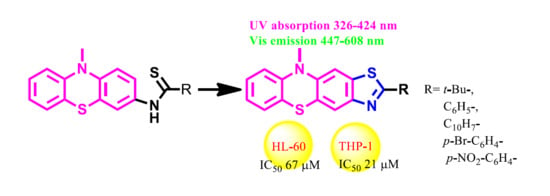
Novel Thiazolo[5,4-b]phenothiazine Derivatives: Synthesis, Structural Characterization, and In Vitro Evaluation of Antiproliferative Activity against Human Leukaemia
Abstract
:1. Introduction
2. Results
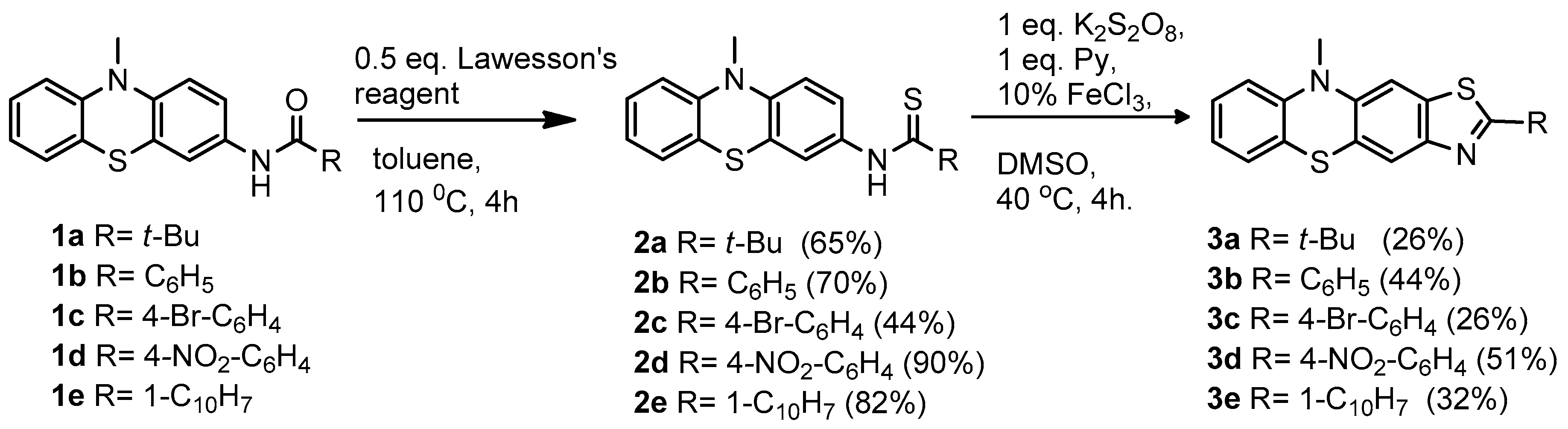
2.1. Synthesis of Thiazolo[5,4-b]phenothiazine Derivatives
2.2. Spectral Properties
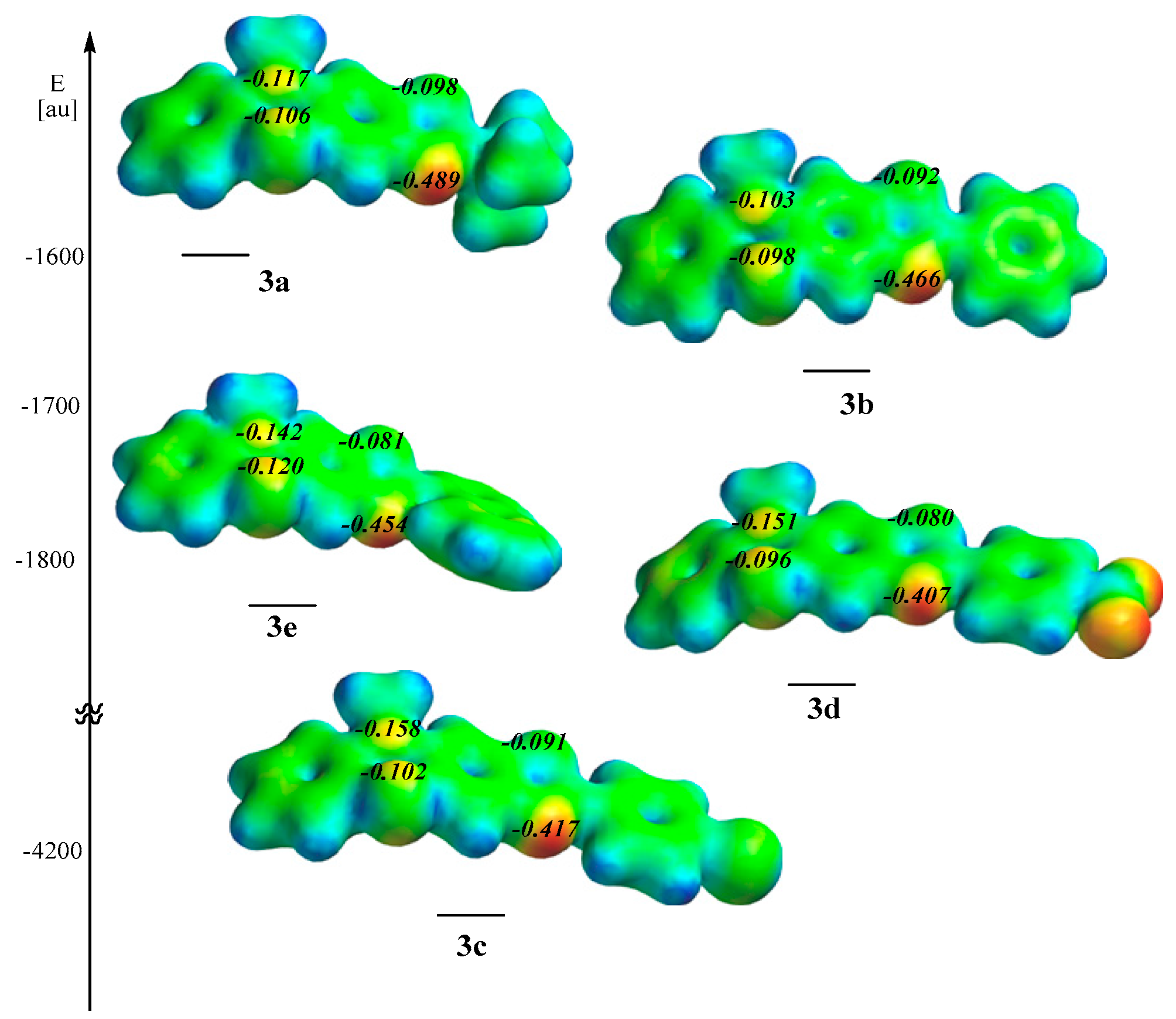
2.3. Computational Data
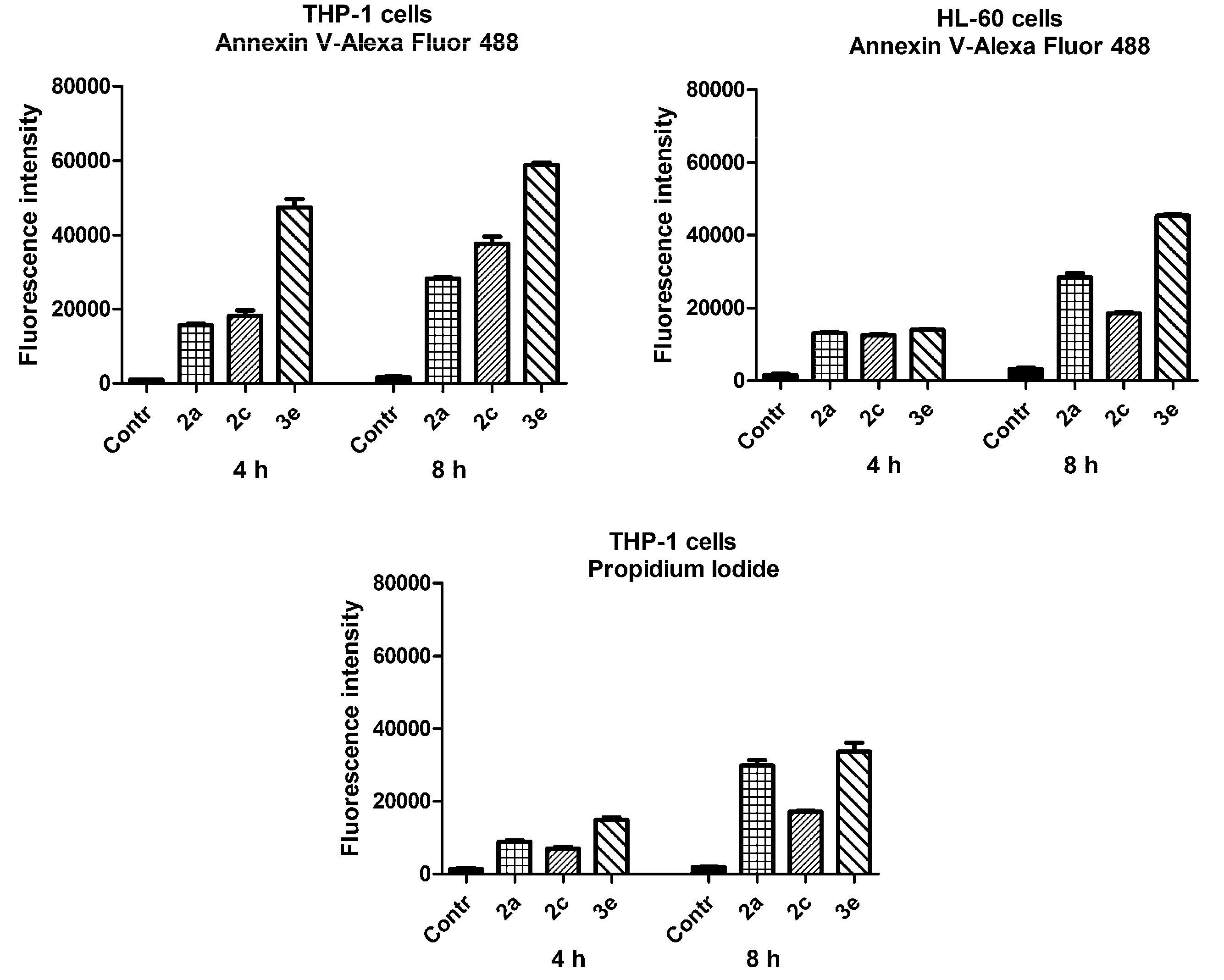
2.4. Biological Properties
3. Discussion
4. Materials and Methods
4.1. General Procedure for Obtaining N-Acyl-3-Aminophenothiazines (1a–e)
4.2. General Procedure for Obtaining N-(Phenothiazin-3-yl)tioamides (2a–e)
4.3. General Procedure for Obtaining Thiazolo[5,4-b]phenothiazine (3a–e)
4.4. Biologic Assay
4.4.1. Cell Cultures
4.4.2. Cell Viability Tests
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 2011, 46, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagupi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Phenothiazinium salts as antimicrobial photosensitizing agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Hamblin, M.R., Jori, G., Eds.; RSC Publishing: Cambbridge, UK, 2011; Volume 11, pp. 19–43. [Google Scholar]
- Pereteanu, I.S.; Müller, T.J.J. Synthesis and electronic properties of 3,7-dianilino substituted N-hexyl phenothiazines. Org. Biomol. Chem. 2013, 11, 5127–5135. [Google Scholar] [CrossRef] [PubMed]
- Sailer, M.; Franz, A.W.; Müller, T.J.J. Synthesis and electronic properties of monodisperse oligophenothiazines. Chem. Eur. J. 2008, 14, 2602–2614. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Ogermann, D.; Pankrath, A.; Müller, T.J.J. Phenothiazinyl rhodanylidene merocyanines for dye-sensitized solar cells. J. Org. Chem. 2012, 77, 3704–3715. [Google Scholar] [CrossRef] [PubMed]
- Jaszczyszyn, A.; Gąsiorowski, K.; Świątek, P.; Malinka, W.; Cieślik-Boczula, K.; Petrus, J.; Czarnik-Matusewicz, B. Chemical structure of phenothiazines and their biological activity. Pharmacol. Rep. 2012, 64, 16–23. [Google Scholar] [CrossRef]
- Hendrich, A.B.; Wesolowska, O.; Motohashi, N.; Molnar, J.; Michalak, K. New phenothiazine-type multidrug resistance modifiers: Anti-MDR activity versus membrane perturbing potency. Biochem. Biophys. Res. Commun. 2003, 304, 260–265. [Google Scholar] [CrossRef]
- Seelig, A. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 1998, 251, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Pluta, K.; Jeleñ, M.; Morak-Modawska, B.; Zimecki, M.; Artym, J.; Kociêba, M. Anticancer activity of newly synthesized azaphenothiazines from NCI’s anticancer screening bank. Pharmacol. Rep. 2010, 62, 319–332. [Google Scholar] [CrossRef]
- Motohashi, N.; Kawase, M.; Satoh, K.; Sakagami, H. Cytotoxic potential of phenothiazines. Curr. Drug Targets 2006, 7, 1055–1066. [Google Scholar] [CrossRef]
- Shin, S.Y.; Choi, B.H.; Kim, J.R.; Kim, J.H.; Lee, Y.H. Suppression of P-glycoprotein expression by antipsychotics trifluoperazine in adriamycin-resistant L1210 mouse leukemia cells. Eur. J. Pharm. Sci. 2006, 28, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.K.; Christopherson, R.I.; Roufogalis, B.D. Reversal of vinblastine transport by chlorpromazine in membrane vesicles from multidrug-resistant human CCRF-CEM leukaemia cells. Br. J. Cancer 1998, 78, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Jena, J. Significance of benzothiazole moiety in the field of cancer. Int. J. Pharm. Pharm. Sci. 2014, 6, 16–22. [Google Scholar]
- Li, H.; Wang, X.-M. Combination of 2-methoxy-3-phenylsulfonylaminobenzamide and 2-aminobenzothiazole to discover novel anticancer agents. Bioorg. Med. Chem. 2014, 22, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.B.; de Brito, M.A.; Vasconcelos, T.R.A.; de Moraes, M.O.; Montenegro, R.C.; Yoneda, J.D.; Leal, K.Z. Synthesis and anticancer activity of (E)-2-benzothiazole hydrazones. Eur. J. Med. Chem. 2014, 86, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Weekes, A.A.; Westwell, A.D. 2-Arylbenzothiazole as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, N.P.; Vekariya, R.H.; Borad, M.A.; Patel, H.D. Recent advances in the synthesis of 2-substituted benzothiazoles: A review. RSC Adv. 2014, 4, 60176–60208. [Google Scholar] [CrossRef]
- Saha, P.; Ramana, T.; Purkait, N.; Ashif, A.M.; Paul, R.; Punniyamurthy, T. Ligand-free copper-catalyzed synthesis of substituted benzimidazoles, 2-aminobenzimidazoles, 2-aminobenzothiazoles, and benzoxazoles. J. Org. Chem. 2009, 74, 8719–8725. [Google Scholar] [CrossRef] [PubMed]
- Evindar, G.; Batey, R.A. Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides. J. Org. Chem. 2006, 71, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Copper-catalyzed synthesis of benzimidazoles via cascade reactions of o-haloacetanilide derivatives with amidine hydrochlorides. J. Org. Chem. 2008, 73, 7841–7844. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. Copper-catalyzed intramolecular C-N bond formation: A straightforward synthesis of benzimidazole derivatives in water. J. Org. Chem. 2011, 76, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Jaseer, E.A.; Prasad, D.J.C.; Dandapat, A.; Sekar, G. An efficient copper(II)-catalyzed synthesis of benzothiazoles through intramolecular coupling-cyclization of N-(2-chlorophenyl)benzothioamides. Tetrahedron Lett. 2010, 51, 5009–5012. [Google Scholar] [CrossRef]
- Ma, D.; Xie, S.; Xue, P.; Zhang, X.; Dong, J.; Jiang, Y. Efficient and economical access to substituted benzothiazoles: Copper-catalyzed coupling of 2-haloanilides with metal sulfides and subsequent condensation. Angew. Chem. Int. Ed. 2009, 48, 4222–4225. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Shang, J.; Li, X.; Wang, H.; Gui, J.; Lei, A. Fe-catalysed oxidative C-H functionalization/C-S bond formation. Chem. Commun. 2012, 48, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Ignat, A.; Lovasz, T.; Vasilescu, M.; Fischer-Fodor, E.; Tatomir, C.B.; Cristea, C.; Silaghi-Dumitrescu, L.; Zaharia, V. Heterocycles 27. Microwave assisted synthesis and antitumour activity of novel phenothiazinyl-thiazolyl-hydrazine derivatives. Arch. Pharm. Chem. Life Sci. 2012, 345, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Brem, B.; Gal, E.; Cristea, C.; Gaina, L.; Grozav, A.; Zaharia, V.; Silaghi-Dumitrescu, L. Synthesis of new benzothiazolyl-phenothiazine derivatives. Studi. Univ. Babes Bolyai Chem. 2015, 60, 371–378. [Google Scholar]
- Grozav, A.; Zaharia, V.; Cristea, C.; Fit, N.I. Antimicrobial activity screening of benzothiazolyl-phenothiazine derivatives. Studia UBB Chem. 2015, 60, 283–289. [Google Scholar]
- Găină, L.I.; Mătărângă-Popa, L.N.; Gal, E.; Boar, P.; Lonnecke, P.; Hey-Hawkins, E.; Silaghi-Dumitrescu, L.; Cristea, C.; Bischin, C.; Lupan, I. Microwave-assisted catalytic amination of phenothiazine; reliable access to phenothiazine analogues of Tröger’s base. Eur. J. Org. Chem. 2013, 24, 5500–5508. [Google Scholar] [CrossRef]
- Yang, L.; Feng, J.K.; Ren, A.M. Theoretical study on electronic structure and optical properties of phenothiazine-containing conjugated oligomers and polymers. J. Org. Chem. 2005, 70, 5987–5996. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, A.M.; Ohba, A.M.; Bakalova, R.; Hadjimitova, V.; Ishikawa, M.; Shinohara, Y.; Baba, Y. Phenothiazines suppress proliferation and induce apoptosis in cultured leukemic cells without any influence on the viability of normal lymphocytes. Cancer Chemother. Pharmacol. 2004, 53, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ad, I.; Shtaif, B.; Levkovitz, Y.; Dayag, M.; Zeldich, E.; Weizman, A. Characterization of phenothiazine-induced apoptosis in neuroblastoma and glioma cell lines: Clinical relevance and possible application for brain-derived tumors. J. Mol. Neurosci. 2004, 22, 189–198. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Takács, D.; Horváth, A.; Riedl, Z.; Hajós, G.; Amaral, L.; Molnár, J. Multidrug resistance reversing activity of newly developed phenothiazines on P-glycoprotein (ABCB1)-related resistance of mouse T-lymphoma cells. Anticancer Res. 2014, 34, 1737–1741. [Google Scholar] [PubMed]
- Cauquil, G.; Casadevall, E.; Casadevall, A. Recherches dans la serie de la phenothiazine(5e memoire). Transposition de Bechmann et reaction de Schmidt appliquées à la synthèse d’amino-methyl 10-phenothiazines. Bull. Soc. Chem. Fr. 1962, 602–616. [Google Scholar]
- Fischer-Fodor, E.; Mot, A.; Deac, F.; Arkosi, M.; Silaghi-Dumitrescu, R. Towards hemerythrin-based blood substitutes: Comparative performance to hemoglobin on human leukocytes and umbilical vein endothelial cells. J. Biosci. 2011, 36, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Miklášová, N.; Fischer-Fodor, E.; Mikláš, R.; Kucková, L.; Kožíšek, J.; Liptaj, T.; Soritau, O.; Valentova, J.; Devinsky, F. Synthesis and characterization of new biologically active palladium(II) complexes with (1E,6E)-1,7-bis(3,4-diethoxyphenyl)-1,6-heptadiene-3,5-dione. Inorg. Chem. Commun. 2014, 46, 229–233. [Google Scholar] [CrossRef]


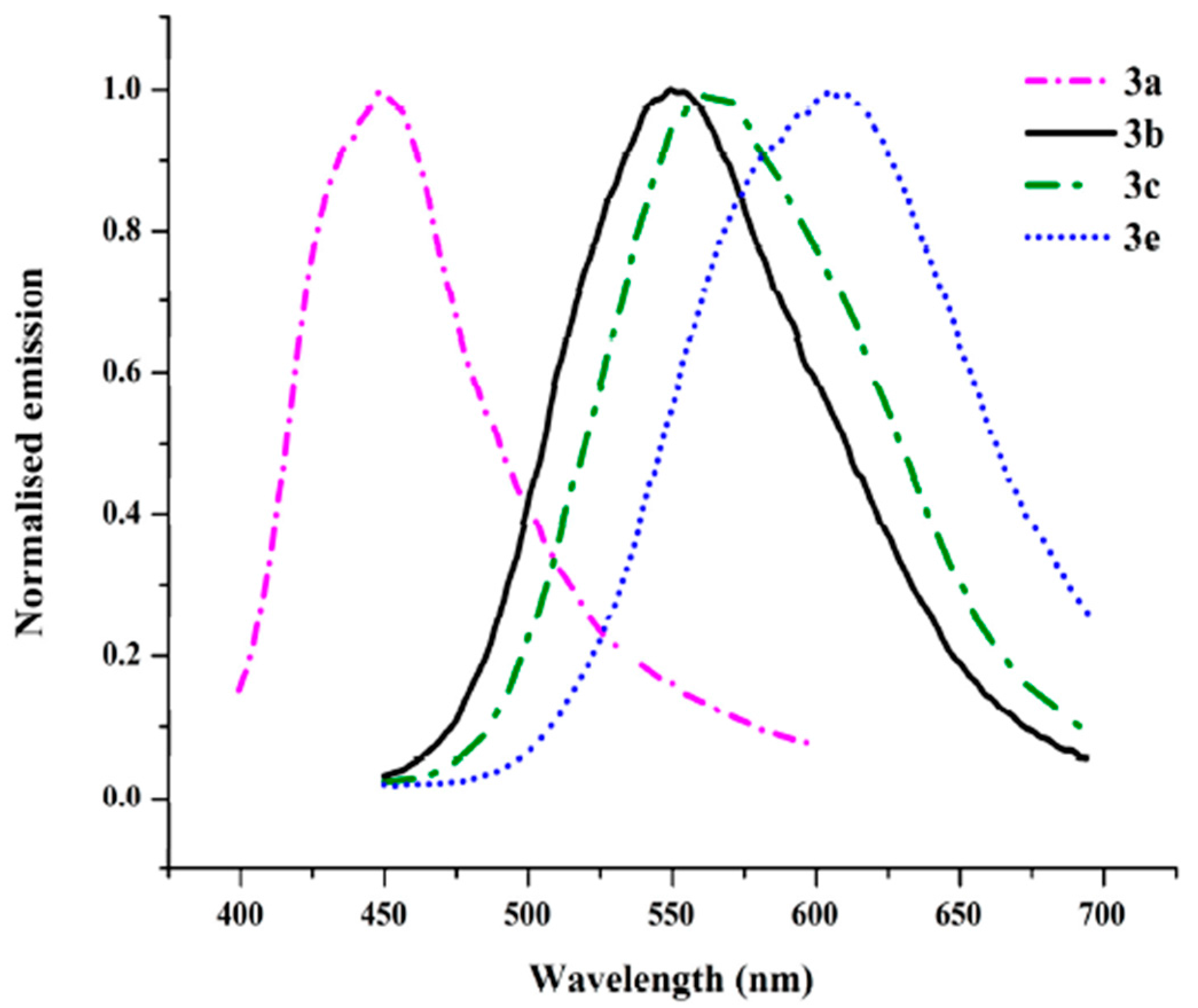

| Cpd | λmax,abs 1 (nm) (ε (M−1 cm−1)) | λmax, em 2 (nm) | Stokes Shift 3 (cm−1) | Egap,opt 4 (eV) | EHOMO 5 (eV) | ELUMO 5 (eV) | Dipole Moment 5 (D) |
|---|---|---|---|---|---|---|---|
| 3a | 263 (28,900) 326 (7400) | 447 | 8300 | 4.49 | −5.07 | −0.78 | 2.68 |
| 3b | 261 (43,400) 370 (15,800) | 550 | 8800 | 4.28 | −5.07 | −1.54 | 2.67 |
| 3c | 262 (32,800) 377 (12,200) | 564 | 8800 | 4.18 | −5.17 | −1.77 | 3.92 |
| 3d | 264 (29,600) 300 (12,600) 424 (9300) | - | - | −5.36 | −2.72 | 7.63 | |
| 3e | 250 (23,300) 294 (8900) 373 (8900) | 608 | 10,400 | 4.07 | −5.07 | −1.69 | 2.55 |
| Cpd | Antiproliferative Activity IC50 (μM) | ||
|---|---|---|---|
| THP-1 | HL-60 | PBMC | |
| 2a | 101.70 ± 0.11 b | 175.8 ± 0.23 b | >2000 |
| 2b | 1481 a | >2000 | 1121 |
| 2c | 88.9 ± 0.03 b | 69.1 ± 0.19 b | >2000 |
| 2e | >2000 | >2000 | 227 a |
| 3a | 463.4 ± 0.16 b | 194.3 ± 0.17 b | >2000 |
| 3b | 1241 a | 338.9 ± 0.14 b | 1906 a |
| 3c | >1000 | 1066 a | >1000 |
| 3e | 21.6 ± 0.06 b | 67.2 ± 0.09 b | >2000 |
| Cytarabine | 9.0 ± 0.05 b | 13.5 ± 0.05 b | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brem, B.; Gal, E.; Găină, L.; Silaghi-Dumitrescu, L.; Fischer-Fodor, E.; Tomuleasa, C.I.; Grozav, A.; Zaharia, V.; Filip, L.; Cristea, C. Novel Thiazolo[5,4-b]phenothiazine Derivatives: Synthesis, Structural Characterization, and In Vitro Evaluation of Antiproliferative Activity against Human Leukaemia. Int. J. Mol. Sci. 2017, 18, 1365. https://doi.org/10.3390/ijms18071365
Brem B, Gal E, Găină L, Silaghi-Dumitrescu L, Fischer-Fodor E, Tomuleasa CI, Grozav A, Zaharia V, Filip L, Cristea C. Novel Thiazolo[5,4-b]phenothiazine Derivatives: Synthesis, Structural Characterization, and In Vitro Evaluation of Antiproliferative Activity against Human Leukaemia. International Journal of Molecular Sciences. 2017; 18(7):1365. https://doi.org/10.3390/ijms18071365
Chicago/Turabian StyleBrem, Balazs, Emese Gal, Luiza Găină, Luminiţa Silaghi-Dumitrescu, Eva Fischer-Fodor, Ciprian Ionuţ Tomuleasa, Adriana Grozav, Valentin Zaharia, Lorena Filip, and Castelia Cristea. 2017. "Novel Thiazolo[5,4-b]phenothiazine Derivatives: Synthesis, Structural Characterization, and In Vitro Evaluation of Antiproliferative Activity against Human Leukaemia" International Journal of Molecular Sciences 18, no. 7: 1365. https://doi.org/10.3390/ijms18071365
APA StyleBrem, B., Gal, E., Găină, L., Silaghi-Dumitrescu, L., Fischer-Fodor, E., Tomuleasa, C. I., Grozav, A., Zaharia, V., Filip, L., & Cristea, C. (2017). Novel Thiazolo[5,4-b]phenothiazine Derivatives: Synthesis, Structural Characterization, and In Vitro Evaluation of Antiproliferative Activity against Human Leukaemia. International Journal of Molecular Sciences, 18(7), 1365. https://doi.org/10.3390/ijms18071365