N-Glycoprofiling Analysis for Carbohydrate Composition and Site-Occupancy Determination in a Poly-Glycosylated Protein: Human Thyrotropin of Different Origins
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. hTSH Preparations
4.2. In Vivo Bioassay
4.3. Immunoradiometric Assay (IRMA)
4.4. Pharmacokinetic Studies
4.5. Mass Spectrometry for Molecular Mass Determination
4.6. N-Glycosylation Profiling by MALDI-TOF-MS
4.6.1. Glycosidase Digestion and Permethylation of N-Glycans
4.6.2. MALDI-TOF Analysis of N-Glycans
4.6.3. Average N-Glycan Mass and Monosaccharide Molar Ratio Determination on the Basis of Glycoprofiling
4.7. Site-Occupancy and Mass of the Carbohydrate Moiety Determination
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Szkudlinski, M.W.; Fremont, V.; Ronin, C.; Weintraub, B.D. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol. Rev. 2002, 82, 473–502. [Google Scholar] [CrossRef] [PubMed]
- Ribela, M.T.C.P.; Gout, P.W.; Oliveira, J.E.; Bartolini, P. HPLC analysis of human pituitary hormones for pharmaceutical applications. Curr. Pharm. Anal. 2006, 2, 103–126. [Google Scholar] [CrossRef]
- Pierce, J.C.; Parsons, F. Glycoprotein hormones: Structure and function. Ann. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Bishop, L.A.; Nguyen, T.V.; Schofield, P.R. Both of the β-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potency. Endocrinology 1995, 136, 2635–2640. [Google Scholar] [PubMed]
- Bousfield, G.R.; Butnev, V.Y.; Walton, W.J.; Nguyen, V.T.; Huneidi, J.; Singh, V.; Kolli, V.S.K.; Harvey, D.J.; Rance, N.E. All-hormone N-glycosylation in primate follicle-stimulating hormone β subunits. Mol. Cell Endocrinol. 2007, 260, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Aguirre, A.; Zariñan, T.; Pasapera, A.M.; Casas-Gonzales, P.; Dias, J.A.Q. Multiple facets of follicle-stimulating receptor function. Endocrine 2007, 32, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.E.; Damiani, R.; Vorauer-Uhl, K.; Bartolini, P.; Ribela, M.T.C.P. Influence of a reduced CO2 environment on the secretion yield, potency and N-glycan structures of recombinant thyrotropin from CHO cells. Mol. Biotechnol. 2008, 30, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Oliveira, J.E.; Almeida, B.E.; Ueda, E.K.M.; Torjesen, P.A.; Bartolini, P.; Ribela, M.T.C.P. Efficient subunit isolation of recombinant and pituitary glycoprotein hormones. J. Chromatogr. A 2009, 1216, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Boime, I.; Ben-Menahem, D. Glycoprotein hormone structure-function and analog design. Recent. Prog. Horm. Res. 1999, 54, 271–287. [Google Scholar] [PubMed]
- Butnev, V.Y.; Gotschall, R.R.; Butnev, V.Y.; Baker, V.L.; Moore, W.T.; Bousfield, G.R. Hormone-specific inhibitory influence of α-subunit Asn56 oligosaccharide on in vivo subunit association and follicle-stimulating hormone receptor binding of equine gonadotropins. Biol. Reprod. 1998, 58, 458–469. [Google Scholar] [CrossRef]
- Amoresano, A.; Siciliano, R.; Orru, S.; Napoleoni, R.; Altarocca, V.; de Luca, E.; Sirna, A.; Pucci, P. Structural characterization of human recombinant glycohormones follitropin, lutropin and choriogonadotropin expressed in Chinese hamster ovary cells. Eur. J. Biochem. 1996, 242, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Ribela, M.T.C.P.; Bianco, A.C.; Bartolini, P. The use of recombinant human thyrotropin produced by Chinese hamster ovary cells for the preparation of immunoassay reagents. J. Clin. Endocrinol. Metab. 1996, 81, 249–256. [Google Scholar] [PubMed]
- Chen, M.K.; Doddamane, I.; Cheng, D.W. Recombinant human thyroid-stimulating hormone as an alternative for thyroid hormone withdrawal in thyroid cancer management. Curr. Opin. Oncol. 2010, 22, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, S.J.; Hegedus, L. Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome. Endocr. Rev. 2010, 33, 920–980. [Google Scholar] [CrossRef] [PubMed]
- Luster, M. Present status of the use of recombinant human TSH in thyroid cancer management. Acta Oncol. 2006, 45, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Sturiale, L.; Garozzo, D. Mass spectrometry in the characterization of human genetic N-glycosylation defects. Mass. Spectrom. Rev. 2009, 28, 517–542. [Google Scholar] [CrossRef] [PubMed]
- Fogli, A.; Merle, C.; Roussel, V.; Schiffmann, R.; Ughetto, S.; Theisen, M.; Boespflug-Tanguy, O. CSF N-glycan profiles to investigate biomarkers in brain developmental disorders: Application to leukodystrophies related to eIF2B mutations. PLoS ONE 2012, 7, e42688. [Google Scholar] [CrossRef] [PubMed]
- Losfeld, M.E.; Soncin, F.; Ng, B.G.; Singec, I.; Freeze, H.H. A sensitive green fluorescent protein biomarker of N-glycosylation site occupancy. FASEB J. 2012, 26, 4210–4217. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Tamura, Y.; Chen, R.; May, D.; McIntosh, M.W.; Brentnall, T.A. Large-scale quantitative glycoproteomics analysis of site-specific glycosylation occupancy. Mol. Biosyst. 2012, 8, 2850–2856. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, A.J.; Milac, A.L.; Petrescu, S.M.; Dwek, R.A.; Wormald, M.R. Statistical analysis of the protein environment of N-glycosylation sites: Implications for occupancy, structure, and folding. Glycobiology 2004, 14, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, H. Glycoproteomics and clinical applications. Proteom. Clin. Appl. 2010, 4, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Hermajakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef]
- Desaire, H. Glycopeptide analysis, recent developments and applications. Mol. Cell. Proteom. 2013, 12, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Ma, Y.C.; Pai, P.J.; Her, G.R. A comparative study of glycoprotein concentration, glycoform profile and glycosylation site occupancy using isotope labeling and electrospray linear ion trap mass spectrometry. Anal. Chim. Acta 2012, 728, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.V.N.; Suzuki, M.F.; Oliveira, J.E.; Damiani, R.; Soares, C.R.J.; Bartolini, P. N-glycoprofiling analysis in a simple glycoprotein model: A comparison between recombinant and pituitary glycosylated human prolactin. J. Biotechnol. 2015, 202, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Szkudlinski, M.W.; Thotakura, N.R.; Bucci, I.; Joshi, L.R.; Tsai, A.; East-Palmer, J.; Shiloach, J.; Weintraub, B.D. Purification and characterization of recombinant human thyrotropin (TSH) isoforms produced by Chinese hanmster ovary cells: The role of sialylation and sulfation in TSH bioactivity. Endocrinology 1993, 133, 1490–1503. [Google Scholar] [PubMed]
- Cole, E.S.; Lee, K.; Lauziere, K.; Kelton, C.; Chappel, S.; Weintraub, B.; Ferrara, D.; Peterson, P.; Bernasconi, R.; Edmunds, T.; et al. Recombinant human thyroid-stimulating hormone: Development of a biotechnolgy product for detection of metastatic lesions of thyroid carcinoma. Nat. Biotechnol. 1993, 11, 1014–1024. [Google Scholar] [CrossRef]
- Morelle, W.; Donadio, S.; Ronin, C.; Michalski, J.-C. Characterization of N-glycans of recombinant human thyrotropin using mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Damiani, R.; Almeida, B.E.; Oliveira, J.E.; Bartolini, P.; Ribela, M.T.C.P. Enhancement of human thyrotropin synthesis by sodium butyrate addition to serum-free CHO cell culture. Appl. Biochem. Biotechnol. 2013, 171, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Damiani, R.; Oliveira, J.E.; Vorauer-Uhl, K.; Peroni, C.N.; Gimbo-Vianna, E.; Bartolini, P.; Ribela, M.T.C.P. Stable expression of a human-like, sialylated recombinant thyrotropin in a chinese hamster ovary cell line expressing α2,6-sialyltransferase. Protein Expr. Purif. 2009, 67, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Thotakura, N.R.; Blithe, D.L. Glycoprotein hormones-Glycobiology of gonadotropins, thyrotropin and free alpha subuhnit. Glycobiology 1995, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, L.; Trojan, J.; Helton, T.E.; Usadel, K.H.; Magner, J.A. Serum thyrotropin (TSH) heterogeneity in euthyroid subjects and patients with subclinical hypothyroidism—The core fucose content of TSH-releasing hormone-released TSH is altered, but not the net charge of TSH. J. Endocrinol. 1995, 144, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Gyves, P.W.; Gesundheit, N.; Thotakura, N.R.; Stannard, B.S.; De Cherney, G.S.; Weintraub, B. Changes in the sialylation and sulfation of secreted thyrotropin in congenital hypothyroidism. Proc. Natl. Acad. Sci. USA 1990, 87, 3792–3796. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.E.; Damiani, R.; Bartolini, P.; Ribela, M.T.C.P. Practical RP-HPLC method for laboratory-scale purification of recombinant human thyrotropin (hTSH). J. Cromatogr. A 2007, 1164, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.; Oliveira, J.E.; Bartolini, P.; Ribela, M.T.C.P. Two-step chromatographic purification of recombinant human thyrotropin and its immunological, biological, physico-chemical and mass spectral characterization. J. Chromatogr. A 2005, 1062, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Parlow, A.F.; Condliffe, P.G.; Reichert, L.E.; Wilhelmi, A.E. Recovery and partial purification of FSH and LH during the purification of TSH from human pituitary glands. Endocrinology 1965, 76, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, P.A.; Sand, T.; Norman, N.; Trygstad, O.; Foss, I. Isolation of LH, FSH and TSH from human pituitaries after the removal of hGH. Acta Endocrinol. 1974, 77, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Ribela, M.T.C.P.; Gout, P.W.; Bartolini, P. Synthesis and chromatographic purification of recombinant human pituitary hormones. J. Chromatogr. 2003, 790, 285–316. [Google Scholar] [CrossRef]
- Peroni, C.N.; Soares, C.R.J.; Gimbo, E.; Morganti, L.; Ribela, M.T.C.P.; Bartolini, P. High-level expression of human thyroid stimulating hormone in Chinese hamster ovary cells by cotransfection of dicistronic expression vectors followed by a dual marker amplification strategy. Biotechnol. Appl. Biochem. 2002, 35, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Damiani, R.; Oliveira, J.E.; Dalmora, S.L.; Torjesen, P.A.; Bartolini, P.; Ribela, M.T.C.P. Reversed-phase high performance liquid chromatography as an alternative to animal bioassay for human thyrotropin potency determination. Anal. Methods 2014, 6, 6688–6694. [Google Scholar] [CrossRef]
- Laidler, P.; Cowan, D.A.; Hider, R.C.; Keane, A.; Kichman, A.T. Tryptic mapping of human chorionic-gonadotropin by matrix-assisted laser-desorption ionization mass-spectrometry. Rapid Commun. Mass Spectrom. 1995, 9, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, R.F.; Oliveira, J.E.; Bartolini, P.; Ribela, M.T.C.P. Analysis of intact human follicle-stimulating hormone (hFSH) preparations by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2006, 1136, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Wu, S.W.; Hsiao, H.H.; Khoo, K.H. Enabling techniques and strategic workflow for sulfoglycomics based on mass spectrometry mapping and sequencing of permethylated sulfated glycans. Glycobiology 2009, 19, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Green, E.D.; Baenziger, J.U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. J. Biol. Chem. 1988, 263, 25–35. [Google Scholar] [PubMed]
- Baenziger, J.U.; Green, E.D. Pituitary glycoprotein hormone oligosaccharides: Structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim. Biophys. Acta 1988, 947, 287–306. [Google Scholar] [CrossRef]
- Baenziger, J.U. Glycoprotein hormone GalNAc-4-sulphotransferase. Biochem. Soc. Trans. 2003, 31, 326–330. [Google Scholar] [CrossRef] [PubMed]

| N-Glycan 1 | Underivatized Mass (-H2O) (Da) | Relative Intensity of Each N-Glycan per Each Preparation (%) | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | R1 | R2 | R3 2 | ||
| (1) 0 | 892.3 | 2.0 | ||||
| (2) F1 | 1038.4 | 1.9 | ||||
| (3) M1 | 1054.4 | 3.7 | ||||
| (4) M2 | 1216.4 | 7.0 | ||||
| (5) N1F1 | 1241.5 | 2.7 | ||||
| (6) N1G1 | 1257.5 | 1.3 | ||||
| (7) N2 | 1298.5 | 0.4 | 0.8 | |||
| (8) N1Gn1(SO4) | 1378.4 | 2.0 | 5.1 | |||
| (9) M3 | 1378.5 | 9.0 | ||||
| (10) M1N1G1/M2N1 | 1419.5 | 0.9 | ||||
| (11) N2F1 | 1444.5 | 3.2 | 0.3 | 0.7 | ||
| (12) N2G1 | 1460.5 | 1.9 | 0.4 | 0.4 | 1.9 | 1.4 |
| (13) N1Gn1F1(SO4) | 1524.5 | 0.8 | 1.5 | |||
| (14) N1G1 Gn1(SO4) | 1540.5 | 2.2 | 5.2 | |||
| (15) M4 | 1540.5 | 1.5 | ||||
| (16) N1G1S1 | 1548.5 | 8.2 | 1.8 | |||
| (17) N2Gn1(SO4) | 1581.5 | 2.1 | 6.7 | |||
| (18) M2N1G1/N1M3 | 1581.6 | 0.8 | ||||
| (19) N2G1F1 | 1606.6 | 2.4 | 1.0 | 1.9 | ||
| (20) N2G2 | 1622.6 | 2.1 | 5.0 | 6.2 | 3.8 | |
| (21) N3F1 | 1647.6 | 2.0 | 1.8 | |||
| (22) M1N1Gn1F1(SO4) | 1686.6 | 0.7 | ||||
| (23) N1G1S1F1 | 1694.6 | 4.1 | 1.1 | |||
| (24) M2N1Gn1(SO4) | 1702.5 | 0.3 | 2.5 | |||
| (25) M1N1G1S1 | 1710.6 | 1.2 | 1.5 | |||
| (26) N2Gn1F1(SO4) | 1727.6 | 1.4 | ||||
| (27) N2G1Gn1(SO4) | 1743.6 | 1.8 | 3.6 | |||
| (28) N2G1S1 | 1751.6 | 1.3 | 0.5 | 3.1 | 12.3 | |
| (29) N2G2F1 | 1768.6 | 0.6 | 0.6 | 1.6 | ||
| (30) N2Gn2(SO4) | 1784.6 | 0.6 | ||||
| (31) N2G1Gn1F1 | 1809.7 | 1.5 | 0.8 | |||
| (32) N2Gn2(SO4)2 | 1864.6 | 3.4 | ||||
| (33) N2G1Gn1F1(SO4) | 1889.6 | 4.6 | ||||
| (34) N2G1S1F1 | 1897.7 | 2.7 | 1.0 | |||
| (35) N2G2S1 | 1913.7 | 1.9 | 0.9 | 36.1 | 26.3 | 21.4 |
| (36) N2Gn2F1(SO4) | 1930.7 | 0.3 | 1.6 | |||
| (37) N2G1Gn1F2 | 1955.7 | 1.8 | 0.8 | |||
| (38) N2G1Gn1S1 | 1970.7 | 1.6 | 0.7 | |||
| (39) N3G3 | 1987.7 | 2.4 | ||||
| (40) N2Gn2F1(SO4)2 | 2010.6 | 0.6 | 4.4 | |||
| (41) N3G2Gn1 | 2028.7 | 1.3 | ||||
| (42) N2G1Gn1S1(SO4) | 2034.7 | 14.0 | ||||
| (43) N2G2S1F1 | 2059.7 | 1.3 | 3.4 | 4.1 | 11.0 | |
| (44) N2G1Gn1S1F1 | 2100.7 | 5.5 | 4.2 | |||
| (45) N3G2F2 | 2117.8 | 0.5 | 1.0 | |||
| (46) N3G2Gn1F1 | 2174.8 | 1.4 | ||||
| (47) N2G1Gn1S1F1(SO4) | 2180.7 | 3.5 | ||||
| (48) N2G2S2 | 2204.8 | 10.0 | 14.1 | 31.9 | 31.2 | 0.6 |
| (49) N2G1Gn1S2 | 2245.8 | 2.6 | 2.3 | |||
| (50) N3G3S1 | 2278.8 | 2.7 | 3.7 | |||
| (51) N2G2S2F1 | 2350.8 | 1.9 | 2.1 | 5.3 | 4.8 | 1.5 |
| (52) N3G3S1F1 | 2424.9 | 0.5 | 1.1 | |||
| (53) N4G4F1 | 2498.9 | 1.0 | ||||
| (54) N3G2S2F1 | 2553.9 | 1.3 | 0.5 | |||
| (55) N3G3S2 | 2569.9 | 6.9 | 7.7 | 1.8 | ||
| (56) N4G3Gn1S1 | 2685.0 | 0.8 | ||||
| (57) N3G3S2F1 | 2716.0 | 1.5 | 1.6 | |||
| (58) N4G3Gn1S1F1 | 2831.0 | 1.0 | ||||
| (59) N3G3S3 | 2861.0 | 0.6 | 3.5 | 4.9 | 5.0 | |
| (60) N4G4S2 | 2935.0 | 2.6 | ||||
| (61) N3G3S3F1 | 3007.1 | 1.4 | 0.9 | 0.7 | 0.9 | 9.5 |
| (62) N4G4S3 | 3226.1 | 3.2 | ||||
| (63) N4G4S4 | 3517.2 | 6.7 | ||||
| (64) N4G4S4F1 | 3663.3 | 10.7 | ||||
| Fucosylated glycans | 36.0 | 35.2 | 11.9 | 13.5 | 37.9 | |
| Sialylated | 41.2 | 50.8 | 94.2 | 90.9 | 86.3 | |
| Sulfated | 13.7 | 55.7 | - | - | - | |
| Bi-antennary | 46.8 | 73.7 | 82.6 | 77.6 | 57.0 | |
| Triantennary | 4.7 | 6.5 | 16.5 | 20.9 | 18.7 | |
| Tetraantennary | - | 1.8 | - | - | 24.2 | |
| hTSH Preparation | Molecular Mass by MALDI-TOF-MS (Da) | Average Glycan Mass (Da) | Monosaccharide or Sulfate Moles/hTSH Mole | Carbohydrate Moiety (%) | Occupancy Occupied Sites | (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fuc | GalNAc | GlcNAc | Gal | Man | SA | Sulfate | |||||||
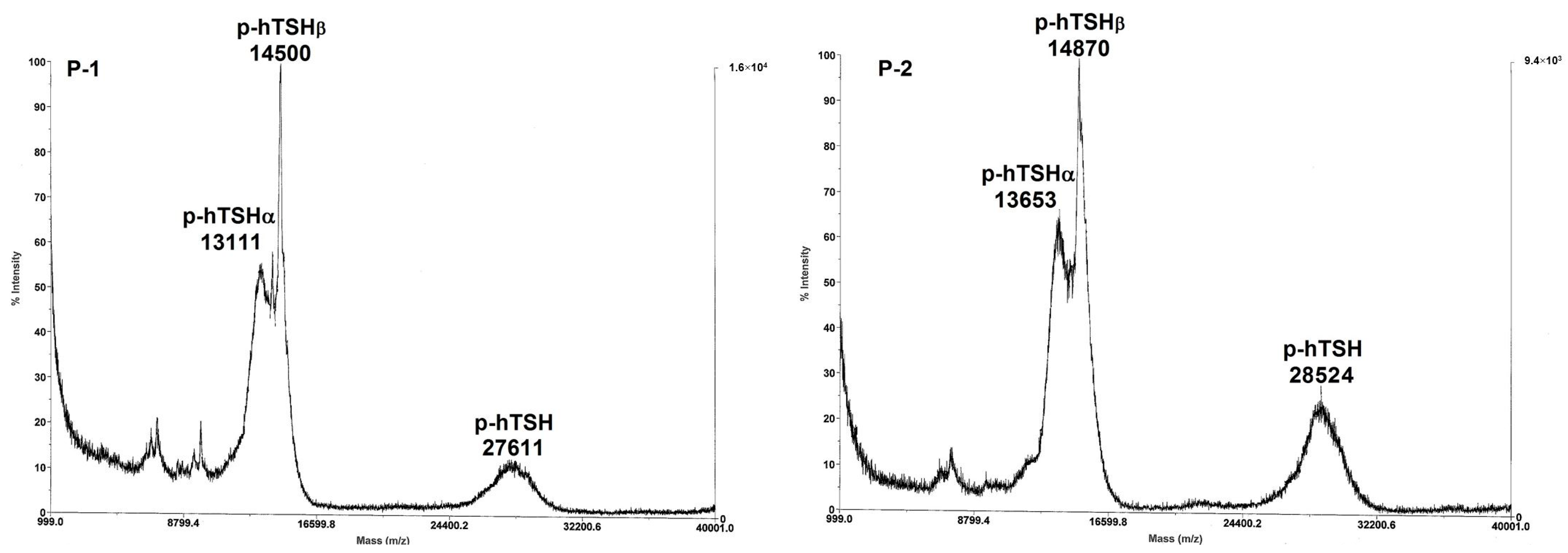
| P-1 | 27,611 | 1670.5 | 0.71 | 0.45 | 6.51 | 1.39 | 7.57 | 0.96 | 0.23 | 11.88 1 | (11.2) 2 | 1.96/3 | 65.3 |
| P-2 | 28,524 | 1931.4 | 0.77 | 1.84 | 8.59 | 1.98 | 6.84 | 1.41 | 1.54 | 14.70 1 | (14.3) 2 | 2.17/3 | 72.3 |
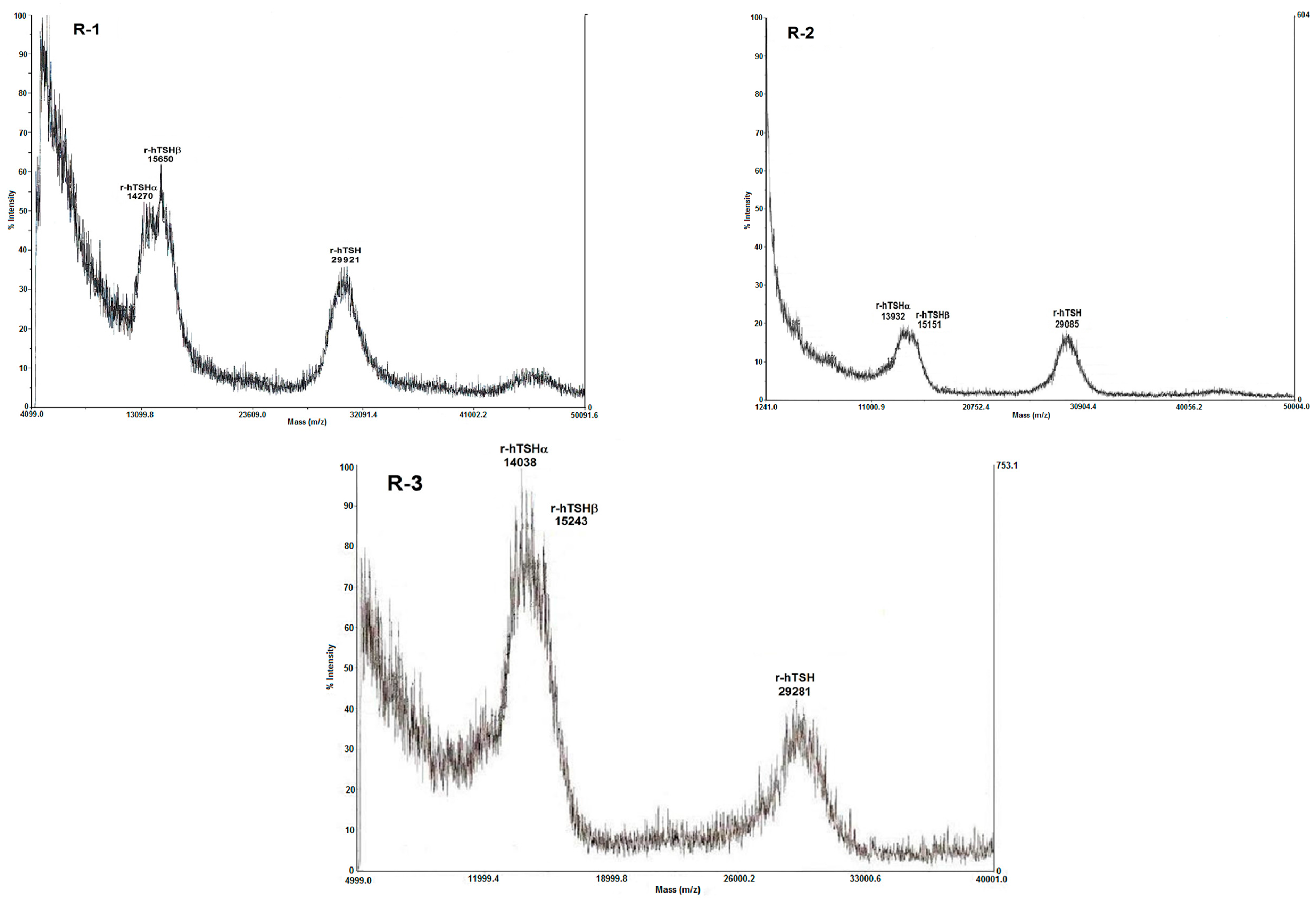
| R-1 | 29,921 | 2128.2 | 0.30 | 0 | 11.04 | 5.63 | 8.08 | 3.76 | 0 | 18.68 1 | (18.0) 2 | 2.63/3 3 | 87.7 |
| R-2 | 29,085 | 2137.6 | 0.30 | 0 | 9.43 | 4.72 | 6.85 | 3.13 | 0 | 16.35 1 | (16.5) 2 | 2.22/3 | 74.0 |
| R-3 | 29,281 | 2416.2 | 0.74 | 0 | 9.93 | 4.92 | 6.67 | 3.25 | 0 | 16.91 1 | (16.5) 2 | 2.05/3 | 68.3 |
| Preparation | T4 Level (µg/dL) |
|---|---|
| P-1 | 1.71 ± 0.13 |
| P-2 | 2.37 ± 0.64 |
| R-1 | 2.85 ± 0.34 |
| R-2 | 3.05 ± 0.70 |
| R-3 | 2.57 ± 0.64 |
| Preparation | No. of Assays | t1/2 1 (min) | Signif. Level | AUC 2 (µg∙min/mL) | Signif. 3 Level |
|---|---|---|---|---|---|
| P-1 | 2 | 56.5 ± 15.7 | p < 0.05 | 11,463 ± 682.0 | p < 0.05 |
| P-2 | 2 | 89.7 ± 0.42 | p < 0.05 | 14,270 ± 2147.1 | NS |
| R-1 | 3 | 115.1 ± 3.12 | - | 16,155 ± 1398.1 | - |
| R-2 | 3 | 105.5 ± 18.4 | NS | 15,555 ± 1197.8 | NS |
| R-3 | 3 | 114.5 ± 26.9 | NS | 16,976 ± 1720.0 | NS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribela, M.T.C.P.; Damiani, R.; Silva, F.D.; Lima, E.R.; Oliveira, J.E.; Peroni, C.N.; Torjesen, P.A.; Soares, C.R.; Bartolini, P. N-Glycoprofiling Analysis for Carbohydrate Composition and Site-Occupancy Determination in a Poly-Glycosylated Protein: Human Thyrotropin of Different Origins. Int. J. Mol. Sci. 2017, 18, 131. https://doi.org/10.3390/ijms18020131
Ribela MTCP, Damiani R, Silva FD, Lima ER, Oliveira JE, Peroni CN, Torjesen PA, Soares CR, Bartolini P. N-Glycoprofiling Analysis for Carbohydrate Composition and Site-Occupancy Determination in a Poly-Glycosylated Protein: Human Thyrotropin of Different Origins. International Journal of Molecular Sciences. 2017; 18(2):131. https://doi.org/10.3390/ijms18020131
Chicago/Turabian StyleRibela, Maria Teresa C. P., Renata Damiani, Felipe D. Silva, Eliana R. Lima, João E. Oliveira, Cibele N. Peroni, Peter A. Torjesen, Carlos R. Soares, and Paolo Bartolini. 2017. "N-Glycoprofiling Analysis for Carbohydrate Composition and Site-Occupancy Determination in a Poly-Glycosylated Protein: Human Thyrotropin of Different Origins" International Journal of Molecular Sciences 18, no. 2: 131. https://doi.org/10.3390/ijms18020131






