Synthesis and Characterization of Dual-Sensitive Fluorescent Nanogels for Enhancing Drug Delivery and Tracking Intracellular Drug Delivery
Abstract
:1. Introduction
2. Result and Discussion
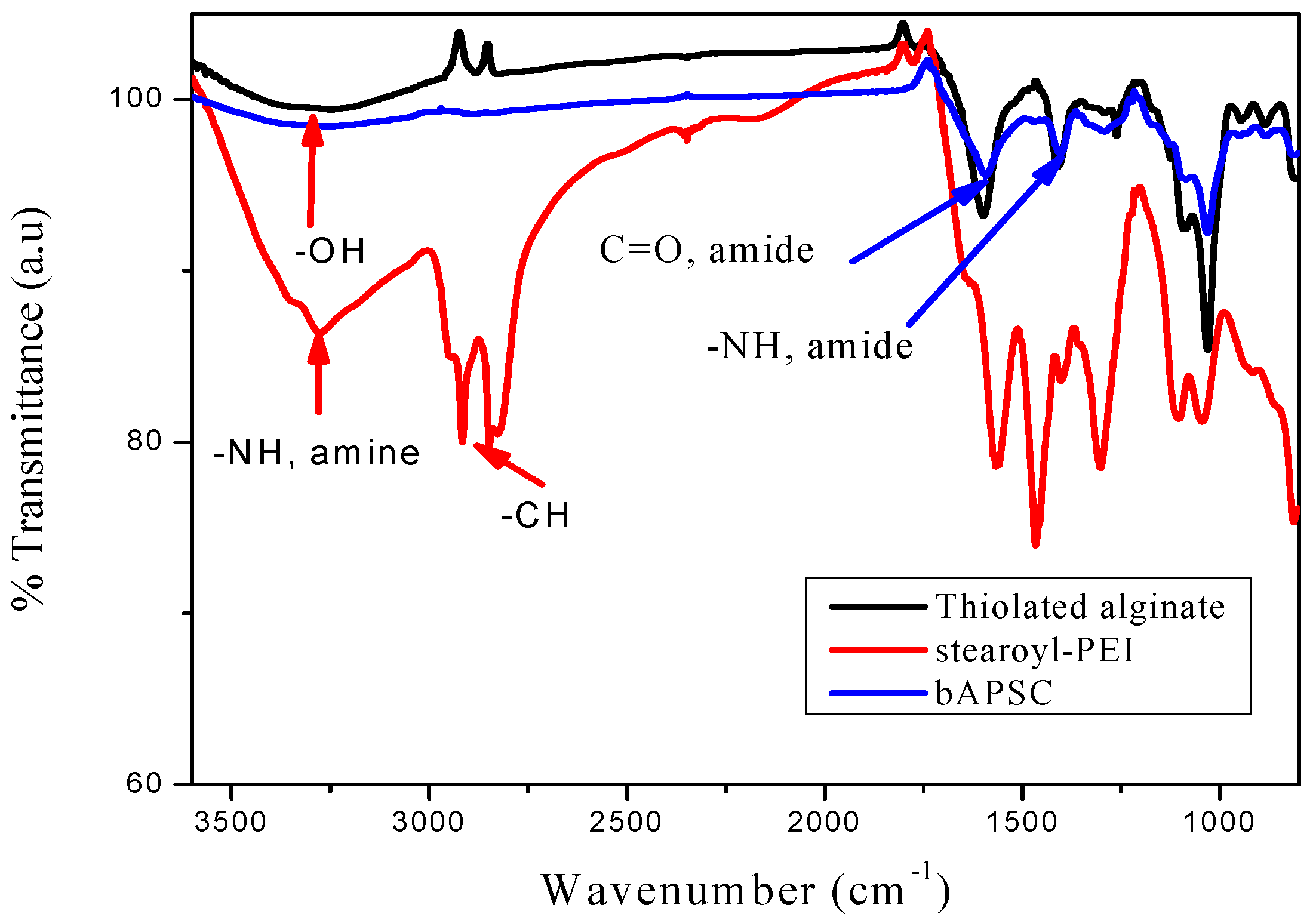
2.1. Characterization of bAPSC Nanogels
2.2. In Vitro Cytotoxicity of bAPSC and Its Precursors
2.3. In Vitro Cytotoxicity of Free Dox and Dox-Loaded bAPSC Nanogels
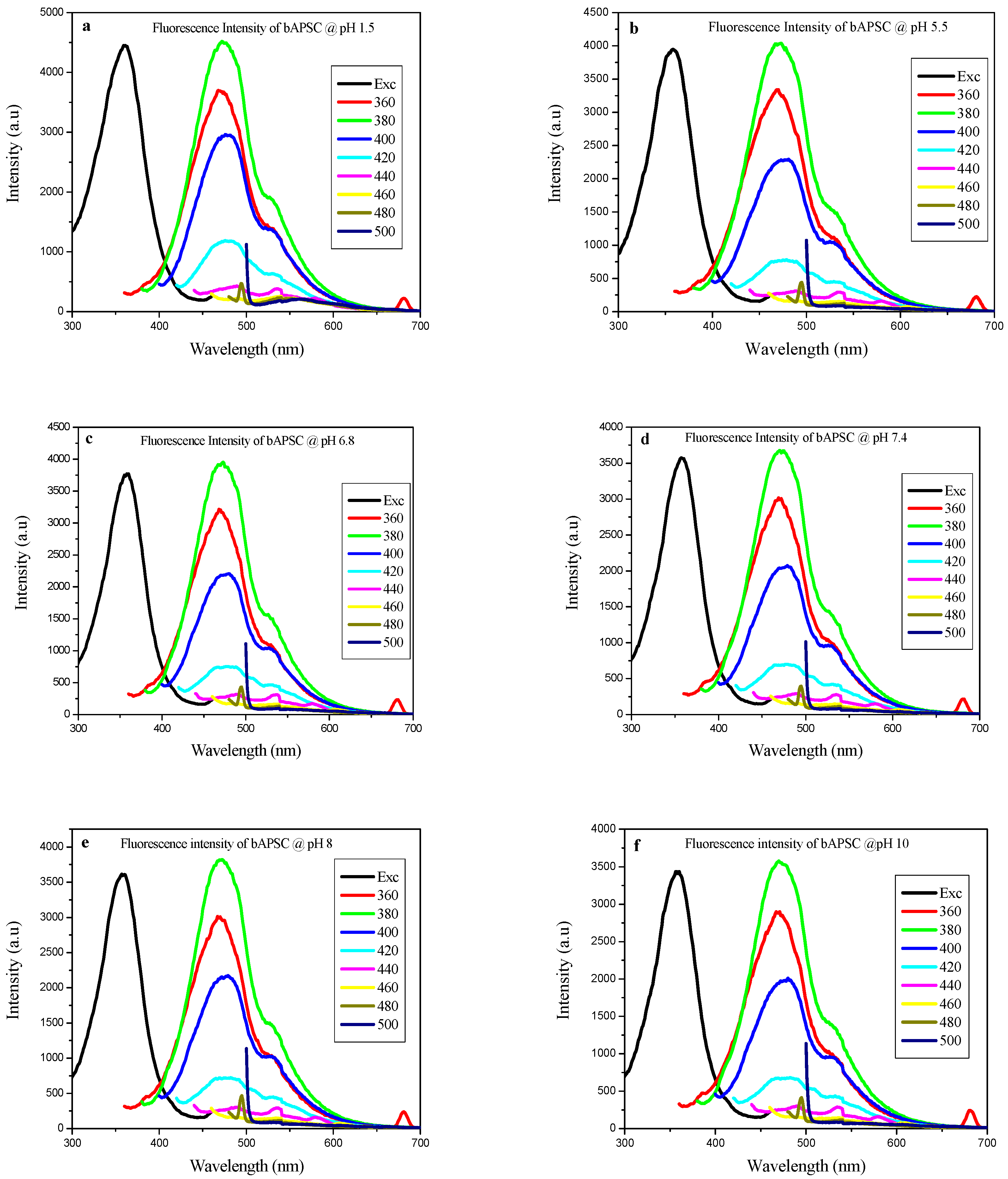
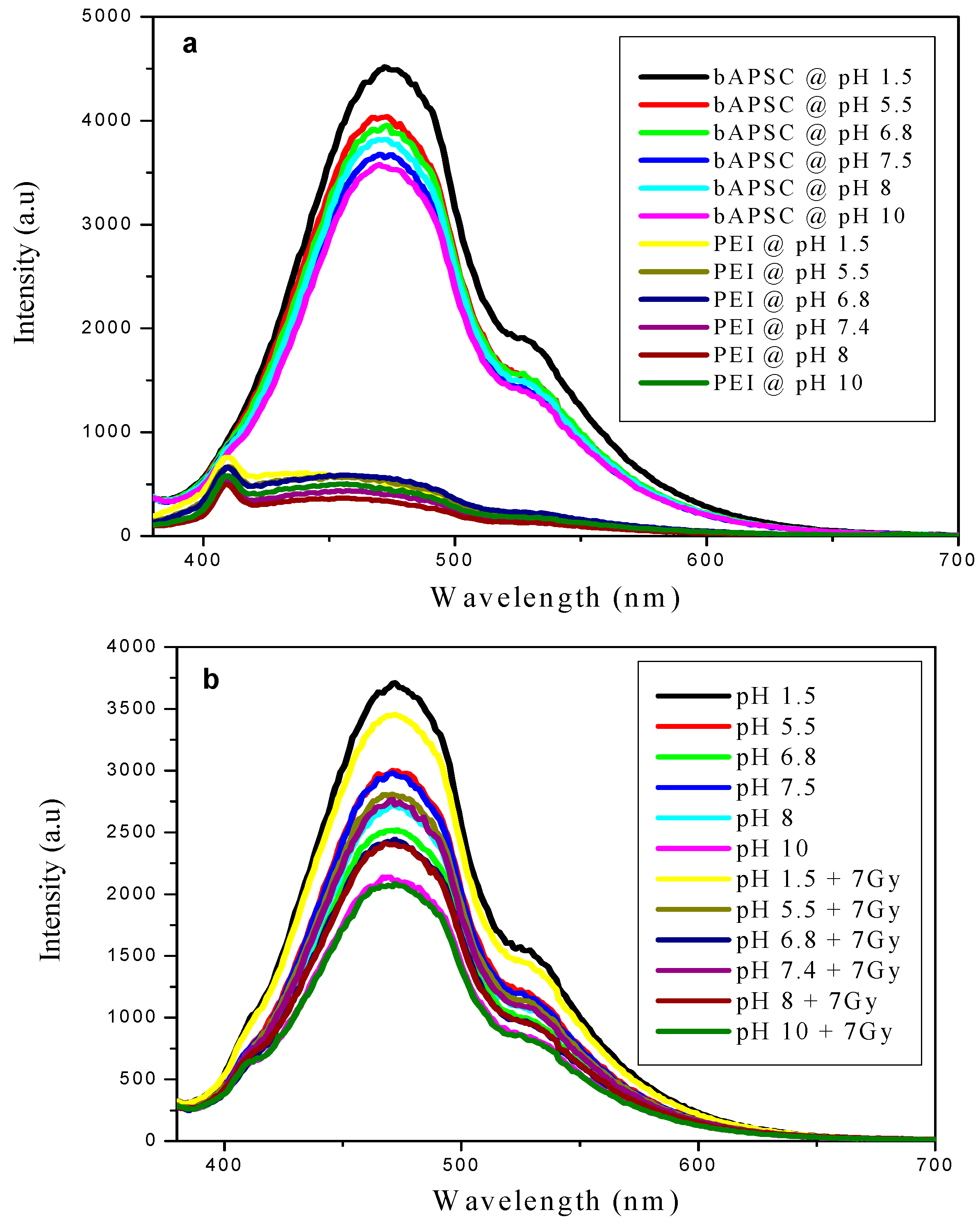
2.4. Fluorescence Properties of bAPSC NPs
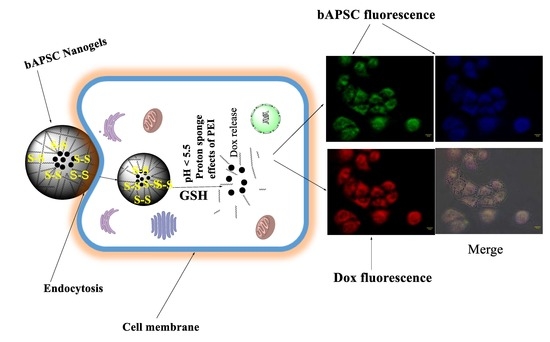
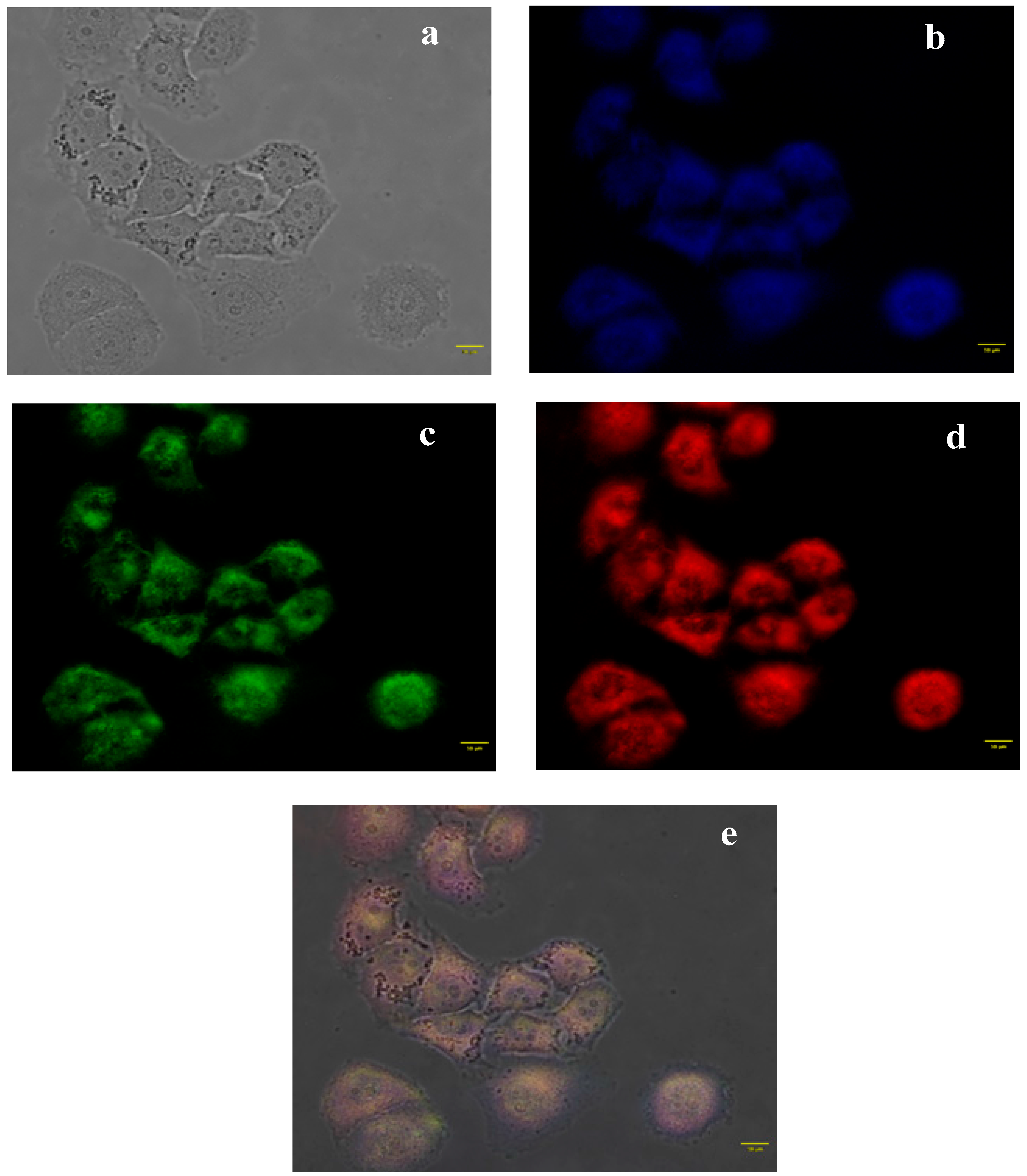
2.5. In Vitro Cellular Uptake Study through Fluorescence Microscopy
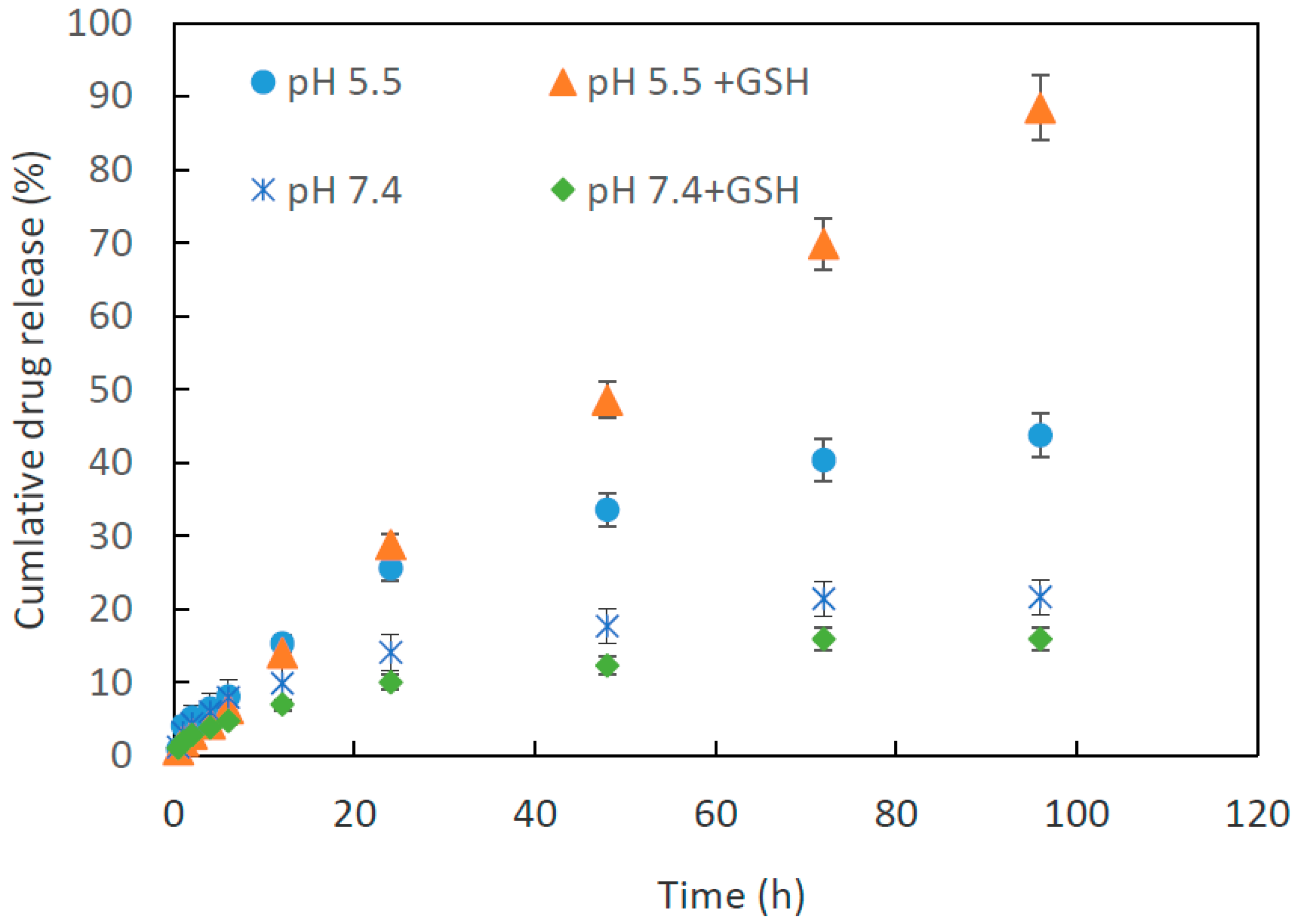
2.6. Dox Loading and Release Study
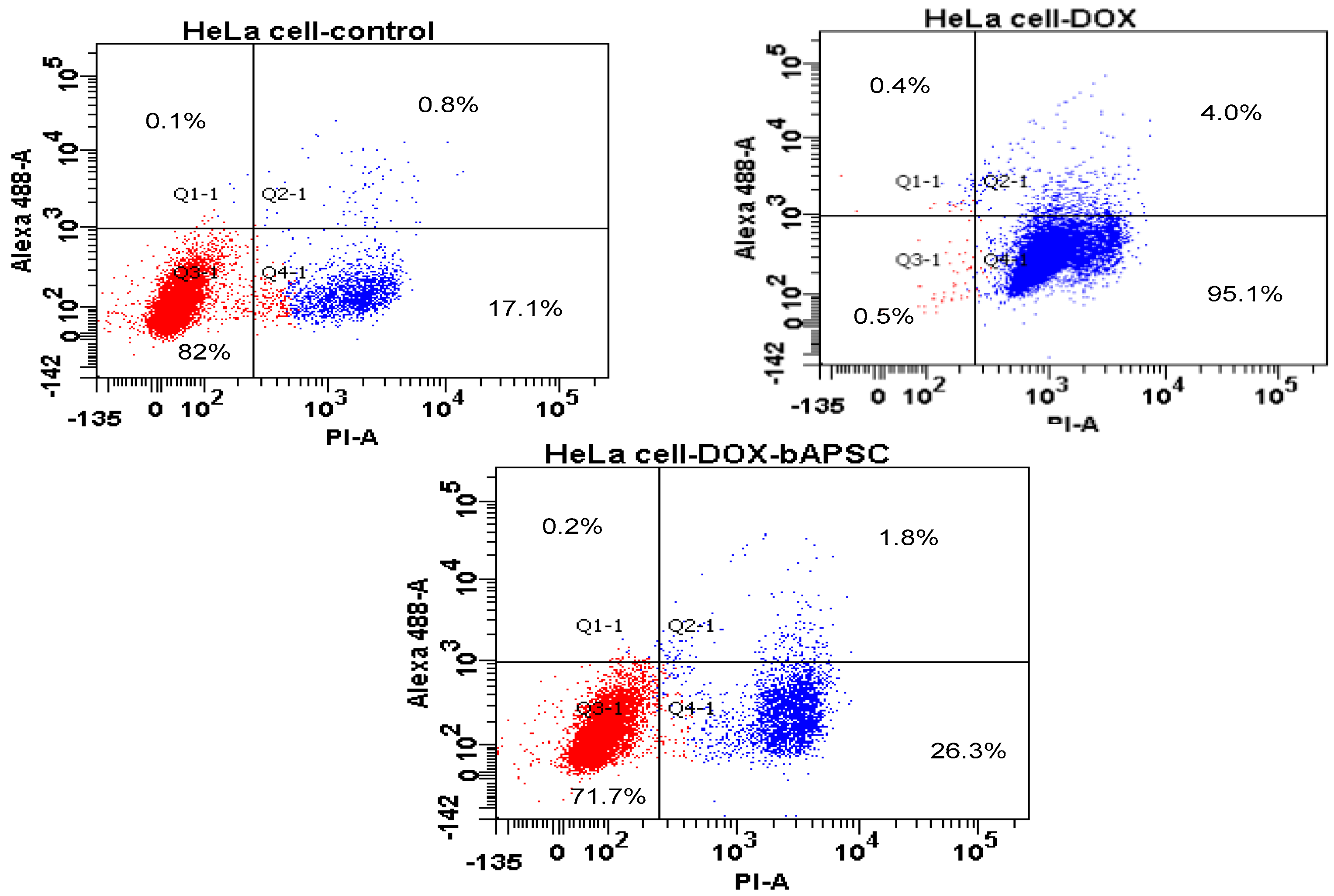
2.7. Cell Apoptosis/Necrosis Quantification through Flow Cytometry
2.8. Hemocompatibility Test
3. Materials and Methods
3.1. Materials
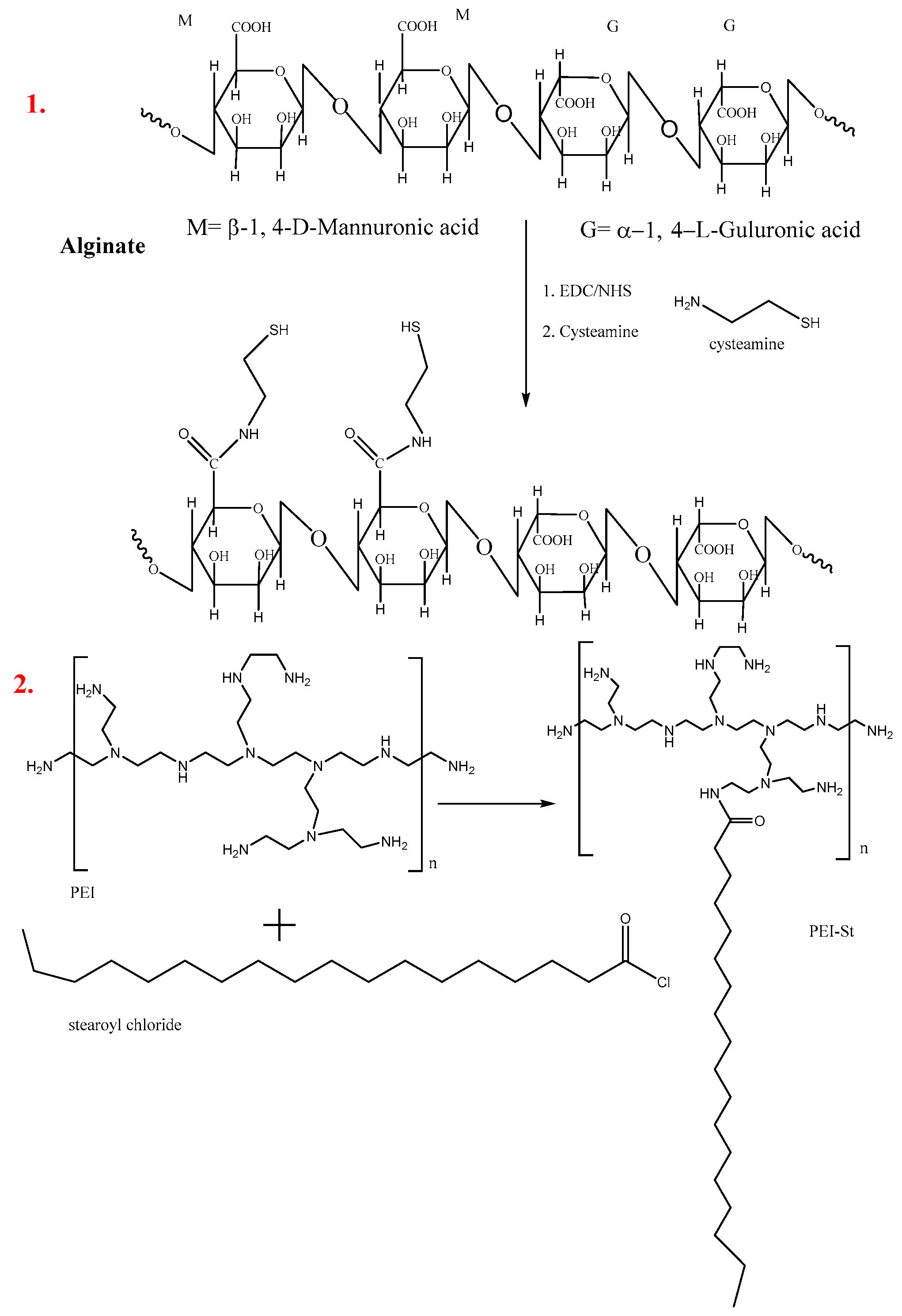
3.2. Synthesis of Thiolated Alginate by Using Cysteamine
3.3. Synthesis of Modified PEI-Stearoyl Chloride
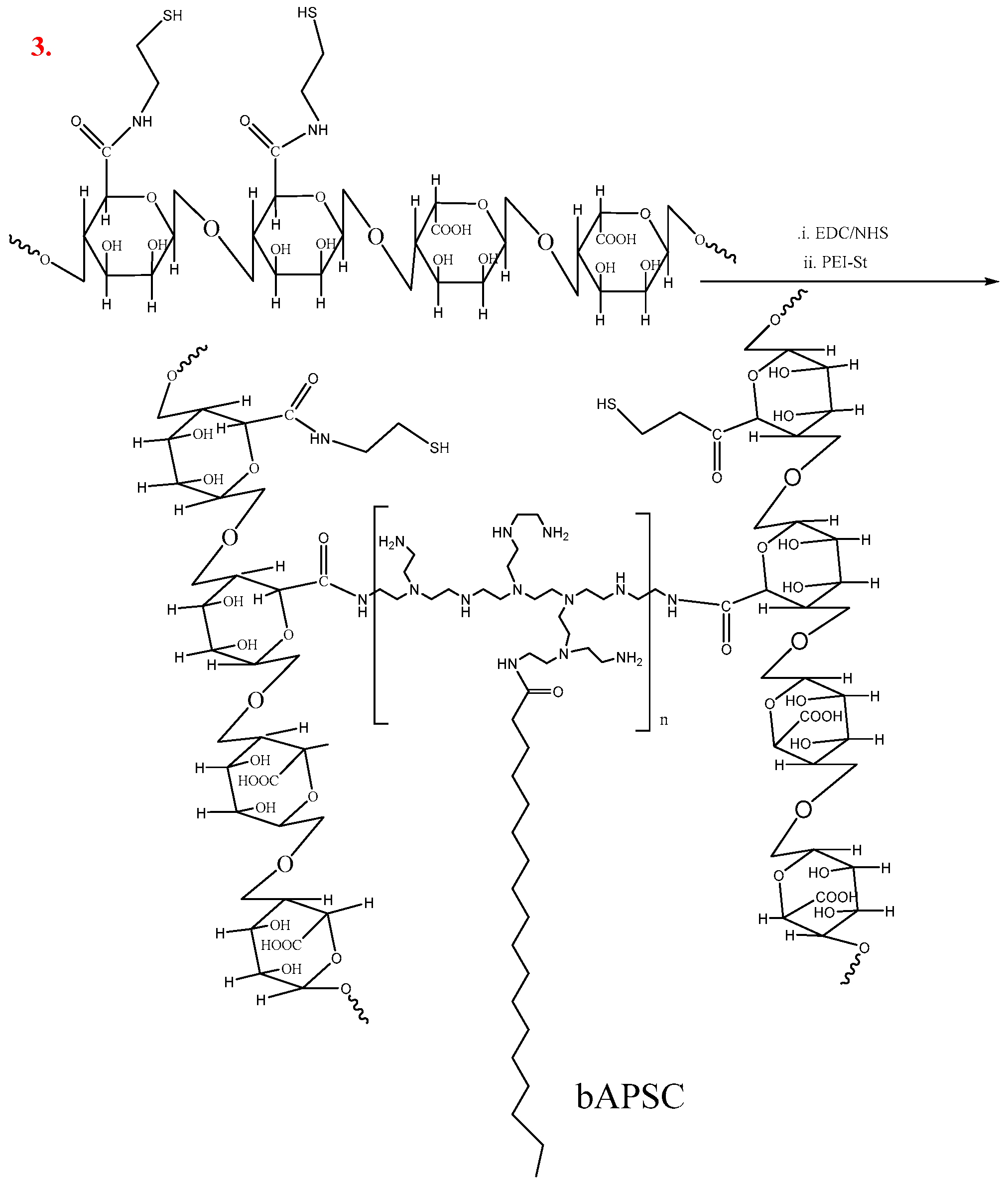
3.4. Synthesis of Redox-Sensitive Alginate-PS Conjugate
3.5. Characterization of bAPSC Nanogels
3.5.1. FTIR Analysis
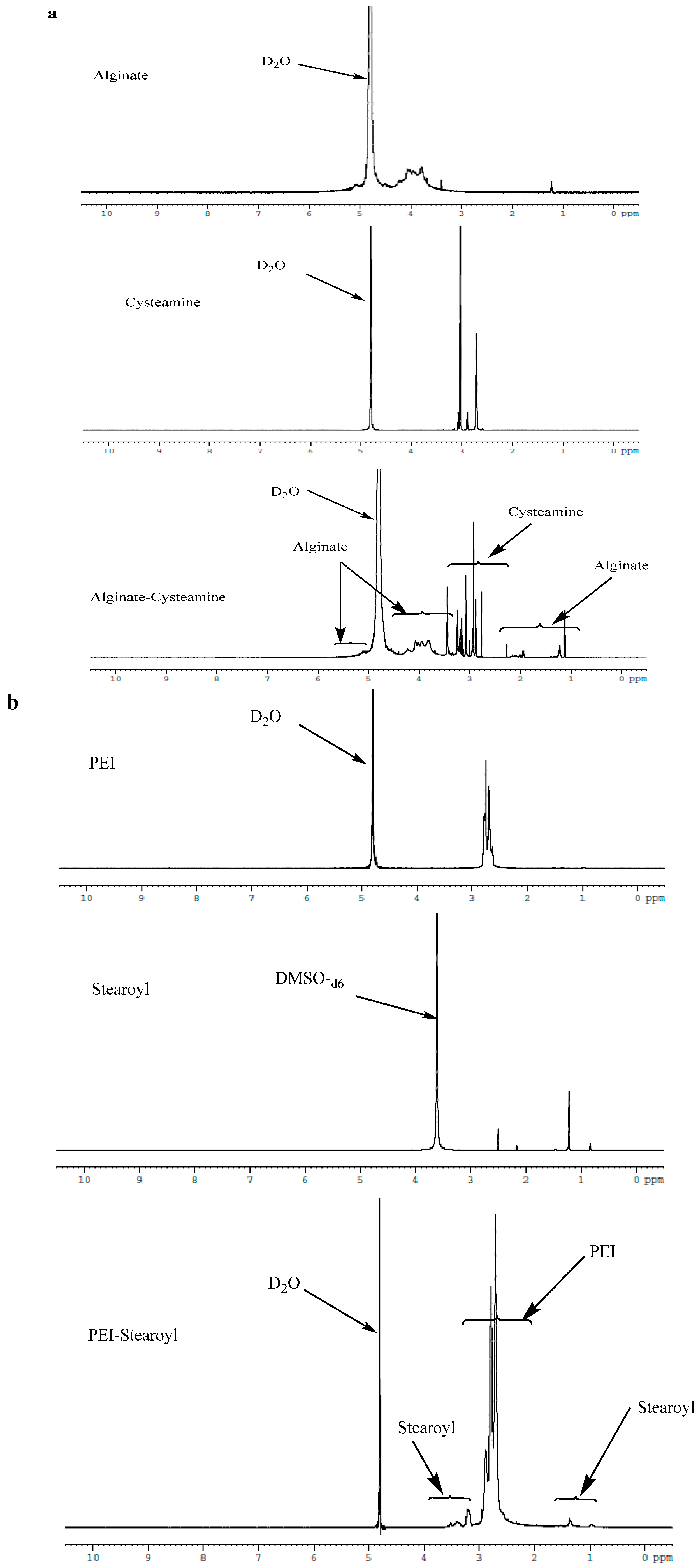
3.5.2. 1H-NMR Analysis
3.6. Synthesis of bAPSC Nanogels
3.6.1. Dynamic Light Scattering
3.6.2. Field-Emission Scanning Electron Microscopy
3.6.3. Transmission Electron Microscopy
3.7. Fluorescence Spectrophotometer
3.8. Drug Loading
3.9. Drug Release Study
3.10. In Vitro Cytotoxicity of Materials, Free Dox, and Dox-Loaded bAPSC Nanogels
3.11. Cellular Uptake Study
3.12. Cell Apoptosis/Necrosis Quantification through Flow Cytometry
3.13. Hemolytic Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Ljubimova, J.Y.; Holler, E. Biocompatible nanopolymers: The next generation of breast cancer treatment? Nanomedicine 2012, 7, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. R. Soc. Interface 2010, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Dutta, D.; Walter, G.A.; Moudgil, B.M. Fluorescent Nanoparticle Probes for Cancer Imaging. Technol.Cancer Res. Treat. 2005, 4, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Licha, K.; Olbrich, C. Optical imaging in drug discovery and diagnostic applications. Adv. Drug Deliv. Rev. 2005, 57, 1087–1108. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.-M.; Chen, Y.; Chen, J.-T.; Liu, Y. Polysaccharide-based Noncovalent Assembly for Targeted Delivery of Taxol. Sci. Rep. 2016, 6, 19212. [Google Scholar] [CrossRef] [PubMed]
- Ziebarth, J.; Wang, Y. Molecular Dynamics Simulations of DNA-Polycation Complex Formation. Biophys. J. 2009, 97, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, C.K.; Roy, S. Structural and dynamical properties of polyethylenimine in explicit water at different protonation states: A molecular dynamics study. Soft Matter 2013, 9, 2269–2281. [Google Scholar] [CrossRef]
- Sun, C.; Tang, T.; Uludağ, H.; Cuervo, Javier, E. Molecular Dynamics Simulations of DNA/PEI Complexes: Effect of PEI Branching and Protonation State. Biophys. J. 2011, 100, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Mekuria, S.L.; Lin, S.-Y.; Tsai, H.-C. Synthesis and characterization of bioreducible heparin-polyethyleneimine nanogels: Application as imaging-guided photosensitizer delivery vehicle in photodynamic therapy. RSC Adv. 2016, 6, 14692–14704. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.; Schubert, S.; Ochrimenko, S.; Fischer, D.; Schubert, U.S. Branched and linear poly(ethylene imine)-based conjugates: Synthetic modification, characterization, and application. Chem. Soc. Rev. 2012, 41, 4755–4767. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Pérez, L.; Chen, Y.; Shen, Z.; Lahoz, A.; Stiriba, S.E. Unprecedented Blue Intrinsic Photoluminescence from Hyperbranched and Linear Polyethylenimines: Polymer Architectures and pH-Effects. Macromol. Rapid Commun. 2007, 28, 1404–1409. [Google Scholar] [CrossRef]
- Lungwitz, U.; Breunig, M.; Blunk, T.; Göpferich, A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005, 60, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-S. Design and Development of Degradable Polyethylenimines for Delivery of DNA and Small Interfering RNA: An Updated Review. ISRN Mater. Sci. 2012, 2012, 24. [Google Scholar] [CrossRef]
- Kafil, V.; Omidi, Y. Cytotoxic Impacts of Linear and Branched Polyethylenimine Nanostructures in A431 Cells. Bioimpacts 2011, 1, 23–30. [Google Scholar] [PubMed]
- Wiseman, J.W.; Goddard, C.A.; McLelland, D.; Colledge, W.H. A comparison of linear and branched polyethylenimine (PEI) with DCChol//DOPE liposomes for gene delivery to epithelial cells in vitro and in vivo. Gene Ther. 2003, 10, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F.R. Carbohydrate nanocarriers in biomedical applications: Functionalization and construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Jo, D.G.; Park, J.H. Polysaccharide-Based Nanoparticles: A Versatile Platform for Drug Delivery and Biomedical Imaging. Curr. Med. Chem. 2012, 19, 3212–3229. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.; De Boisseson, M.R.; Hubert, P.; Dalençon, F.; Dellacherie, E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J. Control. Release 2004, 98, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Vallée, F.; Müller, C.; Durand, A.; Schimchowitsch, S.; Dellacherie, E.; Kelche, C.; Cassel, J.C.; Leonard, M. Synthesis and rheological properties of hydrogels based on amphiphilic alginate-amide derivatives. Carbohydr. Res. 2009, 344, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Bellettini, I.C.; Eising, R.; Felippe, A.C.; Domingos, J.B.; Minatti, E.; Machado, V.G. Association of Branched Polyethylene Imine with Surfactants in Aqueous Solution. Quím. Nova 2015, 38. [Google Scholar] [CrossRef]
- Ling, Y.; Wu, J.J.; Gao, Z.F.; Li, N.B.; Luo, H.Q. Enhanced Emission of Polyethyleneimine-Coated Copper Nanoclusters and Their Solvent Effect. J. Phys. Chem. C 2015, 119, 27173–27177. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, W.; Li, S.; Jin, S.; Hu, K.; Hu, L.; Huang, Y.; Gao, X.; Wu, Y.; Liang, X.-J. Ultrabright and Multicolorful Fluorescence of Amphiphilic Polyethyleneimine Polymer Dots for Efficiently Combined Imaging and Therapy. Sci. Rep. 2013, 3, 3036. [Google Scholar] [CrossRef] [PubMed]
- Grasdalen, H. High-field, 1H-NMR spectroscopy of alginate: Sequential structure and linkage conformations. Carbohydr. Res. 1983, 118, 255–260. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, X.; Yang, L.; Wu, Y.; Liu, H.; Zhang, X.; Wei, Y. Preparation of fluorescent organic nanoparticles from polyethylenimine and sucrose for cell imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, X.; Yang, B.; Li, Z.; Deng, F.; Yang, Y.; Zhang, X.; Wei, Y. Fluorescent nanoparticles from starch: Facile preparation, tunable luminescence and bioimaging. Carbohydr. Polym. 2015, 121, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Liu, M.; Yang, B.; Feng, L.; Ji, Y.; Tao, L.; Wei, Y. Size tunable fluorescent nano-graphite oxides: Preparation and cell imaging applications. Phys. Chem. Chem. Phys. 2013, 15, 19013–19018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Xu, L.; Feng, L.; Ji, Y.; Tao, L.; Li, S.; Wei, Y. Biocompatible polydopamine fluorescent organic nanoparticles: Facile preparation and cell imaging. Nanoscale 2012, 4, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Cheng, W.-Y.; Chen, C.-Y.; Shyue, J.-J.; Nieh, C.-C.; Chou, C.-F.; Lee, J.-R.; Lee, Y.-Y.; Cheng, C.-Y.; Chang, S.Y.; et al. Excitation-dependent visible fluorescence in decameric nanoparticles with monoacylglycerol cluster chromophores. Nat. Commun. 2013, 4, 1544. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794–7807. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Imae, T. Fluorescence Emission from Dendrimers and Its pH Dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef] [PubMed]
- Demadis, K.D.; Paspalaki, M.; Theodorou, J. Controlled Release of Bis(phosphonate) Pharmaceuticals from Cationic Biodegradable Polymeric Matrices. Ind. Eng. Chem. Res. 2011, 50, 5873–5876. [Google Scholar] [CrossRef]
- El-Agramy, A.M.; Henaish, B.A. Study of the effect of gamma-irradiation on the emission spectra of poly(methylmethacrylate) doped with rhodamine dyes. Polym. Degrad. Stab. 1993, 40, 13–17. [Google Scholar] [CrossRef]
- Han, J.-M.; Xu, M.; Wang, B.; Wu, N.; Yang, X.; Yang, H.; Salter, B.J.; Zang, L. Low Dose Detection of γ Radiation via Solvent Assisted Fluorescence Quenching. J. Am. Chem. Soc. 2014, 136, 5090–5096. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-M.; Wu, N.; Wang, B.; Wang, C.; Xu, M.; Yang, X.; Yang, H.; Zang, L. [gamma] radiation induced self-assembly of fluorescent molecules into nanofibers: A stimuli-responsive sensing. J. Mater. Chem. C 2015, 3, 4345–4351. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for Analysis of Nanoparticle Hemolytic Properties In Vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Dannenfelser, R.-M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. A pH-sensitive micelle composed of heparin, phospholipids, and histidine as the carrier of photosensitizers: Application to enhance photodynamic therapy of cancer. Int. J. Biol. Macromol. 2017, 98, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, K.C.R.; Uludağ, H. A Comparative Evaluation of Disulfide-Linked and Hydrophobically-Modified PEI for Plasmid Delivery. J. Biomater. Sci. Polym. Ed. 2011, 22, 873–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, F.; Chen, K.; Chen, G.; Tu, K.; Wang, H.; Wang, L.Q. Synthesis of Hemoglobin Conjugated Polymeric Micelle: A ZnPc Carrier with Oxygen Self-Compensating Ability for Photodynamic Therapy. Biomacromolecules 2015, 16, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Sibata, M.N.; Tedesco, A.C.; Marchetti, J.M. Photophysicals and photochemicals studies of zinc(II) phthalocyanine in long time circulation micelles for photodynamic therapy use. Eur. J. Pharm. Sci. 2004, 23, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yao, W.; Wang, X.; Xie, C.; Zhang, J.; Jiang, X. Bioreducible heparin-based nanogel drug delivery system. Biomaterials 2015, 39, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Mekuria, S.L.; Debele, T.A.; Chou, H.-Y.; Tsai, H.-C. IL-6 Antibody and RGD Peptide Conjugated Poly(amidoamine) Dendrimer for Targeted Drug Delivery of HeLa Cells. J. Phys. Chem. B 2016, 120, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Liu, Y.; Zhang, H.; Zhang, Z.; Gao, H.; He, Q. Antitumor and Antimetastasis Activities of Heparin-based Micelle Served as Both Carrier and Drug. ACS Appl. Mater. Interfaces 2016, 8, 9577–9589. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-Y.; Debele, T.A.; Kao, Y.-C.; Tsai, H.-C. Synthesis and Characterization of Dual-Sensitive Fluorescent Nanogels for Enhancing Drug Delivery and Tracking Intracellular Drug Delivery. Int. J. Mol. Sci. 2017, 18, 1090. https://doi.org/10.3390/ijms18051090
Wu S-Y, Debele TA, Kao Y-C, Tsai H-C. Synthesis and Characterization of Dual-Sensitive Fluorescent Nanogels for Enhancing Drug Delivery and Tracking Intracellular Drug Delivery. International Journal of Molecular Sciences. 2017; 18(5):1090. https://doi.org/10.3390/ijms18051090
Chicago/Turabian StyleWu, Szu-Yuan, Tilahun Ayane Debele, Yu-Chih Kao, and Hsieh-Chih Tsai. 2017. "Synthesis and Characterization of Dual-Sensitive Fluorescent Nanogels for Enhancing Drug Delivery and Tracking Intracellular Drug Delivery" International Journal of Molecular Sciences 18, no. 5: 1090. https://doi.org/10.3390/ijms18051090