Differentiated Thyroid Cancer—Treatment: State of the Art
Abstract
:1. Introduction
2. Epidemiology and Classification
- high-risk group: pT3, pT4, each N1, all M1;
- low-risk group: pT1b, pT2, cN0/pN0, cM0;
- very low risk-group: pT1a, cN0/pN0, cM0.
- high-risk group: gross extrathyreoidal extension, incomplete tumor resection, distant metastases, or lymph node >3 cm;
- intermediate-risk: aggressive histology, minor extrathyreoidal extension, vascular invasion, or >5 involved lymph nodes (0.2–3 cm);
- low-risk: intrathyreoidal DTC, ≤5 lymph nodes micrometastases (<0.2 cm).
2.1. Papillary Thyroid Cancer
2.2. Follicular Thyroid Cancer
2.3. Familial Tumor Syndromes
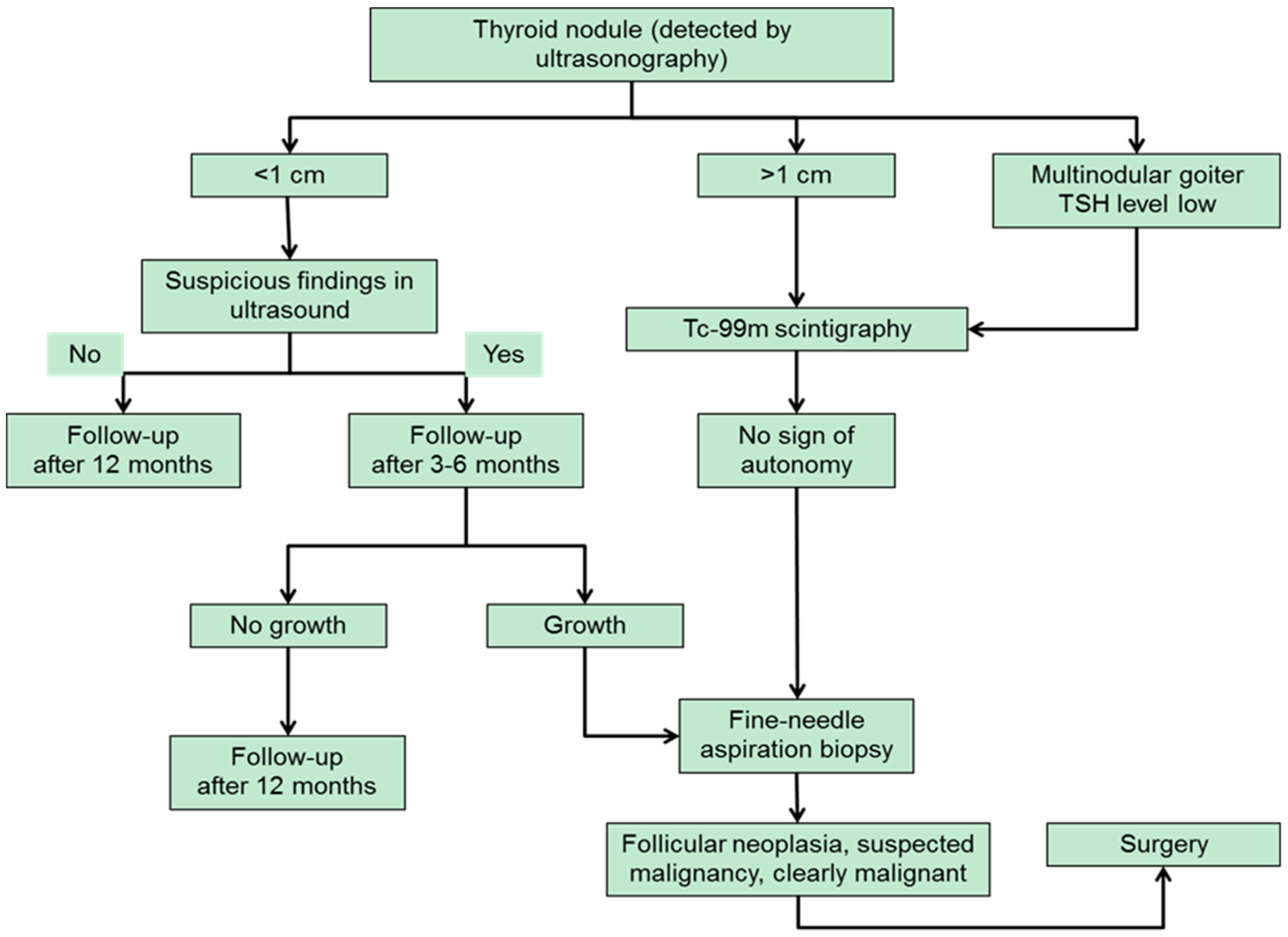
3. Diagnostic Approach to Thyroid Nodules
- I:
- nondiagnostic/unsatisfactory;
- II:
- benign;
- III:
- atypia of undetermined significance/follicular lesion of undetermined significance;
- IV:
- follicular neoplasm/suspicious for follicular neoplasm;
- V:
- suspicious for malignancy;
- VI:
- malignant.
4. Therapy of Differentiated Thyroid Carcinoma
4.1. Surgery
4.2. Adjuvant Radioiodine Therapy
4.3. Metastatic Differentiated Thyroid Carcinoma
4.4. Thyroid Hormone Treatment
4.5. Follow-Up
4.6. Tyrosine Kinase Inhibitors
4.7. Papillary Microcarcinoma
5. Summary and Conclusion
- The value of RIT under the condition of increasing serum level of Tg without a detectable correlatation in the morphological or functional imaging (i.e. iodine-negative whole-body scan);
- The benefit of a remnant ablation in patients with papillary microcarcinoma (very low risk of relapse, lymph node metastasis possible);
- Optimal activities of I-131 for safe and effective radioiodine ablation;
- The role of rhTSH as preparation for RIT to treat incomplete or non-resectable local recurrence or metastases;
- The role of a short LT4 withdrawal to reduce blood levels of iodine before RIT or diagnostic whole-body scan.
Author Contributions
Conflicts of Interest
Abbreviations
| AKT1 | RAC-α serine/threonine-protein kinase 1 |
| ATA | American Thyroid Association |
| CT | computed tomography |
| DTC | differentiated thyroid carcinoma |
| EANM | European Association of Nuclear Medicine |
| ETE | extrathyroidal extension |
| ETA | European Thyroid Association |
| FAP | familial adenomatous polyposis |
| FNA | fine-needle aspiration biopsy |
| FTC | follicular thyroid carcinoma |
| FDG-PET | F-18-fluorodeoxy-glucose positron emission tomography |
| LT4 | levothyroxine |
| MEN2 | multiple endocrine neoplasia type 2 |
| PAX8 | paired box gene 8 |
| PDTC | poorly differentiated thyroid carcinoma |
| PIK3CA | phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit α |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| PTC | papillary thyroid carcinoma |
| PTEN | phosphatase and tensin homolog |
| PTMC | papillary microcarcinoma |
| RET | RET proto-oncogene |
| rhTSH | recombinant thyrotropin |
| RIT | radioiodine therapy |
| SPECT | single photon emission computed tomography |
| TERT | telomerase reverse transcriptase |
| Tg | serum thyroglobulin |
| TKI | receptor tyrosine kinase inhibitors |
| TP53 | tumor protein p53 |
| TSH | thyroid-stimulating hormone (also known as thyrotropin) |
| VEGF | vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Hegedüs, L. Clinical practice. The thyroid nodule. N. Engl. J. Med. 2004, 351, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, L.; Bernier, M.O.; Boin-Pineau, M.H.; Conte Devolx, B.; Maréchaud, R.; Niccoli-Sire, P.; Nocaudie, M.; Orgiazzi, J.; Schlumberger, M.; Wémeau, J.L.; et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur. J. Endocrinol. 2004, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I. Thyroid carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Schechter, R.B.; Shih, Y.C.; Kaplan, E.L.; Chiu, B.C.; Angelos, P.; Grogan, R.H. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, L.; Erdogan, M.F.; Hegedus, L.; Mandel, S.J.; Paschke, R.; Rago, T.; Russ, G. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur. Thyroid J. 2013, 2, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B.; Alavi, A.; Balon, H.R.; Clarke, S.E.; Divgi, C.; Gelfand, M.J.; Goldsmith, S.J.; Jadvar, H.; Marcus, C.S.; Martin, W.H.; et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J. Nucl. Med. 2012, 53, 1633–1651. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.; Tennvall, J.; Bombardieri, E.; European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Takami, H.; Ito, Y.; Okamoto, T.; Onoda, N.; Noguchi, H.; Yoshida, A. Revisiting the guidelines issued by the Japanese Society of Thyroid Surgeons and Japan Association of Endocrine Surgeons: A gradual move towards consensus between Japanese and western practice in the management of thyroid carcinoma. World J. Surg. 2014, 38, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Weinheim, Germany, 2017; pp. 69–71. [Google Scholar]
- Armin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; pp. 1–19. [Google Scholar]
- Dietlein, M.; Eschner, W.; Grünwald, F.; Lassmann, M.; Verburg, F.A.; Luster, M. Procedure guidelines for radioiodine therapy of differentiated thyroid cancer. Version 4. Nuklearmedizin 2016, 55, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.C.; Vaisman, F.; Vaisman, M.; Sobrinho-Simões, M.; Soares, P. Molecular Markers Involved in Tumorigenesis of Thyroid Carcinoma: Focus on Aggressive Histotypes. Cytogenet. Genome Res. 2016, 150, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Ohori, N.P. Papillary Carcinoma. In Diagnostic Pathology and Molecular Genetics of the Thyroid, 1st ed.; Nikiforov, Y.E., Biddinger, P.W., Thompson, L.D.R., Eds.; Lippincott: Philadelphia, PA, USA, 2012; pp. 183–262. [Google Scholar]
- Seethala, R.R.; Asa, S.L.; Carty, S.E.; Hodak, S.P.; McHugh, J.B.; Richardson, M.S.; Shah, J.; Thompson, L.D.R.; Nikiforov, Y.E. For the Members of the Cancer Committee, College of American Pathologists. Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland, Based on AJCC/UICC TNM, 7th edition. Version: Thyroid 3.2.0.0. Available online: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-thyroid-16protocol-3200.pdf (accessed on 2 June 2017).
- Nikiforova, M.N.; Kimura, E.T.; Gandhi, M.; Biddinger, P.W.; Knauf, J.A.; Basolo, F.; Zhu, Z.; Giannini, R.; Salvatore, G.; Fusco, A.; et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J. Clin. Endocrinol. Metab. 2003, 88, 5399–5404. [Google Scholar] [CrossRef] [PubMed]
- Asioli, S.; Erickson, L.A.; Sebo, T.J.; Zhang, J.; Jin, L.; Thompson, G.B.; Lloyd, R.V. Papillary thyroid carcinoma with prominent hobnail features: A new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am. J. Surg. Pathol. 2010, 34, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Howe, G.; Ron, E.; Bebeshko, V.; Bogdanova, T.; Bouville, A.; Carr, Z.; Chumak, V.; Davis, S.; Demidchik, Y.; et al. Cancer consequences of the Chernobyl accident: 20 years on. J. Radiol. Prot. 2006, 26, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E. Radiation-induced thyroid cancer: What we have learned from Chernobyl. Endocr. Pathol. 2006, 17, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Jia, Y.; Sholl, L.M.; Barletta, J.A. Molecular alterations in partially-encapsulated or well-circumscribed follicular variant of papillary thyroid carcinoma. Thyroid 2013, 23, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Singh, B.; Tallini, G.; Carlson, D.L.; Katabi, N.; Shaha, A.; Tuttle, R.M.; Ghossein, R.A. Follicular variant of papillary thyroid carcinoma: A clinicopathologic study of a problematic entity. Cancer 2006, 107, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.D.; Bergstralh, E.J.; van Heerden, J.A.; McConahey, W.M. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: Initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin. Proc. 1991, 66, 11–22. [Google Scholar] [CrossRef]
- Collini, P.; Sampietro, G.; Pilotti, S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non-Hürthle cell follicular carcinoma of the thyroid gland: A clinicopathological study of 18 consecutive cases from a single institution with a 11-year median follow-up. Histopathology 2004, 44, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Lang, W.; Choritz, H.; Hundeshagen, H. Risk factors in follicular thyroid carcinomas. A retrospective follow-up study covering a 14-year period with emphasis on morphological findings. Am. J. Surg. Pathol. 1986, 10, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 1998, 83, 2638–2648. [Google Scholar] [CrossRef]
- Haigh, P.I.; Urbach, D.R. The treatment and prognosis of Hürthle cell follicular thyroid carcinoma compared with its non-Hürthle cell counterpart. Surgery 2005, 138, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Shaha, A.R.; Loree, T.R.; Shah, J.P. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery 1995, 118, 1131–1136. [Google Scholar] [CrossRef]
- Cetta, F.; Montalto, G.; Gori, M.; Curia, M.C.; Cama, A.; Olschwang, S. Germline mutations of the APC gene in patients with familial adenomatous polyposis-associated thyroid carcinoma: Results from a European cooperative study. J. Clin. Endocrinol. Metab. 2000, 85, 286–292. [Google Scholar] [PubMed]
- Harach, H.R.; Williams, G.T.; Williams, E.D. Familial adenomatous polyposis associated thyroid carcinoma: A distinct type of follicular cell neoplasm. Histopathology 1994, 25, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyauchi, A.; Ishikawa, H.; Hirokawa, M.; Kudo, T.; Tomoda, C.; Miya, A. Our experience of treatment of cribriform morular variant of papillary thyroid carcinoma; difference in clinicopathological features of FAP-associated and sporadic patients. Endocr. J. 2011, 58, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.C.; Blumenthal, G.M.; Dennis, P.A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 2011, 11, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.R.; Bongiovanni, M.; Tille, J.C.; Kozakewich, H.; Nosé, V. Thyroid pathology in PTEN-hamartoma tumor syndrome: Characteristic findings of a distinct entity. Thyroid 2011, 21, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Nosé, V. Familial thyroid cancer: A review. Mod. Pathol. 2011, 24, S19–S33. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.S.; Gharib, H. Epidemiology of thyroid nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P. The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, J.; Führer, D.; Luster, M.; Musholt, T.J.; Spitzweg, C.; Schott, M. Fine Needle Aspiration in the Investigation of Thyroid Nodules. Dtsch. Arztebl. Int. 2016, 113, 353–359. [Google Scholar] [PubMed]
- Dralle, H.; Musholt, T.J.; Schabram, J.; Steinmüller, T.; Frilling, A.; Simon, D.; Goretzki, P.E.; Niederle, B.; Scheuba, C.; Clerici, T.; et al. German Association of Endocrine Surgeons practice guideline for the surgical management of malignant thyroid tumors. Langenbecks Arch. Surg. 2013, 398, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Samaan, N.A.; Schultz, P.N.; Hickey, R.C.; Goepfert, H.; Haynie, T.P.; Johnston, D.A.; Ordonez, N.G. The results of various modalities of treatment of well differentiated thyroid carcinomas: A retrospective review of 1599 patients. J. Clin. Endocrinol. Metab. 1992, 75, 714–720. [Google Scholar] [PubMed]
- Sawka, A.M.; Thephamongkhol, K.; Brouwers, M.; Thabane, L.; Browman, G.; Gerstein, H.C. Clinical review 170: A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2004, 89, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Sawka, A.M.; Brierley, J.D.; Tsang, R.W.; Thabane, L.; Rotstein, L.; Gafni, A.; Straus, S.; Goldstein, D.P. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Al-Maqbili, T.; Carter, B.; Martin, E.; Campain, N.; Watkinson, J.; McCabe, C.; Boelaert, K.; Franklyn, J.A. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: A systematic review and meta-analysis of 21,329 person-years of follow-up. J. Clin. Endocrinol. Metab. 2014, 99, 2834–2843. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard, B.G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. British Thyroid Association. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81, 1–122. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Sherman, S.I.; Tuttle, R.M.; et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006, 16, 109–142. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.; Felbinger, R.; Dietlein, M.; Reiners, C. Thyroid hormone withdrawal in patients with differentiated thyroid carcinoma: A one hundred thirty-patient pilot survey on consequences of hypothyroidism and a pharmacoeconomic comparison to recombinant thyrotropin administration. Thyroid 2005, 15, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Ladenson, P.W.; Schlumberger, M.; Driedger, A.; Luster, M.; Kloos, R.T.; Sherman, S.; Haugen, B.; Corone, C.; Molinaro, E.; et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: Results of an international, randomized, controlled study. J. Clin. Endocrinol. Metab. 2006, 91, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Ladenson, P.W.; Braverman, L.E.; Mazzaferri, E.L.; Brucker-Davis, F.; Cooper, D.S.; Garber, J.R.; Wondisford, F.E.; Davies, T.F.; DeGroot, L.J.; Daniels, G.H.; et al. Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. N. Engl. J. Med. 1997, 337, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Luster, M. Acta Oncologica Lecture. Present status of the use of recombinant human TSH in thyroid cancer management. Acta Oncol. 2006, 45, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Ricard, M.; De Pouvourville, G.; Pacini, F. How the availability of recombinant human TSH has changed the management of patients who have thyroid cancer. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Mallick, U.; Harmer, C.; Yap, B.; Wadsley, J.; Clarke, S.; Moss, L.; Nicol, A.; Clark, P.M.; Farnell, K.; McCready, R.; et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N. Engl. J. Med. 2012, 366, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Catargi, B.; Borget, I.; Deandreis, D.; Zerdoud, S.; Bridji, B.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N. Engl. J. Med. 2012, 366, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Kukulska, A.; Krajewska, J.; Gawkowska-Suwińska, M.; Puch, Z.; Paliczka-Cieslik, E.; Roskosz, J.; Handkiewicz-Junak, D.; Jarzab, M.; Gubała, E.; Jarzab, B. Radioiodine thyroid remnant ablation in patients with differentiated thyroid carcinoma (DTC): Prospective comparison of long-term outcomes of treatment with 30, 60 and 100 mCi. Thyroid Res. 2010, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, B.; Beiki, D.; Takavar, A.; Fard-Esfahani, A.; Gilani, K.A.; Saghari, M.; Eftekhari, M. Low versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid carcinoma: A large randomized clinical trial. Nucl. Med. Commun. 2012, 33, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J.; Chon, J.T.; Fleisher, M.; Larson, S.M.; Tuttle, R.M. Is the serum thyroglobulin response to recombinant human thyrotropin sufficient, by itself, to monitor for residual thyroid carcinoma? J. Clin. Endocrinol. Metab. 2002, 87, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Rubino, C.; de Vathaire, F.; Dottorini, M.E.; Hall, P.; Schvartz, C.; Couette, J.E.; Dondon, M.G.; Abbas, M.T.; Langlois, C.; Schlumberger, M. Second primary malignancies in thyroid cancer patients. Br. J. Cancer 2003, 89, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.; Benz, E.; Palmedo, H.; Biersack, H.J.; Grünwald, F.; Klingmüller, D. Testicular function after radioiodine therapy for thyroid carcinoma. Eur. J. Nucl. Med. 2000, 27, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Benbassat, C.A.; Mechlis-Frish, S.; Hirsch, D. Clinicopathological characteristics and long-term outcome in patients with distant metastases from differentiated thyroid cancer. World J. Surg. 2006, 30, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Abonowara, A.; Quraishi, A.; Sapp, J.L.; Alqambar, M.H.; Saric, A.; O’Connell, C.M.; Rajaraman, M.M.; Hart, R.D.; Imran, S.A. Prevalence of atrial fibrillation in patients taking TSH suppression therapy for management of thyroid cancer. Clin. Investig. Med. 2012, 35, E152–E156. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, V.; Schmid, K.W.; Weber, F.; Bockisch, A.; Führer, D. Differentiated thyroid cancer. Internist 2015, 56, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.; Wiersinga, W.; European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J.; Wan, Q.; Grewal, R.K.; Reibke, R.; Gonen, M.; Strauss, H.W.; Tuttle, R.M.; Drucker, W.; Larson, S.M. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-d-glucose-positron emission tomography scanning. J. Clin. Endocrinol. Metab. 2006, 91, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I. Early clinical studies of novel therapies for thyroid cancers. Endocrinol. Metab. Clin. N. Am. 2008, 37, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Kreissl, M.C.; Weismann, D.; Allolio, B. New targets and therapeutic approaches for endocrine malignancies. Pharmacol. Ther. 2009, 123, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, L.; Pieruzzi, L.; Biagini, A.; Sabini, E.; Valerio, L.; Giani, C.; Passannanti, P.; Pontillo-Contillo, B.; Battaglia, V.; Mazzeo, S.; et al. Lenvatinib and other tyrosine kinase inhibitors for the treatment of radioiodine refractory, advanced, and progressive thyroid cancer. Onco Targets Ther. 2016, 9, 6467–6477. [Google Scholar] [CrossRef] [PubMed]
- Laursen, R.; Wehland, M.; Kopp, S.; Pietsch, J.; Infanger, M.; Grosse, J.; Grimm, D. Effects and Role of Multikinase Inhibitors in Thyroid Cancer. Curr. Pharm. Des. 2016, 22, 5915–5926. [Google Scholar] [CrossRef] [PubMed]
- Pitoia, F.; Jerkovich, F. Selective use of sorafenib in the treatment of thyroid cancer. Drug Des. Dev. Ther. 2016, 10, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Ancker, O.V.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. The Adverse Effect of Hypertension in the Treatment of Thyroid Cancer with Multi-Kinase Inhibitors. Int. J. Mol. Sci. 2017, 18, 625. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Lückerath, K.; Schmid, J.S.; Higuchi, T.; Kreissl, M.C.; Grelle, I.; Reiners, C.; Buck, A.K.; Lapa, C. Thyroglobulin fluctuations in patients with iodine-refractory differentiated thyroid carcinoma on lenvatinib treatment—initial experience. Sci. Rep. 2016, 6, 28081. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, M.C.; Fassnacht, M.; Mueller, S.P. Systemic treatment of advanced differentiated and medullary thyroid cancer. Overview and practical aspects. Nuklearmedizin 2015, 54, 88–93. [Google Scholar] [PubMed]
- Machens, A.; Holzhausen, H.J.; Dralle, H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer 2005, 103, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, R.; Giacomobono, S.; Capacchione, D.; Nardelli, A.; Barbato, F.; Nappi, A.; Pellegrino, T.; Storto, G. Should patients with remnants from thyroid microcarcinoma really not be treated with iodine-131 ablation? Endocrine 2013, 44, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Fig, L.M.; Frey, K.A.; Gross, M.D.; Wong, K.K. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: What is the impact on staging? J. Clin. Endocrinol. Metab. 2013, 98, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Creach, K.M.; Siegel, B.A.; Nussenbaum, B.; Grigsby, P.W. Radioactive iodine therapy decreases recurrence in thyroid papillary microcarcinoma. ISRN Endocrinol. 2012, 2012, 816386. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar]
| TX | Primary Tumor Cannot be Assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor size maximum 2 cm, limited to the thyroid |
| T1a | Tumor size maximum 1 cm, limited to the thyroid |
| T1b | Tumor size >1 cm up to a maximum of 2 cm, limited to the thyroid |
| T2 | Tumor size >2 cm up to 4 cm, limited to the thyroid |
| T3 | Tumor size >4 cm, limited to the thyroid, or any tumor with macroscopic extrathyroidal extension (Musculus sternohyoideus, Musculus sternothyreoideus, Musculus omohyoideus) |
| T3a | Tumor size >4 cm, limited to the thyroid |
| T3b | Any tumor with macroscopic extrathyroidal extension (M. sternohyoideus, M. sternothyreoideus, M. omohyoideus) |
| T4a | Any tumor size with extrathyroidal extension beyond the thyroid capsule and invasion of subcutaneous soft tissue, larynx, trachea, esophagus and/or recurrent laryngeal nerve |
| T4b | Any tumor size with invasion of prevertebral fascia, mediastinal vessels or carotid artery |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Regional lymph node metastases |
| N1a | Lymph node metastases unilateral in level VI or upper mediastinum |
| N1b | Metastases in other unilateral, bilateral or contralateral cervical lymph nodes (level I, II, III, IV and V) or retropharyngeal |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Mutation | Histology | Clinicopathological Associations |
|---|---|---|
| BRAF | papillary thyroid carcinoma (PTC) | recurrence, multifocality, extrathyreoidal extension, lymph nodes metastasis, advanced stage, absence of capsule, vascular invasion, more aggressive histological subtype |
| BRAF | micro PTC | multifocality, extrathyreoidal extension, advanced stage, lymph node metastasis |
| BRAF | thyroid carcinoma derived from follicular cells | no association |
| TERT | papillary thyroid carcinoma | more advanced stage by tall cell variant, higher tumor size, vascular invasion, older age, poor outcome, lymph node and distant metastasis |
| TERT | thyroid carcinoma derived from follicular cells | more aggressive histologic variants, concomitant presence of mutated RAS/BRAF, age > 45, higher tumor size, vascular invasion, persistent or recurrent disease, lymph node metastasis |
| Histology | Histological Variants |
|---|---|
| Papillary carcinoma | Classic (usual) Clear cell variant Columnar cell variant Cribriform-morular variant Diffuse sclerosing variant Follicular variant Macrofollicular variant Microcarcinoma (occult, latent, small, microtumor) Oncocytic or oxyphilic variant (follicular/nonfollicular variant) Solid variant Tall cell variant Warthin-like variant |
| Follicular carcinoma | Clear cell variant Oncocytic (Hürthle cell) variant Mucinous variant With signet-ring cells |
| Ultrasound Features | Estimated Risk of Malignancy | Sonographic Pattern | FNA Size Cutoff |
|---|---|---|---|
| Solid hypoechogenic nodule or solid hypoechogenic component of a partially cystic nodule with one or more of the following features: irregular margins, microcalcification, taller rather than wide shape, rim calcifications with small extrusive soft tissue component, evidence of extrathyroidal extension (ETE) | >70–90% | Highly suspicious | >1 cm |
| Solid hypoechogenic nodule with smooth margins without microcalcification, taller rather than wide shape or signs of ETE | 10–20% | Intermediate suspicion | >1 cm |
| Isoechogenic solid nodule or partally cystic nodule with eccentric solid areas without microcalcification, taller rather than wide shape or signs of ETE | 5–10% | Low suspicion | >1.5 cm |
| Spongiform or partially cystic nodule without any of the sonographic features described above | <3% | Very low suspicion | >2 cm, alternative: observation without fine needle aspiration (FNA) |
| Purely cystic nodules without solid components | <1% | Benign | No biopsy |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidbauer, B.; Menhart, K.; Hellwig, D.; Grosse, J. Differentiated Thyroid Cancer—Treatment: State of the Art. Int. J. Mol. Sci. 2017, 18, 1292. https://doi.org/10.3390/ijms18061292
Schmidbauer B, Menhart K, Hellwig D, Grosse J. Differentiated Thyroid Cancer—Treatment: State of the Art. International Journal of Molecular Sciences. 2017; 18(6):1292. https://doi.org/10.3390/ijms18061292
Chicago/Turabian StyleSchmidbauer, Benedikt, Karin Menhart, Dirk Hellwig, and Jirka Grosse. 2017. "Differentiated Thyroid Cancer—Treatment: State of the Art" International Journal of Molecular Sciences 18, no. 6: 1292. https://doi.org/10.3390/ijms18061292





