The Effect of Low-Dose Proteasome Inhibition on Pre-Existing Atherosclerosis in LDL Receptor-Deficient Mice
Abstract
:1. Introduction
2. Results
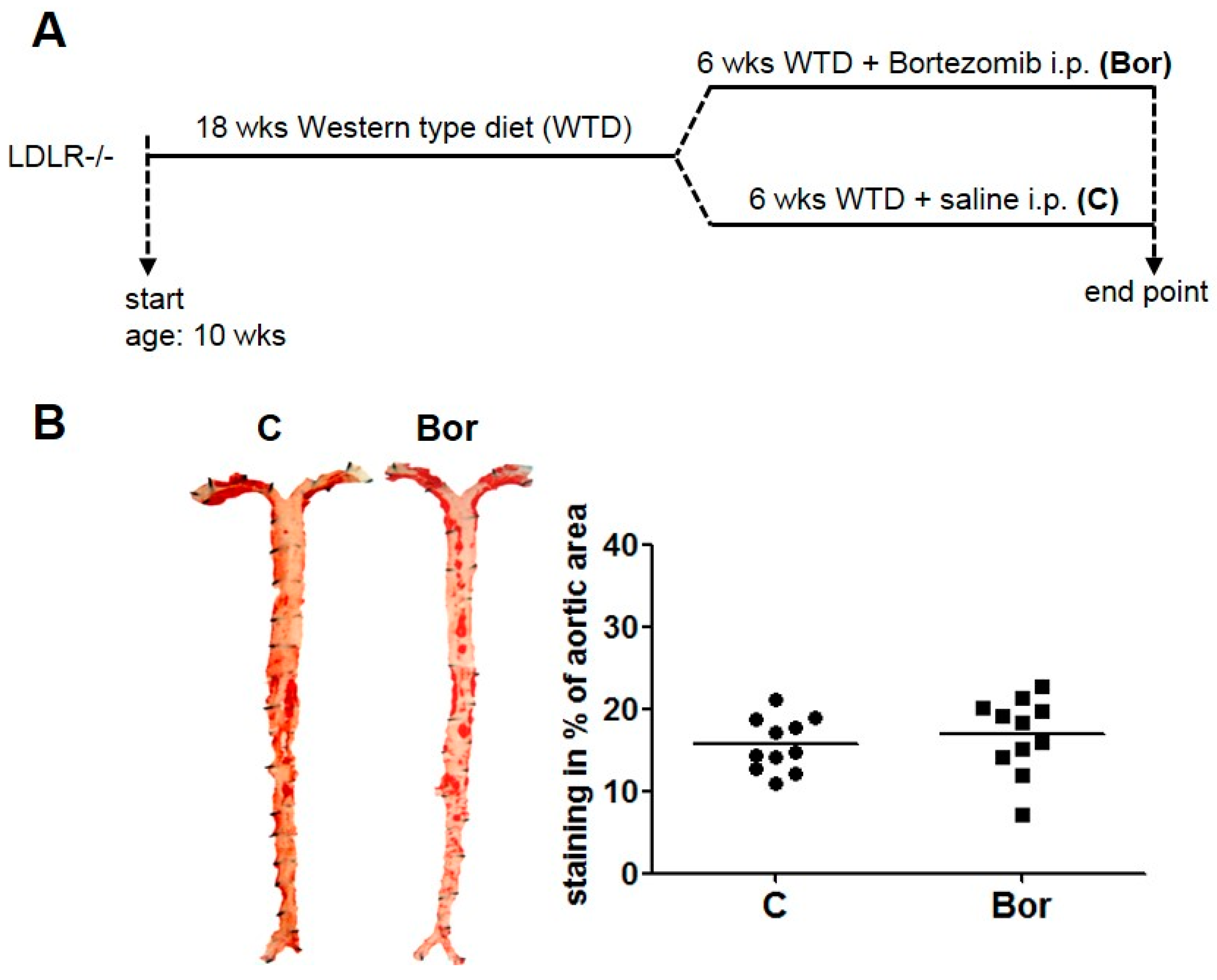
2.1. Low-Dose Bortezomib Treatment Was Efficient and Well-Tolerated
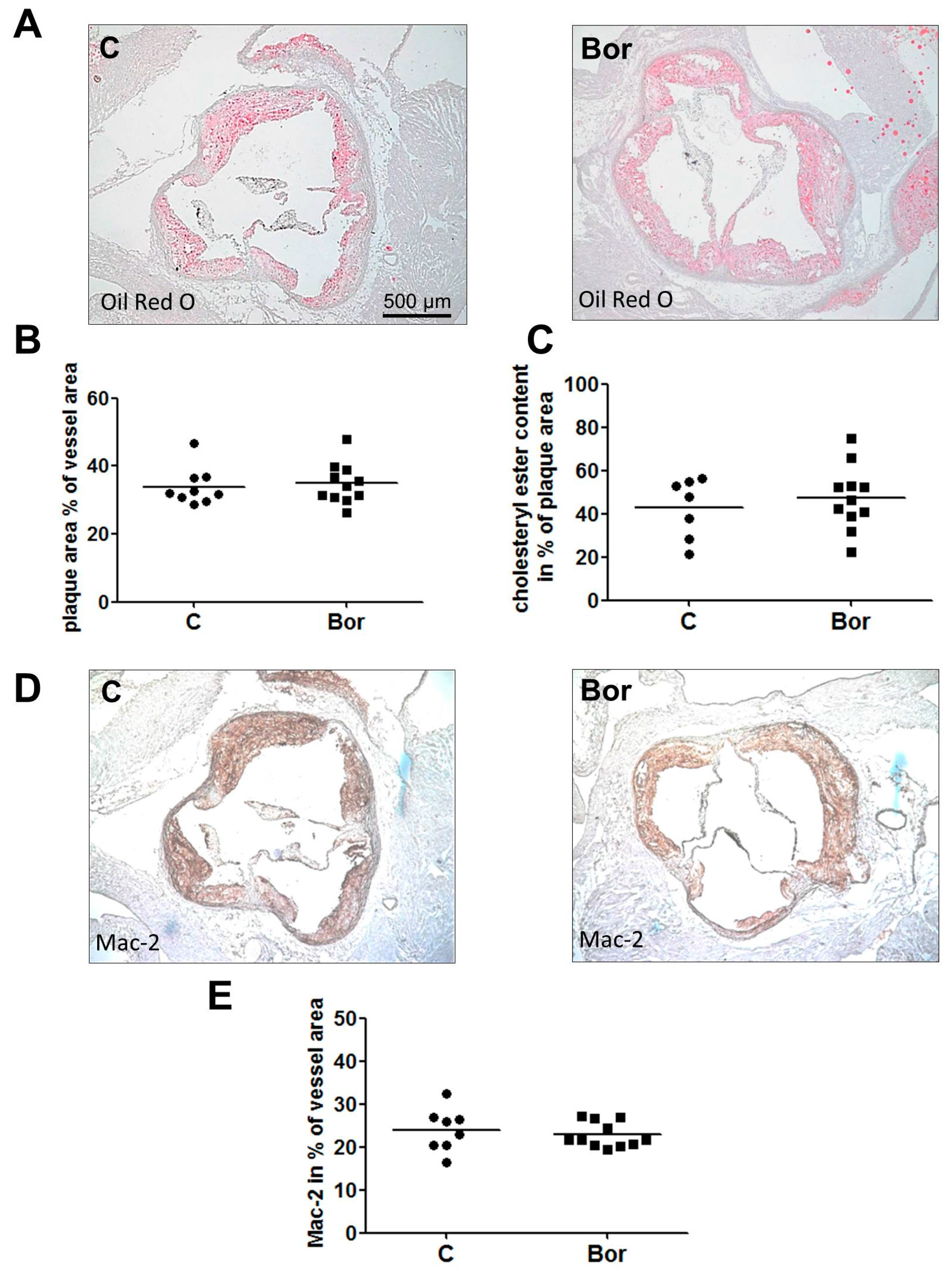
2.2. Bortezomib Had No Effect on Lesion Size and Macrophage Infiltration
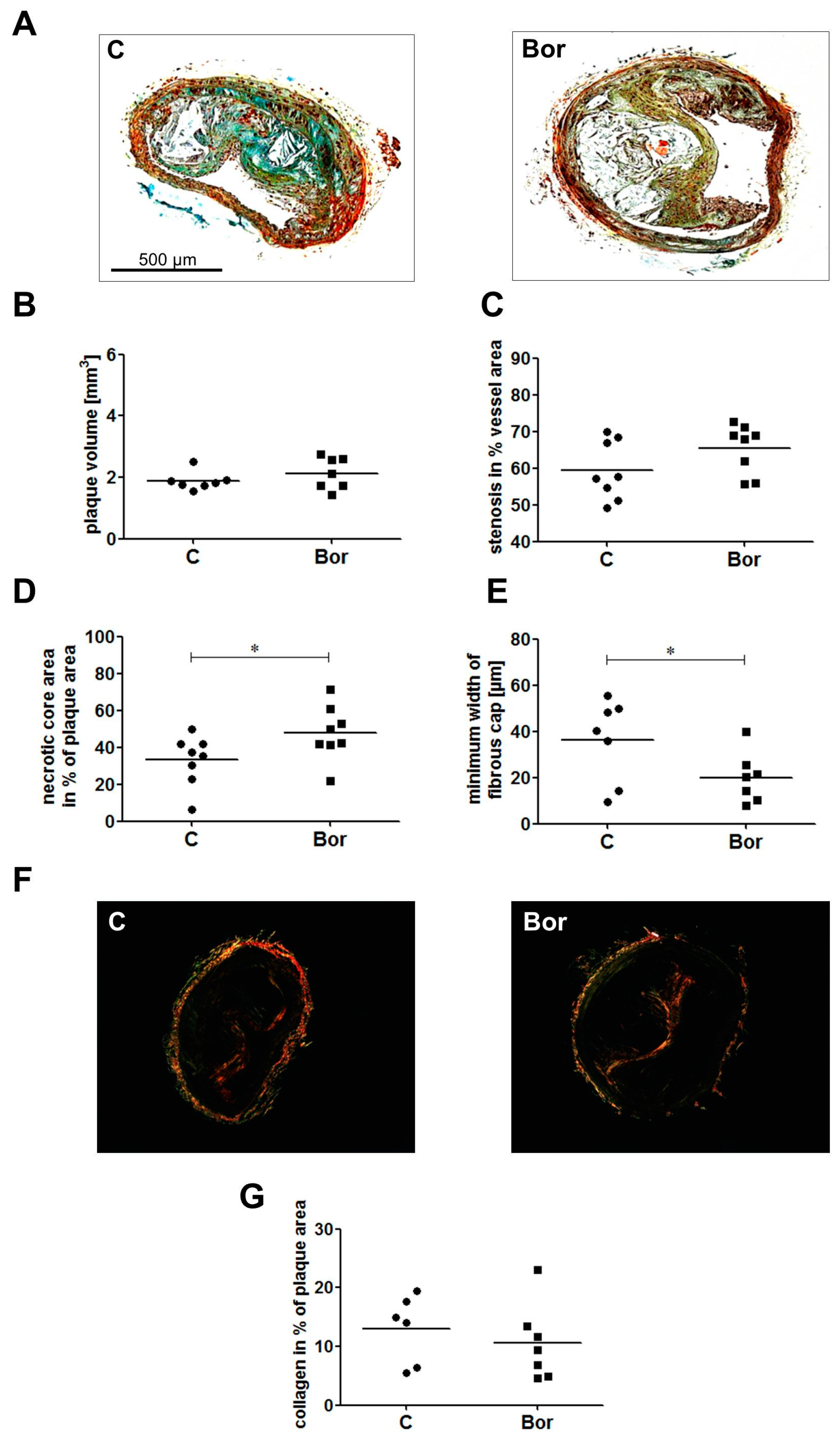
2.3. Bortezomib Influenced Plaque Composition Towards a Vulnerable Phenotype
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experiments
4.3. Measurement of Proteasomal Activity
4.4. Staining and Analysis of Atherosclerotic Lesions
4.5. Immunohistochemistry
4.6. Measurement of Serum Lipids
4.7. Serum Levels of MCP-1 and IL-6
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herrmann, J.; Lerman, L.O.; Lerman, A. On to the road to degradation: Atherosclerosis and the proteasome. Cardiovasc. Res. 2010, 85, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Ludwig, A. Targeting the ubiquitin-proteasome system in atherosclerosis: Status quo, challenges, and perspectives. Antioxid. Redox Signal. 2014, 21, 2344–2363. [Google Scholar] [CrossRef] [PubMed]
- Voges, D.; Zwickl, P.; Baumeister, W. The 26s proteasome: A molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999, 68, 1015–1068. [Google Scholar] [CrossRef] [PubMed]
- Kisselev, A.F.; van der Linden, W.A.; Overkleeft, H.S. Proteasome inhibitors: An expanding army attacking a unique target. Chem. Biol. 2012, 19, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Fechner, M.; Wilck, N.; Meiners, S.; Grimbo, N.; Baumann, G.; Stangl, V.; Stangl, K. Potent anti-inflammatory effects of low-dose proteasome inhibition in the vascular system. J. Mol. Med. 2009, 87, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Meiners, S.; Ludwig, A.; Lorenz, M.; Dreger, H.; Baumann, G.; Stangl, V.; Stangl, K. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic. Biol. Med. 2006, 40, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Fechner, M.; Dreger, H.; Hewing, B.; Arias, A.; Meiners, S.; Baumann, G.; Stangl, V.; Stangl, K.; Ludwig, A. Attenuation of early atherogenesis in low-density lipoprotein receptor-deficient mice by proteasome inhibition. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Marfella, R.; D’Amico, M. Possible dual role of ubiquitin-proteasome system in the atherosclerotic plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1350–1351; author reply 1351. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Soares, S.M.; Lerman, L.O.; Lerman, A. Potential role of the ubiquitin-proteasome system in atherosclerosis: Aspects of a protein quality disease. J. Am. Coll. Cardiol. 2008, 51, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Drews, O.; Tsukamoto, O.; Liem, D.; Streicher, J.; Wang, Y.; Ping, P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ. Res. 2010, 107, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Kloss, A.; Meiners, S.; Ludwig, A.; Dahlmann, B. Multiple cardiac proteasome subtypes differ in their susceptibility to proteasome inhibitors. Cardiovasc. Res. 2010, 85, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Saguner, A.M.; Versari, D.; Peterson, T.E.; Chade, A.; Olson, M.; Lerman, L.O.; Lerman, A. Chronic proteasome inhibition contributes to coronary atherosclerosis. Circ. Res. 2007, 101, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, J.L.; De Meyer, G.R.; Martinet, W.; Bult, H.; Vrints, C.J.; Herman, A.G. Proteasome inhibitor bortezomib promotes a rupture-prone plaque phenotype in apoe-deficient mice. Basic Res. Cardiol. 2010, 105, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Zhang, Y.; Mu, J.; Ye, Z.; Zeng, W.; Qi, W.; Luo, Z.; Guo, Y.; Yang, X.; Yuan, F. Preventive effect of a proteasome inhibitor on the formation of accelerated atherosclerosis in rabbits with uremia. J. Cardiovasc. Pharmacol. 2010, 55, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bross, P.F.; Kane, R.; Farrell, A.T.; Abraham, S.; Benson, K.; Brower, M.E.; Bradley, S.; Gobburu, J.V.; Goheer, A.; Lee, S.L.; et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin. Cancer Res. 2004, 10, 3954–3964. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Meiners, S. Targeting of the proteasome worsens atherosclerosis? Circ. Res. 2008, 102, e37. [Google Scholar] [CrossRef] [PubMed]
- Meiners, S.; Ludwig, A.; Stangl, V.; Stangl, K. Proteasome inhibitors: Poisons and remedies. Med. Res. Rev. 2008, 28, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Versari, D.; Herrmann, J.; Gossl, M.; Mannheim, D.; Sattler, K.; Meyer, F.B.; Lerman, L.O.; Lerman, A. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Edwards, W.D.; Holmes, D.R., Jr.; Shogren, K.L.; Lerman, L.O.; Ciechanover, A.; Lerman, A. Increased ubiquitin immunoreactivity in unstable atherosclerotic plaques associated with acute coronary syndromes. J. Am. Coll. Cardiol. 2002, 40, 1919–1927. [Google Scholar] [CrossRef]
- Marfella, R.; D’Amico, M.; Di Filippo, C.; Baldi, A.; Siniscalchi, M.; Sasso, F.C.; Portoghese, M.; Carbonara, O.; Crescenzi, B.; Sangiuolo, P.; et al. Increased activity of the ubiquitin-proteasome system in patients with symptomatic carotid disease is associated with enhanced inflammation and may destabilize the atherosclerotic plaque: Effects of rosiglitazone treatment. J. Am. Coll. Cardiol. 2006, 47, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Zernecke, A.; Libby, P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nat. Rev. Immunol. 2008, 8, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Hajje, D. Case series discussion of cardiac and vascular events following carfilzomib treatment: Possible mechanism, screening, and monitoring. BMC Cancer 2014, 14, 915. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, H.; Yamashita, T.; Kotani, T.; Sawazaki, A.; Okumura, H.; Nakao, S. Ischemic heart disease associated with bortezomib treatment combined with dexamethasone in a patient with multiple myeloma. Int. J. Hematol. 2010, 91, 903–906. [Google Scholar] [CrossRef] [PubMed]
- ImageJ Software. Available online: https://imagej.nih.gov/ij/index.html (accessed on 5 March 2017).
- Keul, P.; Tolle, M.; Lucke, S.; von Wnuck Lipinski, K.; Heusch, G.; Schuchardt, M.; van der Giet, M.; Levkau, B. The sphingosine-1-phosphate analogue fty720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 607–613. [Google Scholar] [CrossRef] [PubMed]

| C (n = 11) | Bor (n = 11) | |
|---|---|---|
| Body weight (g) | 41.1 ± 1.3 | 42.7 ± 1.4 |
| Total cholesterol (mg/dL) | 1718 ± 154 | 1730 ± 130 |
| Triglycerides (mg/dL) | 1562 ± 186 | 1391 ± 97 |
| Proteasomal activity in liver lysates (RFU) | 1440 ± 51 | 1211 ± 50 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilck, N.; Fechner, M.; Dan, C.; Stangl, V.; Stangl, K.; Ludwig, A. The Effect of Low-Dose Proteasome Inhibition on Pre-Existing Atherosclerosis in LDL Receptor-Deficient Mice. Int. J. Mol. Sci. 2017, 18, 781. https://doi.org/10.3390/ijms18040781
Wilck N, Fechner M, Dan C, Stangl V, Stangl K, Ludwig A. The Effect of Low-Dose Proteasome Inhibition on Pre-Existing Atherosclerosis in LDL Receptor-Deficient Mice. International Journal of Molecular Sciences. 2017; 18(4):781. https://doi.org/10.3390/ijms18040781
Chicago/Turabian StyleWilck, Nicola, Mandy Fechner, Cristian Dan, Verena Stangl, Karl Stangl, and Antje Ludwig. 2017. "The Effect of Low-Dose Proteasome Inhibition on Pre-Existing Atherosclerosis in LDL Receptor-Deficient Mice" International Journal of Molecular Sciences 18, no. 4: 781. https://doi.org/10.3390/ijms18040781





