Joint Effects of PON1 Polymorphisms and Vegetable Intake on Ischemic Stroke: A Family-Based Case Control Study
Abstract
:1. Introduction
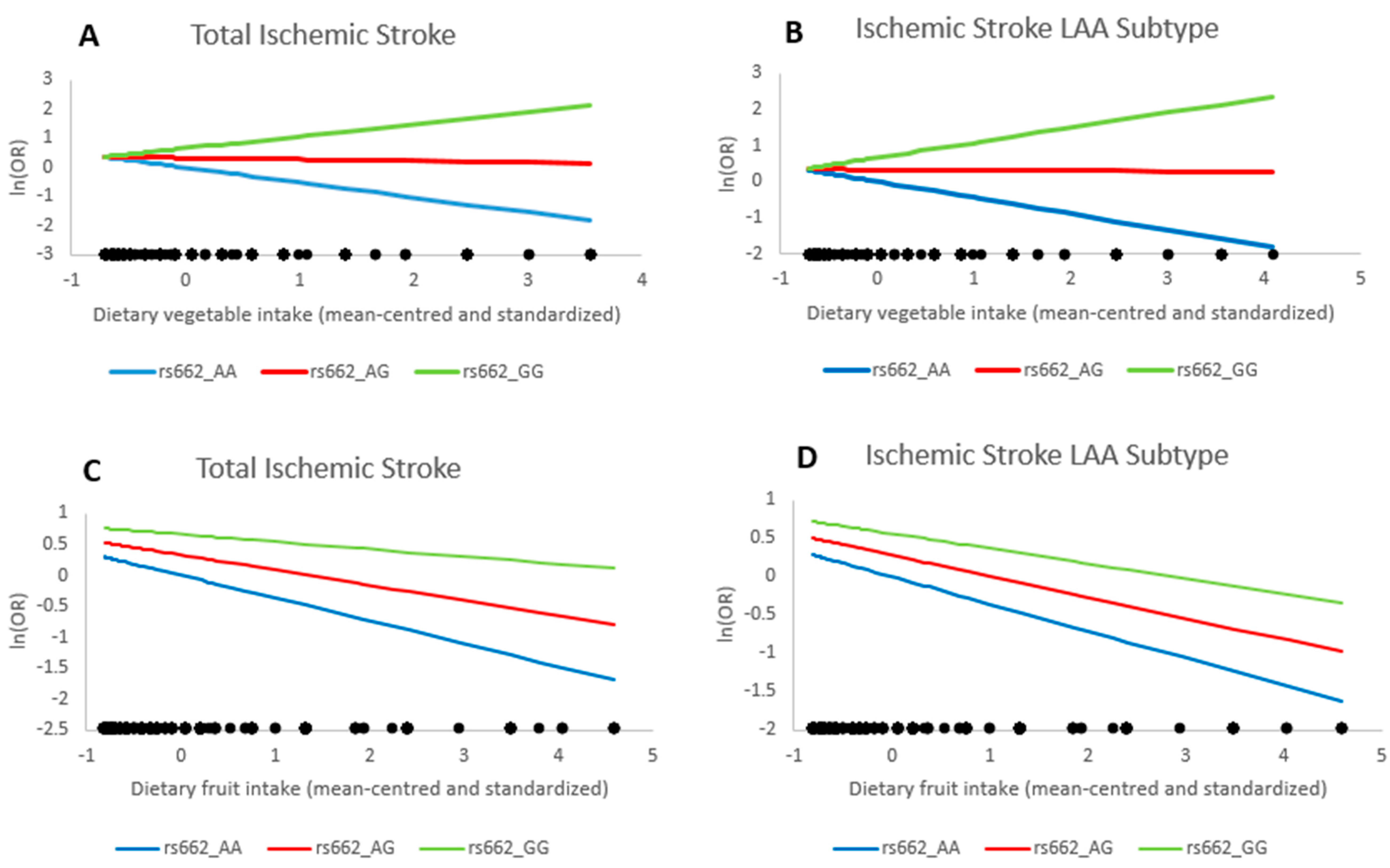
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Ascertainment of Ischemic Stroke Cases and IS-Free Controls
4.3. Assessment of Vegetable and Fruit Consumption
4.4. Genotyping
4.5. Assessment of Covariates
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Bonita, R. Epidemiology of stroke. Lancet 1992, 339, 342–344. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Furlong, C.E.; Suzuki, S.M.; Stevens, R.C.; Marsillach, J.; Richter, R.J.; Jarvik, G.P.; Checkoway, H.; Samii, A.; Costa, L.G.; Griffith, A.; et al. Human PON1, a biomarker of risk of disease and exposure. Chem.-Biol. Interact. 2010, 187, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Hart, K.; Roll, S.; Sperling, M.; Unruh, D.; Davidson, W.S.; Lindsell, C.J.; Adeoye, O. Apolipoprotein A-I and Paraoxonase-1 Are Potential Blood Biomarkers for Ischemic Stroke Diagnosis. J. Stroke Cerebrovasc. Dis. 2016, 25, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Chawhan, S.S.; Mogarekar, M.R.; Wagh, R.V.; Das, R.R.; Pramanik, S.S.; Sonune, S.M.; Chawhan, S.M. Relation of paraoxonase1, arylesterase and lipid profile in ischemic stroke patients. J. Clin. Diagn. Res. 2015, 9, Bc01–Bc03. [Google Scholar] [CrossRef] [PubMed]
- Michalak, S.; Kazmierski, R.; Hellmann, A.; Wysocka, E.; Kocialkowska-Adamczewska, D.; Wencel-Warot, A.; Nowinski, W.L. Serum paraoxonase/arylesterase activity affects outcome in ischemic stroke patients. Cerebrovasc. Dis. 2011, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008, 299, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef]
- Mackness, B.; Durrington, P.N.; Mackness, M.I. Human serum paraoxonase. Gen. Pharmacol. 1998, 31, 329–336. [Google Scholar] [CrossRef]
- Humbert, R.; Adler, D.A.; Disteche, C.M.; Hassett, C.; Omiecinski, C.J.; Furlong, C.E. The molecular basis of the human serum paraoxonase activity polymorphism. Nat. Genet. 1993, 3, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.G.; Richter, R.J.; Keifer, M.; Broomfield, C.A.; Sowalla, J.; Furlong, C.E. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 1996, 14, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Shenhar-Tsarfaty, S.; Waiskopf, N.; Ofek, K.; Shopin, L.; Usher, S.; Berliner, S.; Shapira, I.; Bornstein, N.M.; Ritov, Y.; Soreq, H.; et al. Atherosclerosis and arteriosclerosis parameters in stroke patients associate with paraoxonase polymorphism and esterase activities. Eur. J. Neurol. 2013, 20, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Dahabreh, I.J.; Kitsios, G.D.; Kent, D.M.; Trikalinos, T.A. Paraoxonase 1 polymorphisms and ischemic stroke risk: A systematic review and meta-analysis. Genet. Med. 2010, 12, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xia, P.; Liu, M.; Ji, X.M.; Sun, H.B.; Tao, L.; Mu, Q.W. PON gene polymorphisms and ischaemic stroke: A systematic review and meta analysis. Int. J. Stroke 2013, 8, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruit and vegetable consumption and risk of stroke: A meta-analysis of cohort studies. Neurology 2005, 65, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.P.; Overvad, K.; Stripp, C.; Tjonneland, A.; Husted, S.E.; Sorensen, H.T. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am. J. Clin. Nutr. 2003, 78, 57–64. [Google Scholar] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef]
- Ranade, K.; Kirchgessner, T.G.; Iakoubova, O.A.; Devlin, J.J.; DelMonte, T.; Vishnupad, P.; Hui, L.; Tsuchihashi, Z.; Sacks, F.M.; Sabatine, M.S.; et al. Evaluation of the paraoxonases as candidate genes for stroke: Gln192Arg polymorphism in the paraoxonase 1 gene is associated with increased risk of stroke. Stroke 2005, 36, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Morita, H.; Kurihara, H.; Sugiyama, T.; Kato, N.; Ebihara, A.; Hamada, C.; Kurihara, Y.; Shindo, T.; Oh-hashi, Y.; et al. Evidence for association between paraoxonase gene polymorphisms and atherosclerotic diseases. Atherosclerosis 2000, 149, 435–442. [Google Scholar] [CrossRef]
- Mahrooz, A.; Gohari, G.; Hashemi, M.B.; Zargari, M.; Musavi, H.; Abedini, M.; Alizadeh, A. R-carrying genotypes of serum paraoxonase (PON1) 192 polymorphism and higher activity ratio are related to susceptibility against ischemic stroke. Mol. Biol. Rep. 2012, 39, 11177–11185. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I. Relationship between Paraoxonase 1 (PON1) gene polymorphisms and susceptibility of stroke: A meta-analysis. Eur. J. Epidemiol. 2010, 25, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.J.; Chen, J.; Sun, Y.; Zheng, Z.J. Lack of association between the paraoxonase 1 Q/R192 single nucleotide polymorphism and stroke in a Chinese cohort. Acta Neurol. Belg. 2009, 109, 205–209. [Google Scholar] [PubMed]
- Zhang, G.; Li, W.; Li, Z.; Lv, H.; Ren, Y.; Ma, R.; Li, X.; Kang, X.; Shi, Y.; Sun, Y. Association between paraoxonase gene and stroke in the Han Chinese population. BMC Med. Genet. 2013, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, Y.H.; Yang, Q.D.; Xiao, B.; Ge, L.; Zhang, N.; Xia, J.; Zhang, L.; Liu, Z.J. Human serum paraoxonase gene polymorphisms, Q192R and L55M, are not associated with the risk of cerebral infarction in Chinese Han population. Neurol. Res. 2006, 28, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Mackness, M.I.; Arrol, S.; Turkie, W.; Durrington, P.N. Effect of the human serum paraoxonase 55 and 192 genetic polymorphisms on the protection by high density lipoprotein against low density lipoprotein oxidative modification. FEBS Lett. 1998, 423, 57–60. [Google Scholar] [CrossRef]
- Mackness, M.I.; Arrol, S.; Mackness, B.; Durrington, P.N. Alloenzymes of paraoxonase and effectiveness of high-density lipoproteins in protecting low-density lipoprotein against lipid peroxidation. Lancet 1997, 349, 851–852. [Google Scholar] [CrossRef]
- Mackness, M.I.; Mackness, B.; Durrington, P.N.; Connelly, P.W.; Hegele, R.A. Paraoxonase: Biochemistry, genetics and relationship to plasma lipoproteins. Curr. Opin. Lipidol. 1996, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.F.; Hornung, S.; Furlong, C.E.; Anderson, J.; Giblett, E.R.; Motulsky, A.G. Plasma paraoxonase polymorphism: A new enzyme assay, population, family, biochemical, and linkage studies. Am. J. Hum. Genet. 1983, 35, 393–408. [Google Scholar] [PubMed]
- Kim, N.S.; Kang, K.; Cha, M.H.; Kang, B.J.; Moon, J.; Kang, B.K.; Yu, B.C.; Kim, Y.S.; Choi, S.M.; Bang, O.S. Decreased paraoxonase-1 activity is a risk factor for ischemic stroke in Koreans. Biochem. Biophys. Res. Commun. 2007, 364, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Abbott, C.; Durrington, P.N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 1993, 104, 129–135. [Google Scholar] [CrossRef]
- Luu, H.N.; Kingah, P.L.; North, K.; Boerwinkle, E.; Volcik, K.A. Interaction of folate intake and the paraoxonase Q192R polymorphism with risk of incident coronary heart disease and ischemic stroke: The atherosclerosis risk in communities study. Ann. Epidemiol. 2011, 21, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Lixandru, D.; Mohora, M.; Coman, A.; Stoian, I.; van Gils, C.; Aerts, P.; Manuel, Y.K.B. Diet and paraoxonase 1 enzymatic activity in diabetic foot patients from Romania and Belgium: Favorable association of high flavonoid dietary intake with arylesterase activity. Ann. Nutr. Metab. 2010, 56, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Kaplan, M.; Rosenblat, M.; Fuhrman, B. Dietary antioxidants and paraoxonases against LDL oxidation and atherosclerosis development. Handb. Exp. Pharmacol. 2005, 170, 263–300. [Google Scholar]
- Jarvik, G.P.; Tsai, N.T.; McKinstry, L.A.; Wani, R.; Brophy, V.H.; Richter, R.J.; Schellenberg, G.D.; Heagerty, P.J.; Hatsukami, T.S.; Furlong, C.E. Vitamin C and E intake is associated with increased paraoxonase activity. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N.; Mackness, B.; Mackness, M.I. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Gabas-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A randomised controlled trial of increasing fruit and vegetable intake and how this influences the carotenoid concentration and activities of PON-1 and LCAT in HDL from subjects with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, D.; Del Bo, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of polyphenols and polyphenol-rich foods in the modulation of PON1 activity and expression. J. Nutr. Biochem. 2017, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leikert, J.F.; Rathel, T.R.; Wohlfart, P.; Cheynier, V.; Vollmar, A.M.; Dirsch, V.M. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 2002, 106, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Tsutsumi, T.; Nakamura, M.; Kitagawa, H.; Fujino, J.; Sasaki, K.; Toyoda, M.; Yoshida, T.; Maitani, T. Activation of the aryl hydrocarbon receptor by some vegetable constituents determined using in vitro reporter gene assay. Biol. Pharm. Bull. 2003, 26, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Gouedard, C.; Barouki, R.; Morel, Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol. Cell. Biol. 2004, 24, 5209–5222. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.P.; Daschner, P.J.; Yeh, G.C. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem. J. 1999, 340 Pt 3, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.M.; Lange, C. Family-based designs in the age of large-scale gene-association studies. Nat. Rev. Genet. 2006, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.H.; Sheehan, N.A.; Cox, A.; Worthington, J.; Cannings, C.; Teare, M.D. Family based studies and genetic epidemiology: Theory and practice. Hum. Hered. 2007, 64, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hu, Y.; Chen, D.; Zhan, S.; Zhang, Z.; Dou, H. the fangshan/family-based ischemic stroke study in China (FISSIC) protocol. BMC Med. Genet. 2007, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Meschia, J.F.; Brown, R.D., Jr.; Brott, T.G.; Chukwudelunzu, F.E.; Hardy, J.; Rich, S.S. The siblings with ischemic stroke study (SWISS) protocol. BMC Med. Genet. 2002, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.E.; Robins, M.; Weinfeld, F.D. The national survey of stroke. Clinical findings. Stroke 1981, 12 (2 Pt 2 Suppl. S1), I13–I44. [Google Scholar] [PubMed]
- Jones, W.J.; Williams, L.S.; Meschia, J.F. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke 2001, 32, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Meschia, J.F.; Lojacono, M.A.; Miller, M.J.; Brott, T.G.; Atkinson, E.J.; O'Brien, P.C. Reliability of the questionnaire for verifying stroke-free status. Cerebrovasc. Dis. 2004, 17, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.O.; Yang, G.; Jin, F.; Liu, D.; Kushi, L.; Wen, W.; Gao, Y.T.; Zheng, W. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. Eur. J. Clin. Nutr. 2004, 58, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.M.; Strader, L.C.; Pratt, J.G.; Maiese, D.; Hendershot, T.; Kwok, R.K.; Hammond, J.A.; Huggins, W.; Jackman, D.; Pan, H.; et al. The PhenX Toolkit: Get the most from your measures. Am. J. Epidemiol. 2011, 174, 253–260. [Google Scholar] [CrossRef] [PubMed]
| Ischemic Stroke Cases (n = 1007) | IS-Free Controls (n = 1151) | p-Value | |
|---|---|---|---|
| Age (years), mean (SD) | 59.3 (8.4) | 56.0 (9.2) | <0.001 |
| Male, % | 65.3 | 52.0 | <0.001 |
| BMI (kg/m2), mean (SD) | 26.3 (3.6) | 26.6 (11.2) | 0.59 |
| Smoking status, % | 0.006 | ||
| Never smoker, % | 40.1 | 51.8 | |
| Past smoker, % | 31.1 | 22.3 | |
| Current smoker, % | 28.8 | 25.9 | |
| Alcohol drinking, % | 0.90 | ||
| Never drinker, % | 55.6 | 59.2 | |
| Past drinker, % | 20.8 | 15.2 | |
| Current drinker, % | 23.6 | 25.6 | |
| Type 2 diabetes, % | 30.0 | 21.9 | <0.001 |
| Hypertension, % | 82.7 | 62.7 | <0.001 |
| fruit intake (g/day), mean (SD) | 178.9 (209.7) | 218.8 (242.7) | 0.004 |
| vegetable intake (g/day), mean (SD) | 664.7 (941.4) | 744.9 (915.2) | 0.11 |
| rs662, % | 0.008 | ||
| GG genotype | 35.9 | 39.8 | |
| AG genotype | 51.0 | 48.6 | |
| AA genotype | 13.1 | 11.6 |
| PON1 rs662_G | n | OR | 95% CI | p |
|---|---|---|---|---|
| Ischemic Stroke | 1007 | 1.28 | 1.07–1.54 | 0.008 |
| LAA | 709 | 1.32 | 1.06–1.64 | 0.013 |
| SAO | 185 | 1.46 | 0.96–2.22 | 0.076 |
| Other subtypes 1 | 113 | 0.80 | 0.41–1.53 | 0.495 |
| Model 1 1 | Model 2 2 | Model 3 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Fruit Intake | |||||||||
| Ischemic Stroke | |||||||||
| rs662 | 1.41 | 1.02–1.95 | 0.04 | 1.39 | 0.97–2.00 | 0.07 | 1.39 | 0.96–2.01 | 0.08 |
| Fruit intake | 0.66 | 0.50–0.89 | 0.006 | 0.69 | 0.51–0.94 | 0.02 | 0.69 | 0.50–0.96 | 0.03 |
| rs662 * Fruit intake | 1.13 | 0.89–1.43 | 0.31 | 1.13 | 0.88–1.46 | 0.34 | 1.15 | 0.89–1.49 | 0.29 |
| LAA | |||||||||
| rs662 | 1.42 | 0.99–2.02 | 0.06 | 1.33 | 0.89–1.98 | 0.17 | 1.31 | 0.87–1.98 | 0.19 |
| Fruit intake | 0.66 | 0.48–0.92 | 0.01 | 0.70 | 0.50–0.99 | 0.05 | 0.70 | 0.48–1.02 | 0.06 |
| rs662 * Fruit intake | 1.08 | 0.83–1.41 | 0.58 | 1.08 | 0.82–1.44 | 0.58 | 1.10 | 0.82–1.48 | 0.51 |
| Vegetable Intake | |||||||||
| Ischemic Stroke | |||||||||
| rs662 | 1.41 | 1.13–1.77 | 0.003 | 1.39 | 1.08–1.80 | 0.01 | 1.43 | 0.99–2.07 | 0.06 |
| Vegetable intake | 0.61 | 0.46–0.81 | 0.001 | 0.60 | 0.43–0.83 | 0.002 | 0.73 | 0.50–1.09 | 0.12 |
| rs662 * Vegetable intake | 1.57 | 1.19–2.08 | 0.001 | 1.59 | 1.14–2.21 | 0.006 | 1.46 | 0.90–2.35 | 0.12 |
| LAA | |||||||||
| rs662 | 1.46 | 1.13–1.88 | 0.004 | 1.39 | 1.04–1.86 | 0.03 | 1.33 | 0.88–2.01 | 0.17 |
| Vegetable intake | 0.64 | 0.47–0.87 | 0.004 | 0.64 | 0.45–0.91 | 0.01 | 0.85 | 0.57–1.28 | 0.43 |
| rs662 * Vegetable intake | 1.58 | 1.16–2.14 | 0.003 | 1.54 | 1.08–2.20 | 0.02 | 1.13 | 0.66–1.94 | 0.65 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan, J.; Jiang, X.; Tang, X.; Wu, Y.; Sun, K.; Xiang, X.; Tian, Y.; Wu, T.; Sun, Q.; Kraft, P.; et al. Joint Effects of PON1 Polymorphisms and Vegetable Intake on Ischemic Stroke: A Family-Based Case Control Study. Int. J. Mol. Sci. 2017, 18, 2652. https://doi.org/10.3390/ijms18122652
Juan J, Jiang X, Tang X, Wu Y, Sun K, Xiang X, Tian Y, Wu T, Sun Q, Kraft P, et al. Joint Effects of PON1 Polymorphisms and Vegetable Intake on Ischemic Stroke: A Family-Based Case Control Study. International Journal of Molecular Sciences. 2017; 18(12):2652. https://doi.org/10.3390/ijms18122652
Chicago/Turabian StyleJuan, Juan, Xia Jiang, Xun Tang, Yiqun Wu, Kexin Sun, Xiao Xiang, Yaohua Tian, Tao Wu, Qi Sun, Peter Kraft, and et al. 2017. "Joint Effects of PON1 Polymorphisms and Vegetable Intake on Ischemic Stroke: A Family-Based Case Control Study" International Journal of Molecular Sciences 18, no. 12: 2652. https://doi.org/10.3390/ijms18122652





