3.1. General
The melting points were measured on a Yanaco Mp-S3 micro melting point apparatus and are uncorrected. The infrared (IR) spectra were recorded on a JASCO FT/IR-460Plus spectrometer, and the ultraviolet-visible (UV-Vis) absorption spectra were recorded on a JASCO V-560 spectrophotometer (JASCO, Sapporo, Japan, cell length: 10 mm). The 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a JEOL JNM-ECA-500 spectrometer (JEOL, Tokyo, Japan). The chemical shifts are reported in ppm on the δ scale relative to CDCl3 (δ = 7.26 for 1H-NMR), CDCl3 (δ = 77.0 for 13C-NMR), CD3OD (δ = 3.31 for 1H-NMR), CD3OD (δ = 49.0 for 13C-NMR), DMSO-d6 (δ = 2.49 for 1H-NMR), and DMSO-d6 (δ = 39.5 for 13C-NMR) as internal references. The signal patterns are as follows: s, singlet; brs, broad singlet; d, doublet; brd, broad doublet; t, triplet; m, multiplet. High-resolution mass spectra (HRMS) were recorded on a Bruker micrOTOF-QII (ESI) spectrometer. Analytical thin-layer chromatography (TLC) was performed on Merck precoated analytical plates (0.25 mm thick, silica gel 60 F254, MERCK MILLIPORE, Darmstadt, Germany). High-performance liquid chromatography (HPLC) was performed using a system equipped with JASCO PU-1580 pumps, an MD-1515 detector, HG-1580-32 mixer, and DG-580-35 degasser (JASCO). An Octadecylsilane (ODS) column (SunShell C18, 2.6 µm, 2.1 inside diameter × 100 mm, ChromaNik Technologies, Osaka, Japan) was eluted at 40 °C using linear gradient elution (0.2 mL/min) over 20 min from 10% to 90% aq. MeCN solution containing 0.5% trifluoroacetic acid (TFA). Flash silica gel chromatography was performed using silica gel PSQ60B (Fuji Silysia Chemical, Kasugai, Japan). All reagents were purchased from commercial sources. All reactions were performed under an argon (Ar) atmosphere unless otherwise noted.
To coat the TiO2 paste (Solaronix Ti-Nanoxide, Aubonne, Switzerland) onto fluorine-doped tin oxide (FTO) glass plates (Astellatech Co., Ltd., Yokohama, Japan, 75 mm × 25 mm × 1.8 mm thick), screen-printing equipment (WHT3, Mino International, Ltd., Tokyo, Japan and HP-320, Newlong Seimitsu Kogyo Co., Ltd., Tokyo, Japan) was used. Calcination of the TiO2-coated FTO glass was performed using a furnace (FT-101W, Full-Tech Co., Ltd., Osaka, Japan). N719 (Aldrich, Tokyo, Japan, 95%) was used as the standard dye.
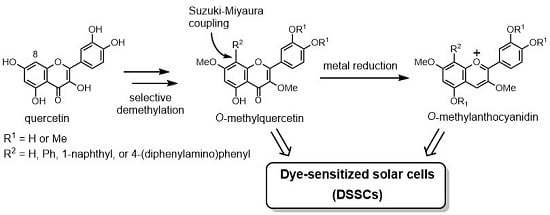
3.2. Synthesis of O-Methylquercetins
3.2.1. 3,7,3′,4′-tetra-O-Methyl-8-phenylquercetin (11)
To a solution of 8 (710 mg, 1.6 mmol) in cyclopentyl methyl ether (CPME) (20 mL) was added BBr3 (4 mL, 4.0 mmol, 1.0 M solution in CH2Cl2) dropwise at 0 °C. After stirring for 5 min at 0 °C and for 60 min at rt, MeOH (25 mL) was poured into the mixture. The reaction mixture was concentrated under reduced pressure and saturated aq. NaCl (30 mL) was added. After the mixture was extracted with CH2Cl2 (15 mL × 3), the combined organic phases were washed with saturated aq. NaCl (30 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was recrystallized from AcOEt (20 mL) to give 11 as a yellow solid (607 mg, 88%). mp: 225–226 °C; IR (KBr) cm−1; 2995, 1585, 1517, 1267, 1217 cm−1; UV-Vis (CDCl3) λmax nm (ε): 276 nm (20,160), 366 nm (26,960); 1H-NMR (CDCl3, 500 MHz) δ 3.61 (3H, s), 3.84 (3H, s), 3.90 (3H, s), 3.91 (3H, s), 6.50 (1H, s), 6.86 (1H, d, J = 9.0 Hz), 7.17 (1H, d, J = 2.5 Hz), 7.3–7.4 (3H, m), 7.4–7.5 (2H, m), 7.76 (1H, dd, J = 9.0, 2.5 Hz); 13C-NMR (CDCl3, 125 MHz) δ 55.8, 55.9, 56.2, 60.0, 94.9, 105.4, 109.5, 110.3, 110.8, 122.7, 123.0, 127.3, 128.1, 131.2, 132.2, 138.8, 148.7, 151.2, 152.8, 155.3, 161.7, 162.3, 179.1; ESI-TOF HRMS Calcd. For C25H23O7 [M + H]+, 435.1438; Found: 435.1440.
3.2.2. 3,7,3′,4′-tetra-O-Methyl-8-(1-naphthyl)quercetin (12)
According to the procedure described for 11, the reaction of 9 (400 mg, 0.80 mmol) was carried out to afford 12 (304 mg, 78%) as a yellow solid. mp: 239–240 °C; IR (KBr) cm−1; 3437, 2935, 1648, 1589, 1218 cm−1; UV-Vis (CDCl3) λmax nm (ε): 275 nm (27,800), 364 nm (20,940); 1H-NMR (CDCl3, 500 MHz) δ 3.16 (3H, s), 3.76 (3H, s), 3.83 (3H, s), 3.90 (3H, s), 6.58 (1H, s), 6.59 (1H, d, J = 2.5 Hz), 6.72 (1H, d, J = 8.5 Hz), 7.3–7.4 (1 H, m), 7.4–7.5 (2H, m), 7.5–7.6 (3H, m), 7.8–7.9 (2H, m); 13C-NMR (CDCl3, 125 MHz) δ 55.2, 55.8, 56.2, 59.9, 95.0, 105.5, 107.2, 109.7, 110.6, 122.6, 122.8, 125.6, 125.9, 126.3, 128.1, 128.2, 129.0, 148.5, 151.0, 153.4, 155.4, 162.2, 163.1, 179.1; ESI-TOF HRMS Calcd. For C29H25O7 [M + H]+, 485.1595; Found: 485.1592.
3.2.3. 3,7,3′,4′-tetra-O-Methyl-8-(4-(diphenylamino)phenyl)quercetin (13)
According to the procedure described for 11, the reaction of 10 (210 mg, 0.34 mmol) was carried out to afford 13 (179 mg, 87%) as a yellow solid. mp: 260–261 °C; IR (KBr) cm−1; 3435, 2992, 1587, 1488, 1266 cm−1; UV-Vis (CDCl3) λmax nm (ε): 254 nm (24,060), 312 nm (39,080), 363 nm (16,600); 1H-NMR (CDCl3, 500 MHz) δ 3.80 (3H, s), 3.87 (3H, s), 3.90 (3H, s), 3.95 (3H, s), 6.50 (1H, s), 6.87 (1H, d, J = 9.0 Hz), 7.0–7.3 (14H, m), 7.54 (1H, d, J = 2.0 Hz), 7.64 (1H, dd, J = 9.0, 2.0 Hz); 13C-NMR (CDCl3, 125 MHz) δ 56.0, 56.2, 56.3, 60.1, 95.0, 105.5, 109.1, 110.7, 111.1, 122.5, 122.6, 123.1, 124.6, 125.5, 129.3, 131.9, 138.7, 146.8, 147.6, 148.7, 151.3, 153.0, 155.6, 161.5, 162.4, 179.1; ESI-TOF HRMS Calcd. For C37H32NO7 [M+H]+, 602.2173; Found: 602.2177.
3.2.4. 3,7-di-O-Methyl-8-phenylquercetin (14)
To a solution of 11 (150 mg, 0.35 mmol) and tetrabutylammonium iodide (TBAI) (0.68 g, 1.83 mmol) in CH2Cl2 (3 mL) was added BCl3 (1.83 mL, 1.83 mmol, 1.0 M solution in CH2Cl2) dropwise at −20 °C. After stirring for 60 min at −20 °C, MeOH (4.5 mL) was poured into the mixture. The reaction mixture was concentrated under reduced pressure and saturated aq. Na2S2O3 (20 mL) was added. After the mixture was extracted with AcOEt (15 mL × 3), the combined organic phases were washed with saturated aq. NaCl (20 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash silica gel chromatography (n-hexane/AcOEt = 1:2 to AcOEt) to give 14 as a yellow solid (119 mg, 85%). mp: 261–262 °C; IR (KBr) cm−1; 3476, 1649, 1595, 1442, 1274 cm−1; UV-Vis (MeOH) λmax nm (ε): 258 nm (35,800), 372 nm (23,800); 1H-NMR (DMSO-d6, 500 MHz) δ 3.80 (3H, s), 3.82 (3H, s), 6.66 (1H, s), 6.70 (1H, d, J = 8.5 Hz), 7.01 (1H, dd, J = 8.5, 2.5 Hz), 7.4–7.5 (6H, m), 9.19 (OH, brs), 9.80 (OH, brs); 13C-NMR (DMSO-d6, 125 MHz) δ 56.4, 59.6, 95.2, 104.6, 108.9, 115.3, 115.9, 120.8, 127.3, 127.9, 131.1, 131.4, 137.5, 145.1, 148.7, 152.2, 156.0, 160.8, 161.9, 178.4; ESI-TOF HRMS Calcd. For C23H19O7 [M + H]+, 407.1125; Found: 407.1133.
3.2.5. 3,7-di-O-Methyl-8-(1-naphthyl)quercetin (15)
According to the procedure described for 14, the reaction of 12 (150 mg, 0.31 mmol) was carried out to afford 15 (88 mg, 62%) as a yellow solid. mp: 309–310 °C; IR (KBr) cm−1; 3479, 3152, 1601, 1438, 1207 cm−1; UV-Vis (MeOH) λmax nm (ε): 258 nm (41,060), 371 nm (29,600); 1H-NMR (DMSO-d6, 500 MHz) δ 3.76 (3H, s), 3.80 (3H, s), 6.4–6.5 (2H, m), 6.75 (1H, s), 7.16 (1H, d, J = 2.0 Hz), 7.3–7.6 (6H, m), 8.0–8.1 (2H, m), 9.05 (OH, brs), 9.72 (OH, brs), 13.03 (OH, brs); 13C-NMR (DMSO-d6, 125 MHz) δ 56.4, 59.6, 95.2, 104.8, 106.7, 115.1, 115.7, 119.6, 120.1, 125.2, 125.5, 125.7, 126.2, 128.0, 128.2, 129.1, 132.8, 137.4, 144.9, 148.6, 152.8, 155.7, 161.3, 162.6, 178.4; ESI-TOF HRMS Calcd. For C27H21O7 [M + H]+, 457.1282; Found: 457.1280.
3.2.6. 3,7-di-O-Methyl-8-(4-(diphenylamino)phenyl)quercetin (16)
According to the procedure described for 14, the reaction of 13 (150 mg, 0.25 mmol) was carried out to afford 16 (117 mg, 82%) as a yellow solid. mp: 271–272 °C; IR (KBr) cm−1; 3434, 1645, 1592, 1489, 1206 cm−1; UV-Vis (MeOH) λmax nm (ε): 257 nm (14,600), 304 nm (22,800), 368 nm (10,000); 1H-NMR (DMSO-d6, 500 MHz) δ 3.80 (3H, s), 3.83 (3H, s), 6.63 (1H, s), 6.76 (1H, d, J = 8.0 Hz), 7.0–7.4 (15H, m), 7.47 (1H, d, J = 2.0 Hz), 9.24 (OH, brs), 9.91 (OH, brs), 13.0 (OH, brs); 13C-NMR (DMSO-d6, 125 MHz) δ 56.5, 59.6, 95.1, 104.7, 108.5, 115.3, 116.0, 120.2, 120.9, 122.8, 123.0, 123.9, 125.6, 129.6, 132.1, 137.5, 145.2, 146.3, 147.2, 148.8, 152.4, 155.8, 160.6, 178.4; ESI-TOF HRMS Calcd. For C35H28NO7 [M + H]+, 574.1860; Found: 574.1849.
3.3. Synthesis of O-Methylanthocyanidins
3.3.1. 3,7,3′,4′-tetra-O-Methylcyanidin (18)
To a suspension of 4 (200 mg, 0.56 mmol) and Zn powder (2.0 g, 31 mmol) in anhydrous MeOH (8.0 mL) and anhydrous THF (2.0 mL) was added 4 N hydrogen chloride-methanol solution (10 mL) at 0 °C. After stirring for 30 min at 0 °C, Zn was removed by suction filtration. The filtrate solution was stirred under air for 20 h. After the reaction solution was added with water containing 0.5% trifluoroacetic acid (TFA) (100 mL), MeOH and THF were removed under reduced pressure. The residual aqueous solution was extracted with CH2Cl2 (20 mL × 4). The combined organic phases were dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash silica gel chromatography (33% AcOEt/Hexane with 0.5% TFA → AcOEt with 0.5% TFA → 5% MeOH/AcOEt with 0.5% TFA) to give 18 as a TFA salt (dark red) solid (210 mg, 83%). mp: 110–111 °C; IR (KBr) cm−1; 2935, 1613, 1589, 1319, 1209 cm−1; UV-Vis (0.1%HCl-MeOH) λmax nm (ε): 279 nm (18,000), 522 nm (26,600); 1H-NMR (10%TFA-d-CDCl3, 500 MHz) δ 4.02 (3H,s), 4.05 (3H, s), 4.08 (3H,s), 4.23 (3H,s), 6.84 (1H, s), 6.95 (1H, s), 7.14 (1H, d, J = 9.0 Hz), 7.97 (1H, d, J = 2.5 Hz), 8.37 (1H, dd, J = 9.0, 2.5 Hz), 8.79 (1H, s), 13C-NMR (10%TFA-d-CDCl3, 125 MHz) δ 56.4, 56.6, 56.8, 57.8, 92.5, 103.3, 112.0, 112.8, 112.9, 120.9, 128.8, 130.3, 147.9, 149.4, 155.3, 155.6, 156.6, 162.6, 169.2; ESI-TOF HRMS Calcd. For C19H19O6 [M]+, 343.1176; Found: 343.1170.
3.3.2. 3,7,4′-tri-O-Methylcyanidin (19)
According to the procedure described for 18, the reaction of 5 (200 mg, 0.58 mmol) was carried out to afford 19 (192 mg, 75%) as a TFA salt (dark red) solid. mp: 144–145 °C; IR (KBr) cm−1; 3284, 1680, 1574, 1375, 1204 cm−1; UV-Vis (0.1%HCl-MeOH) λmax nm (ε): 280 nm (11,080), 525 nm (17,320); 1H-NMR (10%TFA-d-CD3OD, 500 MHz) δ 3.95 (3H, s), 3.98 (3H,s), 4.17 (3H,s), 6.66 (1H, d, J = 2.0 Hz), 7.02 (1H, d, J = 2.0 Hz), 7.03 (1H, d, J = 9.0 Hz), 7.89 (1H, d, J = 2.0 Hz), 8.14 (1H, dd, J = 9.0, 2.0 Hz), 8.70 (1H, s), 13C-NMR (10%TFA-d-CD3OD, 125 MHz) δ 56.9, 57.5, 58.3, 92.7, 103.3, 112.9, 114.3, 117.8, 122.4, 127.9, 130.8, 148.6, 149.4, 156.7, 157.8, 163.8, 170.0; ESI-TOF HRMS Calcd. For C18H17O6 [M]+, 329.1020; Found: 329.1023.
3.3.3. 3,7-di-O-methylcyanidin (20).
According to the procedure described for 18, the reaction of 6 (200 mg, 0.61 mmol) was carried out to afford 20 (183 mg, 71%) as a TFA salt (dark red) solid. mp: 121–122 °C; IR (KBr) cm−1; 3419, 1682, 1455, 1210, 1128 cm−1; UV-Vis (0.1%HCl-MeOH) λmax nm (ε): 280 nm (9600), 527 nm (11,600); 1H-NMR (10%TFA-d-CD3OD, 500 MHz) δ 3.98 (3H, s), 4.18 (3H,s), 6.67 (1H, d, J = 2.0 Hz), 6.98 (1H, d, J = 9.0 Hz), 7.05 (1H, d, J = 2.0Hz), 8.04 (1H, d, J = 2.5 Hz), 8.15 (1H, dd, J = 9.0, 2.5 Hz), 8.68 (1H, s), 13C-NMR (10%TFA-d-CD3OD, 125 MHz) δ 57.4, 58.2, 92.6, 103.1, 113.6, 117.6, 118.7, 121.3, 128.6, 129.7, 147.6, 149.3, 156.6, 157.7, 164.5, 169.5; ESI-TOF HRMS Calcd. For C17H15O6 [M]+, 315.0863; Found: 315.0864.
3.3.4. 3,7-di-O-Methyl-8-phenylcyanidin (24)
According to the procedure described for 18, the reaction of 14 (80 mg, 0.61 mmol) was carried out to afford 24 (38 mg, 38%) as a TFA salt (dark red) solid. mp: 250–251 °C; IR (KBr) cm−1; 3408, 3107, 1679, 1207, 1142 cm−1; UV-Vis (0.1%HCl-MeOH) λmax nm (ε): 281 nm (7700), 538 nm (7100); 1H-NMR (10%TFA-d-CD3OD, 500 MHz) δ 3.98 (3H, s), 4.22 (3H,s), 6.82 (1H, d, J = 8.5 Hz), 6.99 (1H, s), 7.4–7.6 (5H, m), 7.69 (1H, dd, J = 8.5, 2.0 Hz), 7.85 (1H, d, J = 2.0 Hz), 8.80 (1H, s), 13C-NMR (10%TFA-d-CD3OD, 125 MHz) δ 57.5, 58.2, 92.3, 98.9, 111.5, 117.3, 119.6, 121.5, 128.2, 129.4, 129.6, 129.9, 131.6, 132.1, 147.4, 149.1, 165.8, 171.8; ESI-TOF HRMS Calcd. For C23H19O6 [M]+, 391.1176; Found: 391.1181.