrogen and organochlorine pesticide contents in an experimental fish diet on reproduction and hepatic vitellogenin production in medaka (Oryzias latipes). Comp. Med. 2004, 54, 673–680. [Google Scholar] [PubMed]
- Kobayashi, M.; Ishibashi, H.; Moriwaki, T.; Koshiishi, T.; Ogawa, S.; Matsumoto, T.; Arizono, K.; Watabe, S. Production of low-estrogen goldfish diet for in vivo endocrine disrupter test. Environ. Sci. 2006, 13, 125–136. [Google Scholar] [PubMed]
- Bagheri, T.; Imanpoor, M.R.; Jafari, V.; Bennetau-Pelissero, C. Reproductive impairment and endocrine disruption in goldfish by feeding diets containing soybean meal. Anim. Reprod. Sci. 2013, 139, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A. L.; Walker, M.; Morris, K.; Greig, I.; Mason, J.I.; Sharpe, R.M. Infant feeding with soy formula milk: Effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Hum. Reprod. 2006, 21, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Ono, Y.; Koh, K.B.; Takashima, K.; Tainaka, H.; Matsuno, Y.; Nakagawa, S.; Todaka, E.; Sakurai, K.; Fukata, H.; et al. Long-term alteration of gene expression without morphological change in testis after neonatal exposure to genistein in mice: Toxicogenomic analysis using cDNA microarray. Food Chem. Toxicol. 2004, 42, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Persky, R.W.; Turtzo, L.C.; McCullough, L.D. Stroke in women: Disparities and outcomes. Curr. Cardiol. Rep. 2010, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Jarry, H.; Westphalen, S.; Christoffel, V.; Seidlová-Wuttke, D. Phytoestrogens for hormone replacement therapy? J. Steroid Biochem. Mol. Biol. 2002, 83, 133–147. [Google Scholar] [CrossRef]
- Sehmisch, S.; Uffenorde, J.; Maehlmeyer, S.; Tezval, M.; Jarry, H.; Stuermer, K.M.; Stuermer, E.K. Evaluation of bone quality and quantity in osteoporotic mice--the effects of genistein and equol. Phytomedicine 2010, 17, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Raghu Nadhanan, R.; Skinner, J.; Chung, R.; Su, Y.-W.; Howe, P.R.; Xian, C.J. Supplementation with fish oil and genistein, individually or in combination, protects bone against the adverse effects of methotrexate chemotherapy in rats. PLoS ONE 2013, 8, e71592. [Google Scholar] [CrossRef] [PubMed]
- Canal Castro, C.; Pagnussat, A.S.; Orlandi, L.; Worm, P.; Moura, N.; Etgen, A.M.; Alexandre Netto, C. Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res. 2012, 1474, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Leathers, C.; Skinner, M.K. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology 2006, 147, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Salian, S.; Doshi, T.; Vanage, G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 2009, 85, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bosagna, C.M.; Skinner, M.K. Environmental epigenetics and phytoestrogen/phytochemical exposures. J. Steroid Biochem. Mol. Biol. 2014, 139, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod. Toxicol. 2011, 31, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS ONE 2012, 7, e46249. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Gely-Pernot, A.; Kervarrec, C.; Boudjema, M.; Becker, E.; Khil, P.; Tevosian, S.; Jégou, B.; Smagulova, F. Exposure to the widely used herbicide atrazine results in deregulation of global tissue-specific RNA transcription in the third generation and is associated with a global decrease of histone trimethylation in mice. Nucleic Acids Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, T.E.; Hansen, F.T.; Purup, S.; Nielsen, A.K.; Bonefeld-Jørgensen, E.C.; Giese, H.; Sørensen, J.L. Fusarin C acts like an estrogenic agonist and stimulates breast cancer cells in vitro. Toxicol. Lett. 2011, 205, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Khosrokhavar, R.; Rahimifard, N.; Shoeibi, S.; Hamedani, M.P.; Hosseini, M.-J. Effects of zearalenone and α-Zearalenol in comparison with Raloxifene on T47D cells. Toxicol. Mech. Methods 2009, 19, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Zhu, Y.; Kiyama, R. Expression profiling of the genes responding to zearalenone and its analogues using estrogen-responsive genes. FEBS Lett. 2009, 583, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Belli, P.; Bellaton, C.; Durand, J.; Balleydier, S.; Milhau, N.; Mure, M.; Mornex, J.-F.; Benahmed, M.; Le Jan, C. Fetal and neonatal exposure to the mycotoxin zearalenone induces phenotypic alterations in adult rat mammary gland. Food Chem. Toxicol. 2010, 48, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar] [PubMed]
- Wang, T.T. Y.; Sathyamoorthy, N.; Phang, J.M. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 1996, 17, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.P.; Hofland, J.; Foster, P.A. In touch with your feminine side: How oestrogen metabolism impacts prostate cancer. Endocr. Relat. Cancer 2016, 23, R249–R266. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Ahmad, A.E.; Hirata, H.; Kawakami, K.; Shahryari, V.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Khatri, G.; et al. BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis 2009, 30, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.-P.; Fontana, L.; Bignon, Y.-J.; Guy, L.; et al. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. In Vivo 2010, 24, 393–400. [Google Scholar] [PubMed]
- Lim, W.; Jeong, W.; Song, G. Coumestrol suppresses proliferation of ES2 human epithelial ovarian cancer cells. J. Endocrinol. 2016, 228, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Jacquot, Y. Interactions of isoflavones and other plant derived estrogens with estrogen receptors for prevention and treatment of breast cancer-considerations concerning related efficacy and safety. J. Steroid Biochem. Mol. Biol. 2014, 139, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Hedman, A.C.; Ames, J.B.; Sacks, D.B. Calmodulin lobes facilitate dimerization and activation of estrogen receptor-α. J. Biol. Chem. 2017, 292, 4614–4622. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, L.; Lin, S.; Leung, L.K. Dietary flavones and flavonones display differential effects on aromatase (CYP19) transcription in the breast cancer cells MCF-7. Mol. Cell. Endocrinol. 2011, 344, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yang, J.-H.; Sato, E.; Miura, N.; Wu, Y.-X. Effects of hydroxy groups in the A-ring on the anti-proteasome activity of flavone. Biol. Pharm. Bull. 2015, 38, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, R.; Sayad, A.; Brown, K.R.; Sanchez-Garcia, F.; Reimand, J.; Haider, M.; Virtanen, C.; Bradner, J.E.; Bader, G.D.; Mills, G.B.; et al. Functional genomic landscape of human breast cancer drivers, vulnerabilities, and resistance. Cell 2016, 164, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sharma, V.; Verma, V.; Pandey, D.; Yadav, S.K.; Maikhuri, J.P.; Gupta, G. Apigenin manipulates the ubiquitin–proteasome system to rescue estrogen receptor-β from degradation and induce apoptosis in prostate cancer cells. Eur. J. Nutr. 2015, 54, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, S.; Lelong, M.; Bourgine, G.; Efstathiou, T.; Saligaut, C.; Pakdel, F. Assessment of the potential activity of major dietary compounds as selective estrogen receptor modulators in two distinct cell models for proliferation and differentiation. Toxicol. Appl. Pharmacol. 2017, 325, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.K.Y.; Lam, H.-M.; Ng, C.-F.; Lee, A.Y.Y.; Chan, E.S.Y.; Ng, H.-K.; Ho, S.-M.; Lau, K.-M. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G(2) cell-cycle arrest. Cell Death Differ. 2010, 17, 1511–1523. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. https://doi.org/10.3390/ijms18071381
Lecomte S, Demay F, Ferrière F, Pakdel F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? International Journal of Molecular Sciences. 2017; 18(7):1381. https://doi.org/10.3390/ijms18071381
Chicago/Turabian StyleLecomte, Sylvain, Florence Demay, François Ferrière, and Farzad Pakdel. 2017. "Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects?" International Journal of Molecular Sciences 18, no. 7: 1381. https://doi.org/10.3390/ijms18071381