Biomarker and Histopathology Evaluation of Patients with Recurrent Glioblastoma Treated with Galunisertib, Lomustine, or the Combination of Galunisertib and Lomustine
Abstract
:1. Introduction
2. Results
2.1. Outcome from the Tissue Collection
2.2. Description of Standard Pathology Evaluation
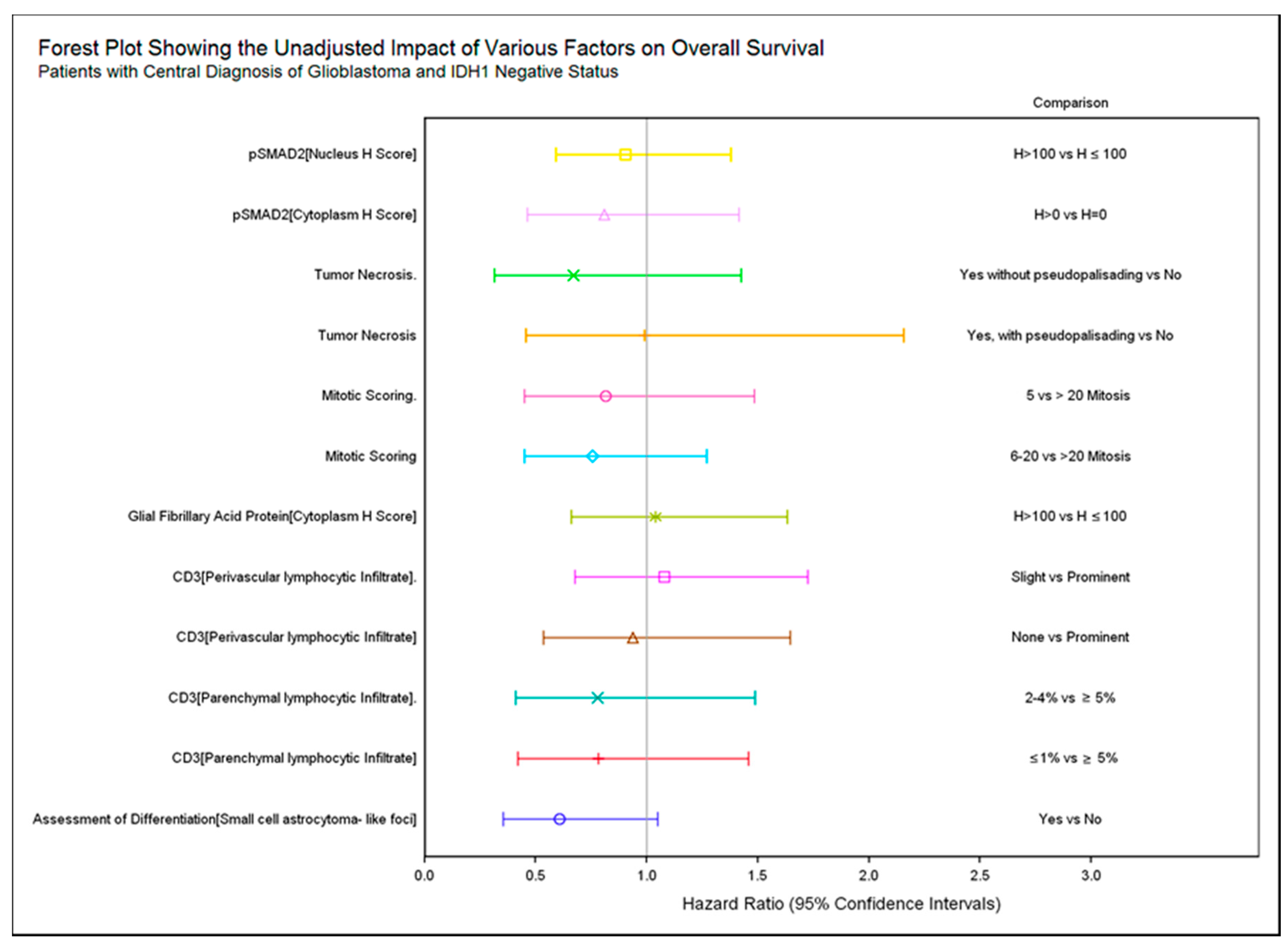
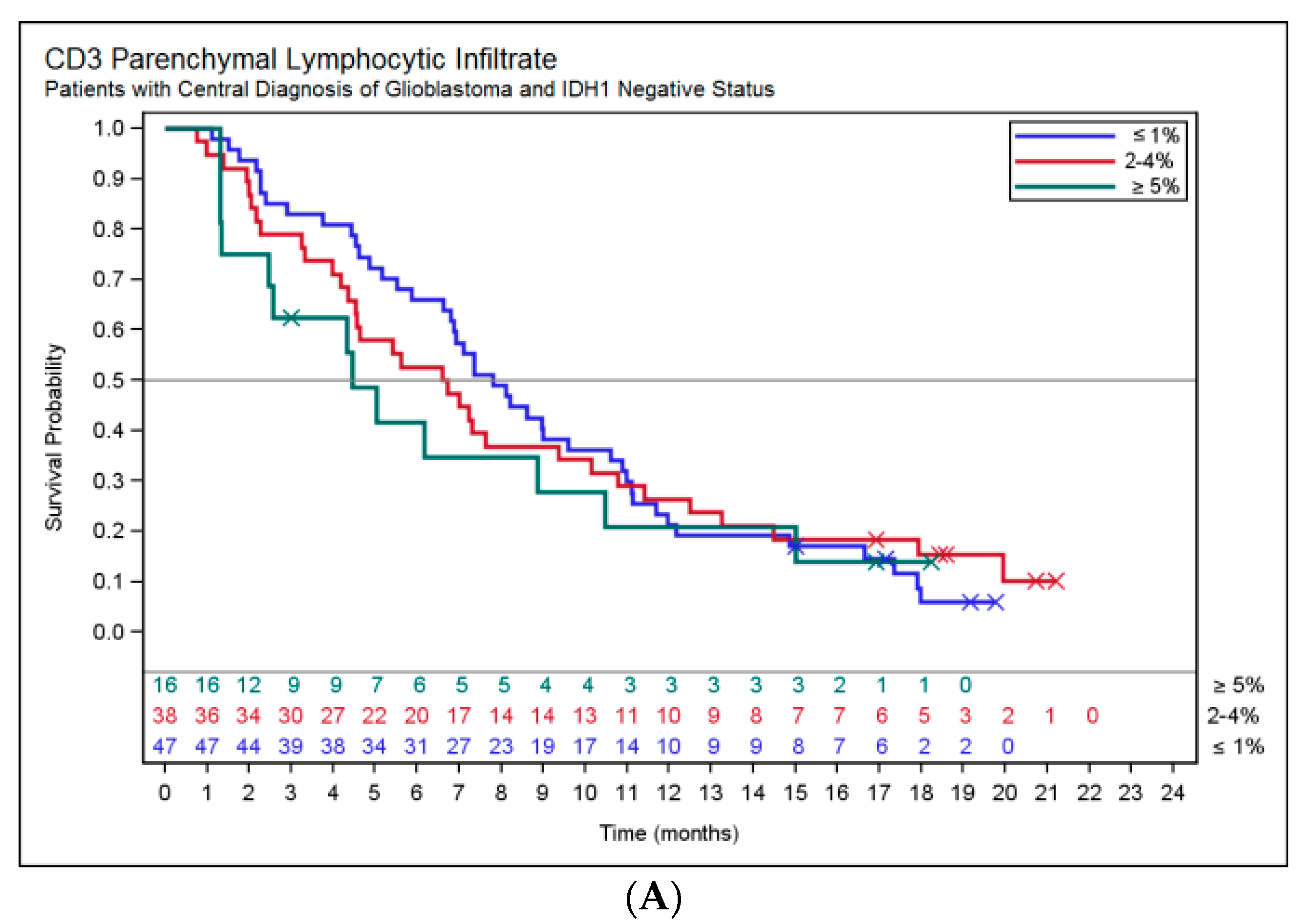
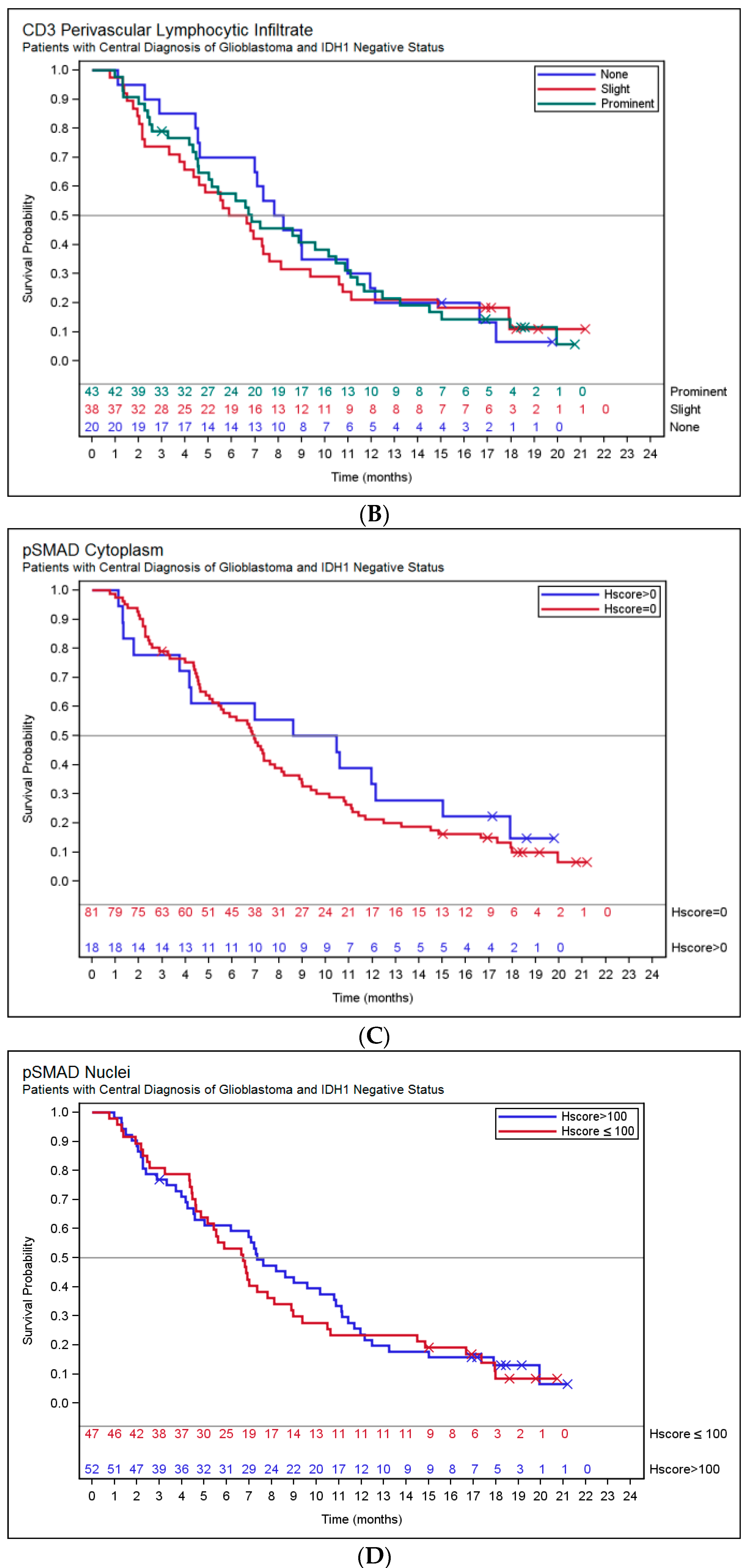
2.3. Association of Histopathology and Overall Survival (OS)
2.4. IDH1 Subgroup Analysis
2.5. Pharmacodynamic Responses
2.5.1. Immune Monitoring and T Cell Subsets by Flow Cytometry
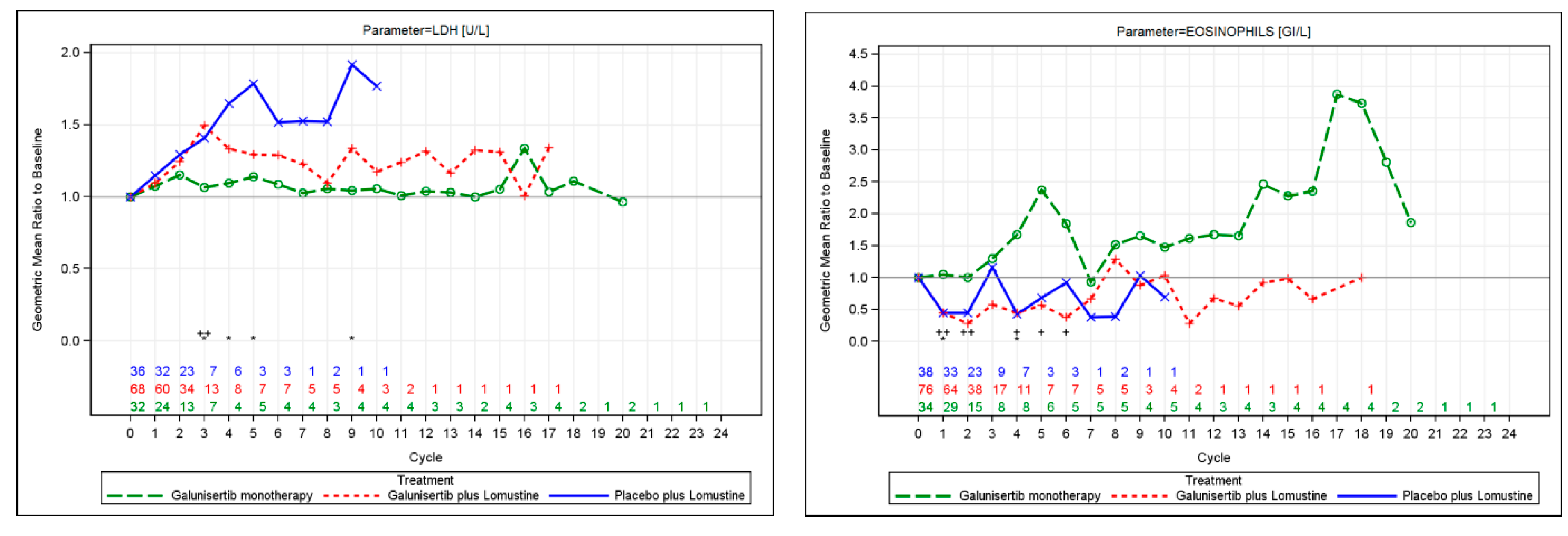
2.5.2. Plasma Markers
2.5.3. Genetic Evaluation
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Study Design
4.2.1. Central Pathology Review and Immunohistochemistry
4.2.2. Central Laboratory Evaluation for Blood Based Markers
4.2.3. Genetic Evaluation
4.3. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Burrell, K.E.; Wolf, A.; Jalali, S.; Hawkins, C.; Rutka, J.T.; Zadeh, G. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch. Immunol. Ther. Exp. 2013, 61, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Wild-Bode, C.; Weller, M.; Rimner, A.; Dichgans, J.; Wick, W. Sublethal irradiation promotes migration and invasiveness of glioma cells: Implications for radiotherapy of human glioblastoma. Cancer Res. 2001, 61, 2744–2750. [Google Scholar] [PubMed]
- Mangani, D.; Weller, M.; Seyed Sadr, E.; Willscher, E.; Seystahl, K.; Reifenberger, G.; Tabatabai, G.; Binder, H.; Schneider, H. Limited role for transforming growth factor-β pathway activation-mediated escape from VEGF inhibition in murine glioma models. Neuro Oncol. 2016, 18, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Fecci, P.E.; Mitchell, D.A.; Whitesides, J.F.; Xie, W.; Friedman, A.H.; Archer, G.E.; Herndon, J.E., 2nd; Bigner, D.D.; Dranoff, G.; Sampson, J.H. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006, 66, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Frei, K.; Gramatzki, D.; Tritschler, I.; Schroeder, J.J.; Espinoza, L.; Rushing, E.J.; Weller, M. Transforming growth factor-β pathway activity in glioblastoma. Oncotarget 2015, 6, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; Darken, R.S.; Rojo, F.; Ocaña, A.; Peñuelas, S.; Arias, A.; Paris, R.; Tortosa, A.; Mora, J.; Baselga, J.; et al. High TGFβ-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 2007, 11, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, S.; Anido, J.; Prieto-Sánchez, R.M.; Folch, G.; Barba, I.; Cuartas, I.; García-Dorado, D.; Poca, M.A.; Sahuquillo, J.; Baselga, J.; et al. TGF-β increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 2009, 15, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Anido, J.; Sáez-Borderías, A.; Gonzàlez-Juncà, A.; Rodón, L.; Folch, G.; Carmona, M.A.; Prieto-Sánchez, R.M.; Barba, I.; Martínez-Sáez, E.; Prudkin, L.; et al. TGF-β receptor inhibitors target the CD44high/Id1high glioma-initiating cell population in human glioblastoma. Cancer Cell 2010, 18, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; Benhadji, K.A.; et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-β signaling pathway. Drug Des. Dev. Ther. 2015, 10, 4479–4499. [Google Scholar]
- Rodon, J.; Carducci, M.A.; Sepulveda-Sánchez, J.M.; Azaro, A.; Calvo, E.; Seoane, J.; Braña, I.; Sicart, E.; Gueorguieva, I.; Cleverly, A.L.; et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res. 2015, 21, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Sanchez, J.; Ramos, A.; Hilario, A.; DE Velasco, G.; Castellano, D.; Garcia de la Torre, M.; Rodon, J.; Lahn, M.F. Brain perfusion and permeability in patients with advanced, refractory glioblastoma treated with lomustine and the transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate. Oncol. Lett. 2015, 9, 2442–2448. [Google Scholar] [PubMed]
- Parsons, S.; Sawyer, S.; Yan, L.; Foreman, R.; Weir, S.; Lahn, M.; Yingling, J.; Graff, J.R. Abstract C201: The combination of the small molecule TGFβR1 inhibitor, LY2157299 monohydrate, with CCNU substantially blocks SMAD phosphorylation and significantly suppresses human glioblastoma xenograft growth. In Proceedings of the 2011 AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, San Francisco, CA, USA, 12–16 November 2011. [Google Scholar]
- Brandes, A.A.; Carpentier, A.F.; Kesari, S.; Sepulveda-Sanchez, J.M.; Wheeler, H.R.; Chinot, O.; Cher, L.; Steinbach, J.P.; Capper, D.; Specenier, P.; et al. A phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016, 18, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar]
- Preusser, M.; Capper, D.; Hartmann, C. Euro-CNS Research Committee. IDH testing in diagnostic neuropathology: Review and practical guideline article invited by the Euro-CNS research committee. Clin. Neuropathol. 2011, 30, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Weller, M.; van den Bent, M.; Sanson, M.; Weiler, M.; von Deimling, A.; Plass, C.; Hegi, M.; Platten, M.; Reifenberger, G. MGMT testing—The challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014, 10, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Dunn, I.F.; Curry, W.T. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007, 7, 12. [Google Scholar] [PubMed]
- Kmiecik, J.; Poli, A.; Brons, N.H.; Waha, A.; Eide, G.E.; Enger, P.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 2013, 264, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wöhrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015, 17, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Döring, C.; Hansmann, M.L.; Agostinelli, C.; Piccaluga, P.P.; Facchetti, F.; Pileri, S.; Küppers, R.; Newrzela, S.; Hartmann, S. A novel immunohistochemical classifier to distinguish Hodgkin lymphoma from ALK anaplastic large cell lymphoma. Mod. Pathol. 2014, 27, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.A.; Dey, M.; Chang, A.; Lesniak, M.S. Targeting tregs in malignant brain cancer: Overcoming IDO. Front. Immunol. 2013, 4, 116. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.K.; Lieu, A.S.; Su, Y.F.; Cheng, C.Y.; Tsai, T.H.; Lin, C.L.; Lee, K.S.; Hwang, S.L.; Kwan, A.L.; Wang, C.J.; et al. Plasma levels of transforming growth factor-β1 before and after removal of low- and high-grade astrocytomas. Cytokine 2013, 61, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Robert, F.; Busby, E.; Marques, M.B.; Reynolds, R.E.; Carey, D.E. Phase II study of docetaxel plus enoxaparin in chemotherapy-naive patients with metastatic non-small cell lung cancer: Preliminary results. Lung Cancer 2003, 42, 237–245. [Google Scholar] [CrossRef]
- Zhou, M.; Bracci, P.M.; McCoy, L.S.; Hsuang, G.; Wiemels, J.L.; Rice, T.; Zheng, S.; Kelsey, K.T.; Wrensch, M.R.; Wiencke, J.K. Serum macrophage-derived chemokine/CCL22 levels are associated with glioma risk, CD4 T cell lymphopenia and survival time. Int. J. Cancer 2015, 137, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.D.; Schulze, J.J.; Kühn, T.; Raschke, E.; Hüsing, A.; Johnson, T.; Kaaks, R.; Olek, S. Treg-mediated immune tolerance and the risk of solid cancers: Findings from EPIC-Heidelberg. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Baron, U.; Floess, S.; Wieczorek, G.; Baumann, K.; Grützkau, A.; Dong, J.; Thiel, A.; Boeld, T.J.; Hoffmann, P.; Edinger, M.; et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur. J. Immunol. 2007, 37, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef] [PubMed]

| Evaluation | Result | All Patients n (%) a | Patients with Glioblastoma, Gliosarcoma or Giant Cell Glioblastoma, and IDH1 R132H Negative n (%) a |
|---|---|---|---|
| Diagnosis | Glioblastoma | 116 (92) | 103 (96) |
| Glioma, astrocytoma | 3 (2) | 0 | |
| Glioma, gliosarcoma | 3 (2) | 3 (3) | |
| Glioma, pleomorphic xanthoastrocytoma | 1 (1) | 0 | |
| Protoplasmic astrocytoma | 1 (1) | 0 | |
| Oligoastrocytoma | 1 (1) | 0 | |
| Giant cell glioblastoma | 1 (1) | 1 (1) | |
| Assessment of differentiation | Perinuclear halos | 11 (9) | 7 (7) |
| Fibrillary astrocytoma-like foci | 9 (7) | 6 (6) | |
| Small cell astrocytoma-like foci | 27 (21) | 21 (20) | |
| Polar spongioblastic foci | 0 | 0 | |
| Protoplasmic astrocytoma-like foci | 1 (1) | 0 | |
| Minigemistocytes | 5 (4) | 2 (2) | |
| Classic gemistocytes | 16 (13) | 12 (11) | |
| Giant cells | 16 (13) | 15 (14) | |
| PNET-like | 13 (10) | 11 (10) | |
| Sarcoma-like | 7 (6) | 7 (7) | |
| Microcysts | 24 (19) | 18 (17) | |
| Mucoid degeneration | 11 (9) | 8 (8) | |
| Calcifications | 2 (2) | 2 (2) | |
| Vessel structure | Abnormal number of vessels | 117 (93) | 101 (94) |
| Any endothelial hypertrophy | 124 (100) | 107 (100) | |
| Glomeruloid blood vessel | 69 (56) | 62 (58) | |
| Multi-layering blood vessel | 116 (94) | 103 (96) | |
| Vascular abnormalities | 124 (98) | 107 (100) | |
| Vessel thrombosis | 87 (69) | 80 (75) | |
| Cellular density | Low (like diffuse astrocytoma) | 5 (4) | 5 (5) |
| Medium (like classical glioblastoma) | 100 (79) | 86 (80) | |
| High (like PNET) | 21 (17) | 16 (15) | |
| Tumor necrosis | Yes, with pseudopalisading | 36 (29) | 34 (32) |
| Yes, without pseudopalisading | 72 (57) | 64 (60) | |
| No | 18 (14) | 9 (8) | |
| Mitotic scoring | ≤5 Mitoses (per 10 Highpower fields) | 31 (25) | 24 (23) |
| 6–20 Mitoses (per 10 Highpower fields) | 66 (53) | 59 (56) | |
| >20 Mitoses (per 10 Highpower fields) | 28 (22) | 23 (22) | |
| Nuclear abnormalities | Low (nuclear aspect as in normal glial cells) | 4 (3) | 1 (1) |
| Medium (abnormal nuclear shape) | 92 (73) | 78 (73) | |
| High (bizarre nuclei) | 30 (24) | 28 (26) | |
| CD3 parenchymal lymphocytic infiltrate | ≤1% | 54 (47) | 47 (45) |
| 2–4% | 41 (36) | 40 (38) | |
| ≥5% | 20 (17) | 18 (17) | |
| CD3 perivascular lymphocytic infiltrate | None (≤4 perivascular positive cells per vessel) | 24 (21) | 21 (20) |
| Slight (≥1 vessel with ≥5 and <30 positive perivascular cells) | 43 (37) | 38 (36) | |
| Prominent (≥1 vessel with ≥5 and ≥30 positive perivascular cells) | 48 (42) | 46 (44) | |
| IDH1 R132H | Positive | 8 (7) | 0 |
| Negative | 108 (93) | 107 (100) | |
| Ki67 | ≤5% | 6 (6) | 6 (6) |
| 6–10% | 25 (24) | 23 (24) | |
| 11–20% | 43 (41) | 37 (39) | |
| >20% | 32 (30) | 30 (31) | |
| Glial fibrillary acid protein | Cytoplasm Total Detected (H score > 0) | 124 (100) | 105 (100) |
| Cytoplasm H score Median (25th percentile, 75th percentile) | 140 (70, 210) | 150 (90 210) | |
| pSMAD2 | Cytoplasm Total Detected (H score > 0) | 22 (18) | 18 (18) |
| Cytoplasm H score Median (25th percentile, 75th percentile) | 0 (0, 0) | 0 (0, 0) | |
| Nuclei Total Detected (H score > 0) | 119 (100) | 102 (100) | |
| Nuclei H score Median (25th percentile, 75th percentile) | 100 (70, 160) | 110 (75, 160) |
| Parameter | IDH1 Positive (n = 8) | IDH1 Negative (n = 108) | p-Value |
|---|---|---|---|
| Tissue CD3+ Parenchymal infiltrate, n (%) | ≤1%, 7 (88) | ≤1%, 47 (44) | 0.026 |
| 2–4%, 0 | 2–4%, 40 (38) | ||
| ≥5%, 1 (13) | ≥5%, 19 (18) | ||
| Tissue CD3+ Perivascular infiltrate, n (%) | None, 3 (38) | None, 21 (20) | 0.0267 |
| Slight, 5 (63) | Slight, 38 (36) | ||
| Prominent, 0 | Prominent, 47 (44) | ||
| Blood FOXP3 (%), n Median (range) | n = 8 | n = 101 | 0.0394 |
| 1.4 (0.3, 3.2) | 0.7 (0.1, 2.7) | ||
| Plasma MDC/CCL22 (pg/mL), n Median (range) | n = 8 | n = 104 | 0.2533 |
| 491 (64, 879) | 208 (24, 1220) | ||
| Blood neutrophils (GI/L), n Median (range) | n = 8 | n = 98 | 0.1505 |
| 3.50 (2.65, 11.23) | 5.53 (2.01, 16.81) | ||
| Blood neutrophil/lymphocyte ratio, n, Median (range) | n = 8 | n = 98 | 0.0938 |
| 2.63 (1.52, 18.11) | 5.74 (0.81, 35.75) | ||
| Plasma TGF-β1 (pg/mL), n Median (range) | n = 8 | n = 100 | 0.1027 |
| 2984 (654, 19774) | 2031 (25, 11325) | ||
| Blood CD4+ (cells/uL), n Median (range) | n = 8 | n = 96 | 0.1714 |
| 602 (108, 659) | 309 (30, 1208) | ||
| Blood CD3+ (%), n Median (range) | n = 8 | n = 101 | 0.3016 |
| 27.5 (3.1, 42.8) | 13.6 (2.6, 75.4) | ||
| Blood lymphocytes (GI/L), n Median (range) | n = 8 | n = 98 | 0.2562 |
| 1.33 (0.57, 1.99) | 0.93 (0.22, 2.74) | ||
| Blood CD4+/CD8+ Ratio, n Median (range) | n = 8 | n = 96 | 0.368 |
| 1.75 (0.40, 2.58) | 1.27 (0.29, 6.31) | ||
| Tissue pSMAD2 cytoplasm H score, n (%) | H = 0, 6 (75) | H = 0, 85 (83) | 0.6334 |
| H > 0, 2 (25) | H > 0, 18 (18) | ||
| Blood eosinophils (GI/L), n Median (range) | n = 8 | n = 98 | 0.7284 |
| 0.06 (0.00, 0.16) | 0.05 (0.00, 0.23) | ||
| Tissue pSMAD2 nucleus H score, n (%) | H ≤ 100, 4 (50) | H ≤ 100, 50 (49) | >0.9999 |
| H > 100, 4 (50) | H > 100, 53 (52) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capper, D.; Von Deimling, A.; Brandes, A.A.; Carpentier, A.F.; Kesari, S.; Sepulveda-Sanchez, J.M.; Wheeler, H.R.; Chinot, O.; Cher, L.; Steinbach, J.P.; et al. Biomarker and Histopathology Evaluation of Patients with Recurrent Glioblastoma Treated with Galunisertib, Lomustine, or the Combination of Galunisertib and Lomustine. Int. J. Mol. Sci. 2017, 18, 995. https://doi.org/10.3390/ijms18050995
Capper D, Von Deimling A, Brandes AA, Carpentier AF, Kesari S, Sepulveda-Sanchez JM, Wheeler HR, Chinot O, Cher L, Steinbach JP, et al. Biomarker and Histopathology Evaluation of Patients with Recurrent Glioblastoma Treated with Galunisertib, Lomustine, or the Combination of Galunisertib and Lomustine. International Journal of Molecular Sciences. 2017; 18(5):995. https://doi.org/10.3390/ijms18050995
Chicago/Turabian StyleCapper, David, Andreas Von Deimling, Alba A. Brandes, Antoine F. Carpentier, Santosh Kesari, Juan M. Sepulveda-Sanchez, Helen R. Wheeler, Olivier Chinot, Lawrence Cher, Joachim P. Steinbach, and et al. 2017. "Biomarker and Histopathology Evaluation of Patients with Recurrent Glioblastoma Treated with Galunisertib, Lomustine, or the Combination of Galunisertib and Lomustine" International Journal of Molecular Sciences 18, no. 5: 995. https://doi.org/10.3390/ijms18050995
APA StyleCapper, D., Von Deimling, A., Brandes, A. A., Carpentier, A. F., Kesari, S., Sepulveda-Sanchez, J. M., Wheeler, H. R., Chinot, O., Cher, L., Steinbach, J. P., Specenier, P., Rodon, J., Cleverly, A., Smith, C., Gueorguieva, I., Miles, C., Guba, S. C., Desaiah, D., Estrem, S. T., ... Wick, W. (2017). Biomarker and Histopathology Evaluation of Patients with Recurrent Glioblastoma Treated with Galunisertib, Lomustine, or the Combination of Galunisertib and Lomustine. International Journal of Molecular Sciences, 18(5), 995. https://doi.org/10.3390/ijms18050995





