Probing Protein Glycation by Chromatography and Mass Spectrometry: Analysis of Glycation Adducts
Abstract
:1. Introduction
2. Methods of Amino Acid Analysis in Glycation Research
2.1. GC-MS Analysis of Free Glycated Amino Acids
2.2. Exhaustive Degradation of Proteins to Obtain Amino Acid Glycation Adducts
2.3. Analysis of Protein-Bound and Free Glycation Adducts by HPLC-ESI-MS
2.4. Mass Spectrometry in Detection of Glycated Adducts
3. Analysis of Glycation Adducts as a Diagnostic Tool
4. Analysis of Glycation Adducts in Foods
5. Analysis of Glycation Adducts in Glyoxalase Research
6. Further Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3-DG | 3-deoxyglucosone |
| 3-DG-H | 3-deoxyglucosone-derived hydroimidazolone |
| 3-DP | 3-deoxypentosone |
| ACN | acetonitrile |
| AGEs | advanced glycation end products |
| AM | ammonium formate |
| AP | argpyrimidine |
| aq. | aqueous |
| AQC | 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate |
| BAC | boronic acid affinity chromatography |
| BLG | β-lactoglobulin |
| BSA | bovine serum albumin |
| CAD | collision-activated dissociation |
| CEA | Nδ-(carboxyethyl)arginine |
| CEL | Nε-(carboxyethyl)lysine |
| CMA | Nδ-(carboxymethyl)arginine |
| CM-Ala | Nα-(carboxymethyl)alanine |
| CM-Gly | Nα-(carboxymethyl)glycine |
| CM-Ile | Nα-(carboxymethyl)isoleucine |
| CML | Nε-(carboxymethyl)lysine |
| CM-Leu | Nα-(carboxymethyl)leucine |
| CML-OH | Nε-(carboxymethyl)hydroxylysine |
| CM-Phe | Nα-(carboxymethyl)phenylalanine |
| CMPM | [(3-hydroxy-5-hydroxymethyl-2-methyl-pyridin-4-ylmethyl)amino]acetic acid |
| CM-Val | Nα-(carboxymethyl)valine |
| CXC | cation exchange chromatography |
| DM | diabetes mellitus |
| DOLD | 3-deoxyglucosone-derived lysine dimer |
| EI | electron (impact) ionization |
| ELBIA | enzyme-linked boronate-immunoassay |
| ELISA | enzyme-linked immunosorbent assay |
| ESI | electrospray ionization |
| Ex/Em | excitation/emission wavelengths |
| FA | formic acid |
| FFL | Nα-formyl-Nε-(fructosyl)lysine |
| FID | flame ionization detector |
| FIP-U | Fédération Internationale Pharmaceutique unit |
| FL | Nε-(fructosyl)lysine |
| Fmoc | 9-fluorenylmethoxycarbonyl |
| FT-MS | Fourier transform MS |
| GALA | Nε-(glycoloyl)lysine |
| GC-MS | gas chromatography–mass spectrometry |
| GFC | gel-filtration chromatography |
| GLAP | glyceraldehyde-derived pyridinium compound |
| Glarg | 1-(4-amino-4-carboxybutyl)-2-imino-5-oxo-imidazolidine, glyoxal-derived hydroimidazolone |
| GLO1 | glyoxalase-I |
| GLO2 | glyoxalase-II |
| GO | glyoxal |
| GODIC | 2-ammonio-6-([2-[(4-ammonio-5-oxido-5-oxopentyl)amino]-4,5-dihydro-1H-imidazol-5-ylidene]amino)-hexanoate |
| GOLA | Nε-[2-[(5-amino-5-carboxypentyl)amino]-2-oxoethyl]lysine |
| GOLD | glyoxal-derived lysine dimer |
| GSH | glutathione |
| HbA1C | glycated hemoglobin |
| HESI | heated electrospray ionization |
| HFBA | heptafluorobutyric acid |
| HILIC | hydrophilic interaction liquid chromatography |
| HPLC | high-performance liquid chromatography |
| HR-MS | high resolution MS |
| HSA | human serum albumin |
| i-but-OH | isobutanol |
| IHC | immunohistochemistry |
| IL-1α | interleukin 1α |
| IP-RPC | ion pair-reversed phase chromatography |
| IS | internal standard |
| IT | ion trap |
| LC | liquid chromatography |
| LOD | limit of detection |
| L-VDVA | Nα-(2,4-dinitro-5-fluorophenyl)-l-valinamide |
| MALDI | matrix assisted laser desorption/ionization |
| MCA | multichannel acquisition |
| MCP-1 | monocyte chemoattractant protein-1 |
| MeOH | methanol |
| MG-H | methylglyoxal-derived hydroimidazolone |
| MG-H1 | Nδ-(5-methyl-4-oxo-5-hydroimidazo-linone-2-yl)-l-ornithine |
| MG-H2 | 2-amino-5-(2-amino-5-hydro-5-methyl-4-imidazolon-1-yl)pentanoic acid |
| MG-H3 | 2-amino-5-(2-amino-4-hydro-4-methyl-5-imidazolon-1-yl)pentanoic acid |
| MGO | methylglyoxal |
| ML | Nε-(maltosyl)lysine |
| MODIC | 2-ammonio-6-([2-[(4-ammonio-5-oxido-5-oxopentyl)amino]-4-methyl-4,5-dihydro-1H-imidazol-5-ylidene]amino)hexanoate |
| MOLD | methylglyoxal-derived lysine dimer |
| MRM | multiple reaction monitoring |
| MS | mass spectrometry |
| MS/MS | tandem mass-spectrometry |
| NAL | Nε-(acetyl)lysine |
| NFL | Nε-(formyl)lysine |
| NFPA | nonafluoropentanoic acid |
| NHDNS | Natural History of Diabetic Nephropathy Study |
| NMR | nuclear magnetic resonance |
| ODS | octadecyl silica |
| OPA | o-phthaldialdehyde |
| PFP | pentafluorophenyl |
| PFPA | perfluoropentanoic acid |
| PICI | positive ion chemical ionization |
| PITC | phenylisothiocyanate |
| PU | papain units |
| Py-GC-MS | pyrolysis GC-MS |
| Q | quadrupole mass analyzer |
| QqQ | triple quadrupole |
| QqTOF | quadrupole-time of flight |
| RAGEs | receptors to advanced glycation end products |
| RP | reversed phase |
| SF | sector field |
| SIM | selected ion monitoring |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| TCA | trichloroacetic acid |
| TEA | trimethylamine |
| TFA | trifluoroacetic acid |
| TFAME | trifluoroacetyl methyl ester |
| THP | Nδ-(4-carboxy-4,6-dimethyl-5,6-dihydroxy-1,4,5,6-tetrahydropyrimidin-2-yl)-ornithine |
| TNF-α | tumor necrosis factor α |
| UHPLC | ultra-high performance liquid chromatography |
| UV | ultra-violet |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VIS | visual light |
| v/v | volume/volume |
| XIC | extracted ion chromatogram |
References
- Milkovska-Stamenova, S.; Schmidt, R.; Frolov, A.; Birkemeyer, C. GC-MS method for the quantitation of carbohydrate intermediates in glycation systems. J. Agric. Food Chem. 2015, 63, 5911–5919. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.E. The Amadori rearrangement. Adv. Carbohydr. Chem. 1955, 10, 169–205. [Google Scholar] [PubMed]
- Heyns, K.; Noack, H. Die Umsetzung von d-Fructose mit l-Lysin und l-Arginin und deren Beziehung zu nichtenzymatischen Bräunungsreaktion. Eur. J. Inorg. Chem. 1962, 95, 720–727. [Google Scholar]
- Ahmad, S.; Siddiqui, Z.; Rehman, S.; Khan, M.Y.; Khan, H.; Khanum, S.; Alouffi, S.; Saeed, M. A glycation angle to look into the diabetic vasculopathy: Cause and cure. Curr. Vasc. Pharmacol. 2017, 15, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, K.J.; Zyzak, D.V.; Litchfield, J.E.; Thorpe, S.R.; Baynes, J.W. Mechanism of autoxidative glycosylation: Identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 1995, 34, 3702–3709. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification. The potential role of “autoxidative glycosylation” in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Jahan, I.; Ng, R. Suppression of the accumulation of triosephosphates and increased formation of methylglyoxal in human red blood cells during hyperglycaemia by thiamine in vitro. J. Biochem. 2001, 129, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Araki, A. Oxidative stress and diabetes mellitus: A possible role of α-dicarbonyl compounds in free radical formation. Nihon Ronen Igakkai Zasshi Jpn. J. Geriatr. 1997, 34, 716–720. [Google Scholar]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Henning, C.; Smuda, M.; Girndt, M.; Ulrich, C.; Glomb, M.A. Molecular basis of Maillard amide-advanced glycation end product (AGE) formation in vivo. J. Biol. Chem. 2011, 286, 44350–44356. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products. Dermatoendocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Comazzi, S.; Bertazzolo, W.; Bonfanti, U.; Spagnolo, V.; Sartorelli, P. Advanced glycation end products and sorbitol in blood from differently compensated diabetic dogs. Res. Vet. Sci. 2008, 84, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sajithlal, G.B.; Chandrakasan, G. Role of lipid peroxidation products in the formation of advanced glycation end products: An in vitro study on collagen. Proc. Indian Acad. Sci.-Chem. Sci. 1999, 111, 215–229. [Google Scholar] [CrossRef]
- Smuda, M.; Henning, C.; Raghavan, C.T.; Johar, K.; Vasavada, A.R.; Nagaraj, R.H.; Glomb, M.A. Comprehensive analysis of Maillard protein modifications in human lenses: Effect of age and cataract. Biochemistry 2015, 54, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol. Metab. 2013, 3, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, U.; Rabbani, N.; Mullineaux, P.M.; Thornalley, P.J. Quantitative measurement of specific biomarkers for protein oxidation, nitration and glycation in Arabidopsis leaves. Plant J. 2009, 59, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Bilova, T.; Lukasheva, E.; Brauch, D.; Greifenhagen, U.; Paudel, G.; Tarakhovskaya, E.; Frolova, N.; Mittasch, J.; Balcke, G.U.; Tissier, A.; et al. A snapshot of the plant glycated proteome: Structural, functional, and mechanistic aspects. J. Biol. Chem. 2016, 291, 7621–7636. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, R.; Mironova, R.; Ivanov, I. Glycation of proteins in Escherichia coli: Effect of nutrient broth ingredients on glycation. Biotechnol. Biotechnol. Equip. 2004, 18, 99–103. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Sun, W.; Gao, X.; Sell, D.R.; Cleary, P.A.; Lachin, J.M.; Genuth, S.; DCCT/EDIC Research Group. Skin collagen advanced glycation endproducts (AGEs) and the long-term progression of sub-clinical cardiovascular disease in type 1 diabetes. Cardiovasc. Diabetol. 2015, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, H.J.; Fages, C.; Rauvala, H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-κB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J. Biol. Chem. 1999, 274, 19919–19924. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Jiang, H.; Ren, H.; Hu, X.; Wang, X.; Han, C. AGEs and chronic subclinical inflammation in diabetes: Disorders of immune system. Diabetes Metab. Res. Rev. 2015, 31, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Skrha, J. Pathogenesis of angiopathy in diabetes. Acta Diabetol. 2003, 40, S324–S329. [Google Scholar] [CrossRef] [PubMed]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. Fundamental role of pan-inflammation and oxidative-nitrosative pathways in neuropathogenesis of Alzheimer’s disease in focal cerebral ischemic rats. Am. J. Neurodegener. Dis. 2016, 5, 102–130. [Google Scholar] [PubMed]
- Chen, H.; O’Reilly, E.J.; Schwarzschild, M.A.; Ascherio, A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am. J. Epidemiol. 2008, 167, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Anitha, B.; Sampathkumar, R.; Balasubramanyam, M.; Rema, M. Advanced glycation index and its association with severity of diabetic retinopathy in type 2 diabetic subjects. J. Diabetes Complicat. 2008, 22, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Karachalias, N.; Babaei-Jadidi, R.; Ahmed, N.; Thornalley, P.J. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem. Soc. Trans. 2003, 31, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Yagihashi, S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann. N. Y. Acad. Sci. USA 2005, 1043, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Pascariu, M.; Ghitescu, L. Early glycation products of endothelial plasma membrane proteins in experimental diabetes. Biochim. Biophys. Acta 2006, 1762, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Abdullah, S.M.S.; Ahmad, S.; Fatma, S.; Baig, M.H.; Iqbal, J.; Madkhali, A.M.; Jerah, A.B.A. Prevalence of autoantibodies against 3-DG-glycated H2A protein in type 2 diabetes. Biochem. Biokhimiia 2017, 82, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Frimat, M.; Daroux, M.; Litke, R.; Nevière, R.; Tessier, F.J.; Boulanger, E. Kidney, heart and brain: Three organs targeted by ageing and glycation. Clin. Sci. 1979 2017, 131, 1069–1092. [Google Scholar] [CrossRef] [PubMed]
- Loomis, S.J.; Chen, Y.; Sacks, D.B.; Christenson, E.S.; Christenson, R.H.; Rebholz, C.M.; Selvin, E. Cross-sectional analysis of AGE-CML, sRAGE, and esRAGE with diabetes and cardiometabolic risk factors in a community-based cohort. Clin. Chem. 2017, 63, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Gandecka, A.; Nowicki, M.; Uruska, A.; Malińska, A.; Kowalska, K.; Wierusz-Wysocka, B.; Zozulińska-Ziółkiewicz, D. Association between small fiber neuropathy and higher skin accumulation of advanced glycation end products in patients with type 1 diabetes. Pol. Arch. Med. Wewn. 2016, 126, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Leone, A.; Raciti, G.A.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-Glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Thorpe, S.R.; Baynes, J.W. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J. Biol. Chem. 1986, 261, 4889–4894. [Google Scholar] [PubMed]
- Ahmed, M.U.; Brinkmann Frye, E.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997, 324, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, L.; Qi, H.; Wan, L.; Cai, P.; Xu, Z.; Li, B. Formation of peptide bound pyrraline in the Maillard model systems with different Lys-containing dipeptides and tripeptides. Molecules 2016, 21, 463. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Henle, T. Formyline, a new glycation compound from the reaction of lysine and 3-deoxypentosone. Eur. Food Res. Technol. 2010, 230, 903–914. [Google Scholar] [CrossRef]
- Usui, T.; Shimohira, K.; Watanabe, H.; Hayase, F. Detection and determination of glyceraldehyde-derived pyridinium-type advanced glycation end product in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2007, 71, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Glomb, M.A.; Pfahler, C. Amides are novel protein modifications formed by physiological sugars. J. Biol. Chem. 2001, 276, 41638–41647. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbolz, U.; Henle, T.; Haebner, R.; Klostermeyer, H. On the reaction of glyoxal with proteins. Z. Leb. -Forsch. A 1997, 205, 121–124. [Google Scholar] [CrossRef]
- Henle, T.; Walter, A.W.; Haessner, R.; Klostermeyer, H. Isolation to AGEs formed endogenously. Corresponding studies, and identification of a protein-bound imidazolone resulting from the reaction of arginine residues and methylglyoxal. Z. Lebensm. Unters. -Forsch. 1997, 204, 95–98. [Google Scholar] [CrossRef]
- Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.A.; Thornalley, P.J. Assay of advanced glycation endproducts (AGEs): Surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 2002, 364, 1–14. [Google Scholar] [PubMed]
- Glomb, M.A.; Lang, G. Isolation and characterization of glyoxal-arginine modifications. J. Agric. Food Chem. 2001, 49, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.; Hofmann, T. Chemoselective synthesis of peptides containing major advanced glycation end-products of lysine and arginine. Chem. Biol. Drug Des. 2005, 66, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Shipanova, I.N.; Glomb, M.A.; Nagaraj, R.H. Protein modification by methylglyoxal: Chemical nature and synthetic mechanism of a major fluorescent adduct. Arch. Biochem. Biophys. 1997, 344, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Oya, T.; Hattori, N.; Mizuno, Y.; Miyata, S.; Maeda, S.; Osawa, T.; Uchida, K. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J. Biol. Chem. 1999, 274, 18492–18502. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Monnier, V.M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J. Biol. Chem. 1989, 264, 21597–21602. [Google Scholar] [PubMed]
- Obayashi, H.; Nakano, K.; Shigeta, H.; Yamaguchi, M.; Yoshimori, K.; Fukui, M.; Fujii, M.; Kitagawa, Y.; Nakamura, N.; Nakamura, K.; et al. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem. Biophys. Res. Commun. 1996, 226, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakazawa, Y.; Ienaga, K. Acid-stable fluorescent advanced glycation end products: Vesperlysines A, B, and C are formed as crosslinked products in the Maillard reaction between lysine or proteins with glucose. Biochem. Biophys. Res. Commun. 1997, 232, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F.; Obrenovich, M.; Monnier, V.M. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 1999, 274, 20796–20804. [Google Scholar] [CrossRef] [PubMed]
- Frye, E.B.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J. Biol. Chem. 1998, 273, 18714–18719. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J. Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence. Biochem. J. 2002, 364, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Luévano-Contreras, C.; Gómez-Ojeda, A.; Macías-Cervantes, M.H.; Garay-Sevilla, M.E. Dietary advanced glycation end products and cardiometabolic risk. Curr. Diabetes Rep. 2017, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Grantham, C.E.; Hull, K.L.; Graham-Brown, M.P.M.; March, D.S.; Burton, J.O. The potential cardiovascular benefits of low-glucose degradation product, biocompatible peritoneal dialysis fluids: A review of the literature. Perit. Dial. Int. J. 2017, 37, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Aragno, M.; Mastrocola, R. Dietary sugars and endogenous formation of advanced glycation end products: Emerging mechanisms of disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.; Diamandis, E.P.; Giesbrecht, E.E.; Daneman, D.; Allen, L.C. An automated “high-pressure” liquid-chromatographic assay for hemoglobin A1c. Clin. Chem. 1984, 30, 1746–1752. [Google Scholar] [PubMed]
- Vidal, P.; Deckert, T.; Hansen, B.; Welinder, B.S. High-performance liquid chromatofocusing and column affinity chromatography of in vitro 14C-glycated human serum albumin. Demonstration of a glycation-induced anionic heterogeneity. J. Chromatogr. 1989, 476, 467–475. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Sakamoto, Y.; Kawasaki, Y.; Miyake, T.; Tanaka, K.; Urata, T.; Katayama, Y.; Ueda, S.; Horiuchi, S. Determination of glycated albumin by enzyme-linked boronate immunoassay (ELBIA). Clin. Chem. 1998, 44, 256–263. [Google Scholar] [PubMed]
- Miyake, T.; Tanaka, K. Enzyme reagent for detecting glycated proteins. J. Pharm. Health Care Sci. 1992, 28, 871–875. [Google Scholar]
- Floridi, A.; Trizza, V.; Paolotti, P.; Lucarelli, C. Analytical strategy for the assessment of the protein glycation status in uremic patients by high-performance liquid chromatography. J. Chromatogr. A 1999, 846, 65–71. [Google Scholar] [CrossRef]
- Resmi, H.; Akhunlar, H.; Temiz Artmann, A.; Güner, G. In vitro effects of high glucose concentrations on membrane protein oxidation, G-actin and deformability of human erythrocytes. Cell Biochem. Funct. 2005, 23, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, K.; Wróbel, K.; Garay-Sevilla, M.E.; Nava, L.E.; Malacara, J.M. Novel analytical approach to monitoring advanced glycosylation end products in human serum with on-line spectrophotometric and spectrofluorometric detection in a flow system. Clin. Chem. 1997, 43, 1563–1569. [Google Scholar] [PubMed]
- Sensi, M.; Pricci, F.; De Rossi, M.G.; Bruno, M.R.; Morano, S.; Capuozzo, E.; Di Mario, U. Formation and ways of detecting advanced glycation end-products in isolated human glomerular basement membrane and human serum albumin nonenzymatically glycated in vitro. J. Diabet. Complicat. 1989, 3, 88–91. [Google Scholar] [CrossRef]
- Makita, Z.; Vlassara, H.; Cerami, A.; Bucala, R. Immunochemical detection of advanced glycosylation end products in vivo. J. Biol. Chem. 1992, 267, 5133–5138. [Google Scholar] [PubMed]
- Miyata, S.; Monnier, V.M. Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J. Clin. Investig. 1992, 89, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Ashour, A.; Thornalley, P.J. Mass spectrometric determination of early and advanced glycation in biology. Glycoconj. J. 2016, 33, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Berning, K.; Quan, C.; Zhang, Y.T. Glycation of antibodies: Modification, methods and potential effects on biological functions. J. mAbs 2017, 9, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Rabbani, N. Detection of oxidized and glycated proteins in clinical samples using mass spectrometry—A user’s perspective. Biochim. Biophys. Acta 2014, 1840, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Nguyen, V.D.; Moschner, J.; Giannis, A.; Frolov, A.; Hoffmann, R. Sensitive and site-specific identification of carboxymethylated and carboxyethylated peptides in tryptic digests of proteins and human plasma. J. Proteome Res. 2015, 14, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Hoffmann, P.; Hoffmann, R. Fragmentation behavior of glycated peptides derived from d-glucose, d-fructose and d-ribose in tandem mass spectrometry. J. Mass Spectrom. JMS 2006, 41, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Monroe, M.E.; Schepmoes, A.A.; Clauss, T.R.W.; Gritsenko, M.A.; Meng, D.; Petyuk, V.A.; Smith, R.D.; Metz, T.O. Comprehensive identification of glycated peptides and their glycation motifs in plasma and erythrocytes of control and diabetic subjects. J. Proteome Res. 2011, 10, 3076–3088. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Böhme, D.; Singer, D.; Frolov, A. Specific tandem mass spectrometric detection of AGE-modified arginine residues in peptides. J. Mass Spectrom. JMS 2015, 50, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Charissou, A.; Ait-Ameur, L.; Birlouez-Aragon, I. Evaluation of a gas chromatography/mass spectrometry method for the quantification of carboxymethyllysine in food samples. J. Chromatogr. A 2007, 1140, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.B.; Poulsen, M.W.; Andersen, S.; Andersen, J.M.; Bak, M.J.; Ritz, C.; Holst, J.J.; Nielsen, J.; Courten, B. de; Dragsted, L.O.; et al. Consumption of a diet low in advanced glycation endproducts for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care 2013. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes—2016: Summary of revisions. Diabetes Care 2016, 39, S4–S5. [Google Scholar] [CrossRef]
- Bhat, S.; Jagadeeshaprasad, M.G.; Venkatasubramani, V.; Kulkarni, M.J. Abundance matters: Role of albumin in diabetes, a proteomics perspective. Expert Rev. Proteom. 2017. [Google Scholar] [CrossRef] [PubMed]
- Furusyo, N.; Hayashi, J. Glycated albumin and diabetes mellitus. Biochim. Biophys. Acta 2013, 1830, 5509–5514. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Quantitation of markers of protein damage by glycation, oxidation, and nitration in peritoneal dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2009, 29, S51–S56. [Google Scholar]
- Sternberg, Z.; Hennies, C.; Sternberg, D.; Wang, P.; Kinkel, P.; Hojnacki, D.; Weinstock-Guttmann, B.; Munschauer, F. Diagnostic potential of plasma carboxymethyllysine and carboxyethyllysine in multiple sclerosis. J. Neuroinflamm. 2010, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Börner, M.; Beer, F.; van Pée, K.-H.; Henle, T. Transformation of free and dipeptide-bound glycated amino acids by two strains of Saccharomyces cerevisiae. ChemBioChem 2017, 18, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Habib, S.; Khan, F.; Alam, K.; Ali, A. Structural changes in histone H2A by methylglyoxal generate highly immunogenic amorphous aggregates with implications in auto-immune response in cancer. Glycobiology 2016, 26, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Slight, S.H.; Prabhakaram, M.; Shin, D.B.; Feather, M.S.; Ortwerth, B.J. The extent of Nε-(carboxymethyl)lysine formation in lens proteins and polylysine by the autoxidation products of ascorbic acid. Biochim. Biophys. Acta 1992, 1117, 199–206. [Google Scholar] [CrossRef]
- Dunn, J.A.; McCance, D.R.; Thorpe, S.R.; Lyons, T.J.; Baynes, J.W. Age-dependent accumulation of Nε-(carboxymethyl)lysine and Nε-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry (Mosc.) 1991, 30, 1205–1210. [Google Scholar] [CrossRef]
- Badoud, R.; Fay, L.B. Mass spectrometric analysis of N-carboxymethylamino acids as periodate oxidation derivatives of Amadori compounds application to glycosylated haemoglobin. Amino Acids 1993, 5, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, T.P.; Grass, L.; Reddy, S.; Thorpe, S.R.; Diamandis, E.P.; Baynes, J.W. Technical note. The serum concentration of the advanced glycation end-product N epsilon-(carboxymethyl)lysine is increased in uremia. Kidney Int. 1997, 52, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Gerhardinger, C.; Baldo, L.; Fedele, D.; Favretto, D.; Seraglia, R.; Traldi, P. Pyrolysis/gas chromatography/mass spectrometry in the analysis of glycated poly-l-lysine. Org. Mass Spectrom. 1992, 27, 183–187. [Google Scholar] [CrossRef]
- Lapolla, A.; Gerhardinger, C.; Baldo, L.; Crepaldi, G.; Fedele, D.; Favretto, D.; Seraglia, R.; Curcuruto, O.; Traldi, P. Pyrolysis—Gas chromatography/mass spectrometry in the characterization of glycated albumin. J. Anal. Appl. Pyrolysis 1992, 24, 87–103. [Google Scholar] [CrossRef]
- Vinale, F.; Fogliano, V.; Schieberle, P.; Hofmann, T. Development of a stable isotope dilution assay for an accurate quantification of protein-bound Nε-(1-deoxy-d-fructos-1-yl)-l-lysine using a 13C-labeled internal standard. J. Agric. Food Chem. 1999, 47, 5084–5092. [Google Scholar] [CrossRef] [PubMed]
- Lederer, M.O.; Klaiber, R.G. Cross-linking of proteins by Maillard processes: Characterization and detection of lysine-arginine cross-links derived from glyoxal and methylglyoxal. Bioorg. Med. Chem. 1999, 7, 2499–2507. [Google Scholar] [CrossRef]
- Chevalier, F.; Chobert, J.-M.; Dalgalarrondo, M.; Haertlé, T. Characterization of the Maillard reaction products of β-lactoglobulin glucosylated in mild conditions. J. Food Biochem. 2001, 25, 33–55. [Google Scholar] [CrossRef]
- Portero-Otin, M.; Nagaraj, R.H.; Monnier, V.M. Chromatographic evidence for pyrraline formation during protein glycation in vitro and in vivo. Biochim. Biophys. Acta 1995, 1247, 74–80. [Google Scholar] [CrossRef]
- Hartkoph, J.; Pahlke, C.; Lüdemann, G.; Erbersdobler, H.F. Determination of Nε-carboxymethyllysine by a reversed-phase high-performance liquid chromatography method. J. Chromatogr. A 1994, 672, 242–246. [Google Scholar] [CrossRef]
- Hellwig, M.; Witte, S.; Henle, T. Free and protein-bound Maillard reaction products in beer: Method development and a survey of different beer types. J. Agric. Food Chem. 2016, 64, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Shirakawa, J.-I.; Ohno, R.-I.; Shinagawa, M.; Hatano, K.; Sugawa, H.; Arakawa, S.; Furusawa, C.; Nagai, M.; Nagai, R. Soft-shelled turtle eggs inhibit the formation of AGEs in the serum and skin of diabetic rats. J. Clin. Biochem. Nutr. 2016, 58, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D.; Fiore, A.; Roviello, G.; Monti, S.M.; Fogliano, V. Simultaneous quantification of amino acids and Amadori products in foods through ion-pairing liquid chromatography-high-resolution mass spectrometry. Amino Acids 2015, 47, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wellner, A.; Huettl, C.; Henle, T. Formation of Maillard reaction products during heat treatment of carrots. J. Agric. Food Chem. 2011, 59, 7992–7998. [Google Scholar] [CrossRef] [PubMed]
- Delatour, T.; Hegele, J.; Parisod, V.; Richoz, J.; Maurer, S.; Steven, M.; Buetler, T. Analysis of advanced glycation endproducts in dairy products by isotope dilution liquid chromatography-electrospray tandem mass spectrometry. The particular case of carboxymethyllysine. J. Chromatogr. A 2009, 1216, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Hegele, J.; Buetler, T.; Delatour, T. Comparative LC-MS/MS profiling of free and protein-bound early and advanced glycation-induced lysine modifications in dairy products. Anal. Chim. Acta 2008, 617, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hegele, J.; Parisod, V.; Richoz, J.; Förster, A.; Maurer, S.; Krause, R.; Henle, T.; Bütler, T.; Delatour, T. Evaluating the extent of protein damage in dairy products: Simultaneous determination of early and advanced glycation-induced lysine modifications. Ann. N. Y. Acad. Sci. 2008, 1126, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Frolov, A.; Hoffmann, R. Oxidative degradation of Nε-fructosylamine-substituted peptides in heated aqueous systems. Amino Acids 2015, 47, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Bassette, R. Detection of intermediate compounds in the early stages of browning reaction in milk products. J. Dairy Sci. 1959, 42, 945–960. [Google Scholar] [CrossRef]
- Heyns, K.; Heukeshoven, J.; Brose, K.-H. Der Abbau von Fructose-Aminosäuren zu N-(2-Furoylmethyl)-Aminosäuren. Zwischenprodukte der Bräunungsreaktionen. Angew. Chem. 1968, 80, 627. [Google Scholar] [CrossRef]
- Finot, P.A.; Bricout, J.; Viani, R.; Mauron, J. Identification of a new lysine derivative obtained upon acid hydrolysis of heated milk. Experientia 1968, 24, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Erbersdobler, H.F.; Zucker, H. Untersuchungen zum Gehalt an Lysin und verfügbarem Lysin in Trockenmagermilch. Milchwissenschaft 1966, 21, 564–568. [Google Scholar]
- Erbersdobler, H.F.; Somoza, V. Forty years of furosine-forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol. Nutr. Food Res. 2007, 51, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D.; Fiore, A.; Wiltafsky, M.; Fogliano, V. Quantification of Nε-(2-Furoylmethyl)-l-lysine (furosine), Nε-(Carboxymethyl)-l-lysine (CML), Nε-(Carboxyethyl)-l-lysine (CEL) and total lysine through stable isotope dilution assay and tandem mass spectrometry. Food Chem. 2015, 188, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Rückriemen, J.; Sandner, D.; Henle, T. Unique pattern of protein-bound Maillard reaction products in manuka (Leptospermum scoparium) honey. J. Agric. Food Chem. 2017, 65, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Murata, M.; Takahara, H.; Irie, S.; Fujimoto, D. Identification of Nω-carboxymethylarginine as a novel acid-labile advanced glycation end product in collagen. Biochem. J. 2000, 347, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Schmidt, R.; Spiller, S.; Greifenhagen, U.; Hoffmann, R. Arginine-derived advanced glycation end products generated in peptide-glucose mixtures during boiling. J. Agric. Food Chem. 2014, 62, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Henle, T.; Walter, H.; Klostermeyer, H. Evaluation of the extent of the early Maillard-reaction in milk products by direct measurement of the Amadori-product lactuloselysine. Z. Lebensm. Unters. -Forsch. 1991, 193, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Van de Merbel, N.C.; Mentink, C.J.A.L.; Hendriks, G.; Wolffenbuttel, B.H.R. Liquid chromatographic method for the quantitative determination of Nepsilon-carboxymethyllysine in human plasma proteins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 808, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, Q.; Jin, C.; Cheng, L.; Zheng, X.; Dai, M.; Zhang, Y. Simultaneous analysis of Nε-(carboxymethyl)lysine and Nε-(carboxyethyl)lysine in foods by ultra-performance liquid chromatography-mass spectrometry with derivatization by 9-fluorenylmethyl chloroformate. J. Food Sci. 2015, 80, C207–C217. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Hanke, T.; Simon, P.; Born, R.; Fischer, C.; Frolov, A.; Langrock, T.; Hoffmann, R.; Schwarzenbolz, U.; Henle, T.; et al. Carboxymethylation of the fibrillar collagen with respect to formation of hydroxyapatite. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Hanke, T.; Frolov, A.; Langrock, T.; Hoffmann, R.; Fischer, C.; Schwarzenbolz, U.; Henle, T.; Born, R.; Worch, H. Modification of collagen in vitro with respect to formation of Nepsilon-carboxymethyllysine. Int. J. Biol. Macromol. 2009, 44, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, C.; Iwaihara, Y.; Chen, S.J.; Tanaka, M.; Watanabe, T.; Matsui, T. Highly-sensitive detection of free advanced glycation end-products by liquid chromatography-electrospray ionization-tandem mass spectrometry with 2,4,6-trinitrobenzene sulfonate derivatization. Anal. Chem. 2013, 85, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- Penndorf, I.; Li, C.; Schwarzenbolz, U.; Henle, T. N-terminal glycation of proteins and peptides in foods and in vivo: Evaluation of N-(2-furoylmethyl)valine in acid hydrolyzates of human hemoglobin. Ann. N. Y. Acad. Sci. 2008, 1126, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Singer, D.; Hoffmann, R. Solid-phase synthesis of glucose-derived Amadori peptides. J. Pept. Sci. 2007, 13, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Hoffmann, R. Separation of Amadori peptides from their unmodified analogs by ion-pairing RP-HPLC with heptafluorobutyric acid as ion-pair reagent. Anal. Bioanal. Chem. 2008, 392, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.; Liehr, K.; Henning, C.; Fiedler, R.; Girndt, M.; Gebert, M.; Hulko, M.; Storr, M.; Glomb, M.A. Detection of free advanced glycation end products in vivo during hemodialysis. J. Agric. Food Chem. 2017, 65, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, C.T.; Smuda, M.; Smith, A.J.O.; Howell, S.; Smith, D.G.; Singh, A.; Gupta, P.; Glomb, M.A.; Wormstone, I.M.; Nagaraj, R.H. AGEs in human lens capsule promote the TGFβ2-mediated EMT of lens epithelial cells: Implications for age-associated fibrosis. Aging Cell 2016, 15, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakete, S.; Klaus, A.; Glomb, M.A. Investigations on the Maillard reaction of dextrins during aging of Pilsner type beer. J. Agric. Food Chem. 2014, 62, 9876–9884. [Google Scholar] [CrossRef] [PubMed]
- Haucke, E.; Navarrete Santos, A.; Simm, A.; Henning, C.; Glomb, M.A.; Gürke, J.; Schindler, M.; Fischer, B.; Navarrete Santos, A. Accumulation of advanced glycation end products in the rabbit blastocyst under maternal diabetes. Reprod. Camb. Engl. 2014, 148, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Reneker, L.W.; Obrenovich, M.E.; Strauch, C.; Cheng, R.; Jarvis, S.M.; Ortwerth, B.J.; Monnier, V.M. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 16912–16917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, G.; Xiao, L.; Mitchell, A.E. Determination of advanced glycation endproducts by LC-MS/MS in raw and roasted almonds (Prunus dulcis). J. Agric. Food Chem. 2011, 59, 12037–12046. [Google Scholar] [CrossRef] [PubMed]
- Nomi, Y.; Annaka, H.; Sato, S.; Ueta, E.; Ohkura, T.; Yamamoto, K.; Homma, S.; Suzuki, E.; Otsuka, Y. Simultaneous quantitation of advanced glycation end products in soy sauce and beer by liquid chromatography-tandem mass spectrometry without ion-pair reagents and derivatization. J. Agric. Food Chem. 2016, 64, 8397–8405. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Babaei-Jadidi, R.; Howell, S.K.; Beisswenger, P.J.; Thornalley, P.J. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia 2005, 48, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5287–5292. [Google Scholar] [CrossRef]
- Anwar, A.; Marini, M.; Abruzzo, P.M.; Bolotta, A.; Ghezzo, A.; Visconti, P.; Thornalley, P.J.; Rabbani, N. Quantitation of plasma thiamine, related metabolites and plasma protein oxidative damage markers in children with autism spectrum disorder and healthy controls. Free Radic. Res. 2016, 50, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Thornalley, P.J.; Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 2016, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Costa, M.L.; Mackay, N.; Filer, A.; Raza, K.; Watts, R.A.; Winyard, P.G.; Tarr, J.; et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci. Rep. 2015, 5, 9259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbani, N.; Shaheen, F.; Anwar, A.; Masania, J.; Thornalley, P.J. Assay of methylglyoxal-derived protein and nucleotide AGEs. Biochem. Soc. Trans. 2014, 42, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Agalou, S.; Ahmed, N.; Babaei-Jadidi, R.; Dawnay, A.; Thornalley, P.J. Profound mishandling of protein glycation degradation products in uremia and dialysis. J. Am. Soc. Nephrol. 2005, 16, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Engelbrecht, B.; Espelage, B.C.; Klusmeier, N.; Tiemann, J.; Gawlowski, T.; Mattern, Y.; Eisenacher, M.; Meyer, H.E.; Rabbani, N.; et al. Glyoxalase 1-knockdown in human aortic endothelial cells-effect on the proteome and endothelial function estimates. Sci. Rep. 2016, 6, 37737. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl proteome and genome damage in metabolic and vascular disease. Biochem. Soc. Trans. 2014, 42, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Deyl, Z.; Miksík, I. Post-translational non-enzymatic modification of proteins. I. Chromatography of marker adducts with special emphasis to glycation reactions. J. Chromatogr. B Biomed. Sci. Appl. 1997, 699, 287–309. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Russell, G.; Miller, M.E.; Rich, S.S.; Mauer, M. Detection of diabetic nephropathy from advanced glycation end products (AGEs) differs in plasma and urine, and is dependent on the method of preparation. Amino Acids 2014, 46, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem. Soc. Trans. 2003, 31, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbolz, U.; Mende, S.; Henle, T. Model studies on protein glycation: Influence of cysteine on the reactivity of arginine and lysine residues toward glyoxal. Ann. N. Y. Acad. Sci. 2008, 1126, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Chernushevich, I.V.; Loboda, A.V.; Thomson, B.A. An introduction to quadrupole-time-of-flight mass spectrometry. J. Mass Spectrom. 2001, 36, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, R.A.; Makarov, A. Orbitrap mass spectrometry. Anal. Chem. 2013, 85, 5288–5296. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Fiehn, O. Advances in structure elucidation of small molecules using mass spectrometry. Bioanal. Rev. 2010, 2, 23–60. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Frolov, A.; Hoffmann, R. Fragmentation behavior of Amadori-peptides obtained by non-enzymatic glycosylation of lysine residues with ADP-ribose in tandem mass spectrometry. J. Mass Spectrom. JMS 2010, 45, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Spiller, S.; Frolov, A.; Hoffmann, R. Quantification of specific glycation sites in human serum albumin as prospective type 2 diabetes mellitus biomarkers. Protein Pept. Lett. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Bennett, B.D.; Rabinowitz, J.D. Analytical strategies for LC-MS-based targeted metabolomics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 236–242. [Google Scholar] [CrossRef] [PubMed]
- MacNair, J.E.; Lewis, K.C.; Jorgenson, J.W. Ultrahigh-pressure reversed-phase liquid chromatography in packed capillary columns. Anal. Chem. 1997, 69, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Chawla, G.; Ranjan, C. Principle, instrumentation, and applications of UPLC: A novel technique of liquid chromatography. Open Chem. J. 2016. [Google Scholar] [CrossRef]
- Mittasch, J.; Böttcher, C.; Frolov, A.; Strack, D.; Milkowski, C. Reprogramming the phenylpropanoid metabolism in seeds of oilseed rape by suppressing the orthologs of reduced epidermal fluorescence1. Plant Physiol. 2013, 161, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Modzel, M.; Didio, A.; Płóciennik, H.; Kijewska, M.; Grischina, T.; Karonova, T.; Bilova, T.; Stefanov, V.; Stefanowicz, P.; et al. Quantification of prospective type 2 diabetes mellitus biomarkers by stable isotope dilution with bi-labeled standard glycated peptides. Anal. Methods 2017, 9, 409–418. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mirshekar-Syahkal, B.; Kennish, L.; Karachalias, N.; Babaei-Jadidi, R.; Thornalley, P.J. Assay of advanced glycation endproducts in selected beverages and food by liquid chromatography with tandem mass spectrometric detection. Mol. Nutr. Food Res. 2005, 49, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T.; Barto, R.; Ten Brink, H.J.; Schalkwijk, C.G. Measurement of Nε-(carboxymethyl)lysine and Nε-(carboxyethyl)lysine in human plasma protein by stable-isotope-dilution tandem mass spectrometry. Clin. Chem. 2004, 50, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Ahmed, N.; Song, L.; Eboigbodin, K.E.; Thornalley, P.J. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes 2006, 55, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Clelland, J.D.; Thornalley, P.J. Synthesis of 14C-labelled methylglyoxal and S-d-lactoylglutathione. J. Label. Compd. Radiopharm. 1990, 28, 1455–1464. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Nedić, O.; Rattan, S.I.S.; Grune, T.; Trougakos, I.P. Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic. Res. 2013, 47 (Suppl. S1), 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kouidrat, Y.; Amad, A.; Arai, M.; Miyashita, M.; Lalau, J.-D.; Loas, G.; Itokawa, M. Advanced glycation end products and schizophrenia: A systematic review. J. Psychiatr. Res. 2015, 66–67, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.-Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y.; Ko, H.-A.; Chu, K.-H.; Shieh, T.-M.; Chi, T.-C.; Chen, H.-I.; Chang, W.-C.; Chang, S.-S. The possible mechanism of advanced glycation end products (AGEs) for Alzheimer’s disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vicente Miranda, H.; Outeiro, T.F. The sour side of neurodegenerative disorders: The effects of protein glycation. J. Pathol. 2010, 221, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Ahmad, S.; Choi, I.; Ahmad, N.; Farhan, M.; Tatyana, G.; Shahab, U. Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches. IUBMB Life 2015, 67, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Kase, S.; Ishida, S.; Rao, N.A. Immunolocalization of advanced glycation end products in human diabetic eyes: An immunohistochemical study. J. Diabetes Mellit. 2011, 1, 57. [Google Scholar] [CrossRef]
- De Vos, L.C.; Lefrandt, J.D.; Dullaart, R.P.F.; Zeebregts, C.J.; Smit, A.J. Advanced glycation end products: An emerging biomarker for adverse outcome in patients with peripheral artery disease. Atherosclerosis 2016, 254, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Nickeleit, V.; James, L.R.; Maeda, N. α-Lipoic acid protects diabetic apolipoprotein E-deficient mice from nephropathy. J. Diabetes Complicat. 2011, 25, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Min, D.; Twigg, S.M.; Shackel, N.A.; Warner, F.J.; Yue, D.K.; McLennan, S.V. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: Possible role in diabetic complications. Am. J. Physiol. Cell Physiol. 2010, 299, C1212–C1219. [Google Scholar] [CrossRef] [PubMed]
- Zuwała-Jagiełło, J.; Pazgan-Simon, M.; Simon, K.; Warwas, M. Advanced oxidation protein products and inflammatory markers in liver cirrhosis: A comparison between alcohol-related and HCV-related cirrhosis. Acta Biochim. Pol. 2011, 58, 59–65. [Google Scholar] [PubMed]
- Škrha, J.; Šoupal, J.; Prázný, M.; Škrha, J. Glycation of lens proteins in diabetes and its non-invasive assessment-first experience in the Czech Republic. Vnitr. Lek. 2015, 61, 346–350. [Google Scholar] [PubMed]
- Januszewski, A.S.; Sachithanandan, N.; Karschimkus, C.; O’Neal, D.N.; Yeung, C.K.; Alkatib, N.; Jenkins, A.J. Non-invasive measures of tissue autofluorescence are increased in Type 1 diabetes complications and correlate with a non-invasive measure of vascular dysfunction. Diabet. Med. J. Br. Diabet. Assoc. 2012, 29, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, R.; Kapsokalyvas, D.; De Giorgi, V.; Maio, V.; Van Wiechen, A.; Massi, D.; Lotti, T.; Pavone, F.S. Scoring of collagen organization in healthy and diseased human dermis by multiphoton microscopy. J. Biophotonics 2010, 3, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; van de Waarenburg, M.P.; Stehouwer, C.D.; Schalkwijk, C.G. Measurement of pentosidine in human plasma protein by a single-column high-performance liquid chromatography method with fluorescence detection. J. Chromatogr. B 2009, 877, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; Schalkwijk, C.G. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: Evaluation of blood specimen. Clin. Chem. Lab. Med. 2014, 52, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yuzawa, H.; Nohara, I.; Ohnishi, T.; Obata, N.; Iwayama, Y.; Haga, S.; Toyota, T.; Ujike, H.; Arai, M.; et al. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch. Gen. Psychiatry 2010, 67, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R.; Naudí, A.; Gavín, R.; Pastrana, M.A.; Sajnani, G.; Ilieva, E.V.; Del Río, J.A.; Portero-Otín, M.; Ferrer, I.; Requena, J.R. Increased oxidation, glycoxidation, and lipoxidation of brain proteins in prion disease. Free Radic. Biol. Med. 2008, 45, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Jaisson, S.; Souchon, P.-F.; Desmons, A.; Salmon, A.-S.; Delemer, B.; Gillery, P. Early formation of serum advanced glycation end-products in children with type 1 diabetes mellitus: Relationship with glycemic control. J. Pediatr. 2016, 172, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Arai, M.; Kobori, A.; Ichikawa, T.; Toriumi, K.; Niizato, K.; Oshima, K.; Okazaki, Y.; Yoshikawa, T.; Amano, N.; et al. Clinical features of schizophrenia with enhanced carbonyl stress. Schizophr. Bull. 2014, 40, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem. Soc. Trans. 2014, 42, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, Q.-D.; Zhu, L.; Peppa, M.; He, C.; Vlassara, H. Oxidative stress-inducing carbonyl compounds from common foods: Novel mediators of cellular dysfunction. Mol. Med. 2002, 8, 337–346. [Google Scholar] [PubMed]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Goodman, S.; Pyzik, R.; Yong, A.; Chen, X.; Zhu, L.; Neade, T.; Beeri, M.; Silverman, J.M.; et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J. Clin. Endocrinol. Metab. 2009, 94, 4483–4491. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.; Huang, C.; Hsu, C.; Yin, M.; Guo, Y. Association of dietary AGEs with circulating AGEs, glycated LDL, IL-1α and MCP-1 levels in type 2 diabetic patients. Eur. J. Nutr. 2010, 49, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, M.M.; Kiesner, C.; Meissner, K.; Hartkopf, J.; Erbersdobler, H.F. Effects of UHT heating of milk in an experimental plant on several indicators of heat treatment. J. Dairy Res. 1994, 61, 305–309. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Renzone, G.; D’Ambrosio, C.; Salzano, A.M.; Scaloni, A. Dairy products and the Maillard reaction: A promising future for extensive food characterization by integrated proteomics studies. Food Chem. 2017, 219, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.Á.; Guerra-Hernández, E.; García-Villanova, B. Pyrraline content in enteral formula processing and storage and model systems. Eur. Food Res. Technol. 2004, 219, 42–47. [Google Scholar] [CrossRef]
- Henle, T.; Schwarzenbolz, U.; Klostermeyer, H. Detection and quantification of pentosidine in foods. Z. Leb. -Forsch. A 1997, 204, 95–98. [Google Scholar] [CrossRef]
- Baxter, J.H.; Lai, C.-S.; Phillips, R.R.; Dowlati, L.; Chio, J.J.; Luebbers, S.T.; Dimler, S.R.; Johns, P.W. Direct determination of methionine sulfoxide in milk proteins by enzyme hydrolysis/high-performance liquid chromatography. J. Chromatogr. A 2007, 1157, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Assar, S.H.; Moloney, C.; Lima, M.; Magee, R.; Ames, J.M. Determination of Nɛ-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids 2009, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Howell, S.K.; Russell, G.B.; Miller, M.E.; Rich, S.S.; Mauer, M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care 2013, 36, 3234–3239. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Faist, V.; Erbersdobler, H.F. Determination of Nϵ-carboxymethyllysine in milk products by a modified reversed-phase HPLC method. Food Chem. 1999, 65, 547–553. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical characterization and antioxidant properties of coffee melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar] [CrossRef] [PubMed]
- Hull, G.L.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl) lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Wetzels, S.; Wouters, K.; Schalkwijk, C.G.; Vanmierlo, T.; Hendriks, J.J.A. Methylglyoxal-derived advanced glycation endproducts in multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 421. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Bichler, J.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry 1995, 34, 10872–10878. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, Z.; Ostrow, P.; Vaughan, M.; Chichelli, T.; Munschauer, F. AGE-RAGE in multiple sclerosis brain. Immunol. Investig. 2011, 40, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Activity, regulation, copy number and function in the glyoxalase system. Biochem. Soc. Trans. 2014, 42, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Glyoxalase I—Structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Rabbani, N.; Thornalley, P.J. Glyoxalase in ageing. Semin. Cell Dev. Biol. 2011, 22, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Jack, M.; Wright, D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl. Res. J. Lab. Clin. Med. 2012, 159, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glyoxalase Centennial conference: Introduction, history of research on the glyoxalase system and future prospects. Biochem. Soc. Trans. 2014, 42, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Mashima, T.; Sato, S.; Hashimoto, Y.; Yamori, T.; Tsuruo, T. Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin. Cancer Res. 2001, 7, 2513–2518. [Google Scholar] [PubMed]
- Santarius, T.; Bignell, G.R.; Greenman, C.D.; Widaa, S.; Chen, L.; Mahoney, C.L.; Butler, A.; Edkins, S.; Waris, S.; Thornalley, P.J.; et al. GLO1-A novel amplified gene in human cancer. Genes Chromosom. Cancer 2010, 49, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Rabbani, N.; Walter, M.; Bonin, M.; Thornalley, P.; Auburger, G.; Gispert, S. α-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell. Mol. Life Sci. 2011, 68, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wollmer, M.A.; Hoerndli, F.; Münch, G.; Kuhla, B.; Rogaev, E.I.; Tsolaki, M.; Papassotiropoulos, A.; Götz, J. Role for glyoxalase I in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Godfrey, L.; Xue, M.; Shaheen, F.; Geoffrion, M.; Milne, R.; Thornalley, P.J. Glycation of LDL by methylglyoxal increases arterial atherogenicity: A possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes 2011, 60, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Jakas, A.; Horvat, S. Study of degradation pathways of Amadori compounds obtained by glycation of opioid pentapeptide and related smaller fragments: Stability, reactions, and spectroscopic properties. Biopolymers 2003, 69, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Milic, I.; Hoffmann, R.; Fedorova, M. Simultaneous detection of low and high molecular weight carbonylated compounds derived from lipid peroxidation by electrospray ionization-tandem mass spectrometry. Anal. Chem. 2013, 85, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Treutler, H.; Tsugawa, H.; Porzel, A.; Gorzolka, K.; Tissier, A.; Neumann, S.; Balcke, G.U. Discovering regulated metabolite families in untargeted metabolomics studies. Anal. Chem. 2016, 88, 8082–8090. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Frolov, A.; Blüher, M.; Hoffmann, R. Site-specific analysis of advanced glycation end products in plasma proteins of type 2 diabetes mellitus patients. Anal. Bioanal. Chem. 2016, 408, 5557–5566. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Frolov, A.; Blüher, M.; Hoffmann, R. Plasma proteins modified by advanced glycation end products (AGEs) reveal site-specific susceptibilities to glycemic control in patients with type 2 diabetes. J. Biol. Chem. 2016, 291, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Blüher, M.; Hoffmann, R. Glycation sites of human plasma proteins are affected to different extents by hyperglycemic conditions in type 2 diabetes mellitus. Anal. Bioanal. Chem. 2014, 406, 5755–5763. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicová-Kudlácková, M.; Sebeková, K.; Schinzel, R.; Klvanová, J. Advanced glycation end products and nutrition. Physiol. Res. 2002, 51, 313–316. [Google Scholar] [PubMed]
- Bilova, T.; Paudel, G.; Shilyaev, N.; Schmidt, R.; Brauch, D.; Tarakhovskaya, E.; Milrud, S.; Smolikova, G.; Tissier, A.; Vogt, T.; et al. Global proteomic analysis of advanced glycation end products in the Arabidopsis proteome provides evidence for age-related glycation Hotspots. J. Biol. Chem. 2017, 292, 15758–15776. [Google Scholar] [CrossRef] [PubMed]
- Paudel, G.; Bilova, T.; Schmidt, R.; Greifenhagen, U.; Berger, R.; Tarakhovskaya, E.; Stöckhardt, S.; Balcke, G.U.; Humbeck, K.; Brandt, W.; et al. Osmotic stress is accompanied by protein glycation in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6283–6295. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Damasceno, C.M.B.; Saravanan, R.S.; He, Y.; Catalá, C.; Saladié, M.; Rose, J.K.C. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat. Protoc. 2006, 1, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Bilova, T.; Paudel, G.; Berger, R.; Balcke, G.U.; Birkemeyer, C.; Wessjohann, L.A. Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model. J. Plant Physiol. 2017, 208, 70–83. [Google Scholar] [CrossRef] [PubMed]
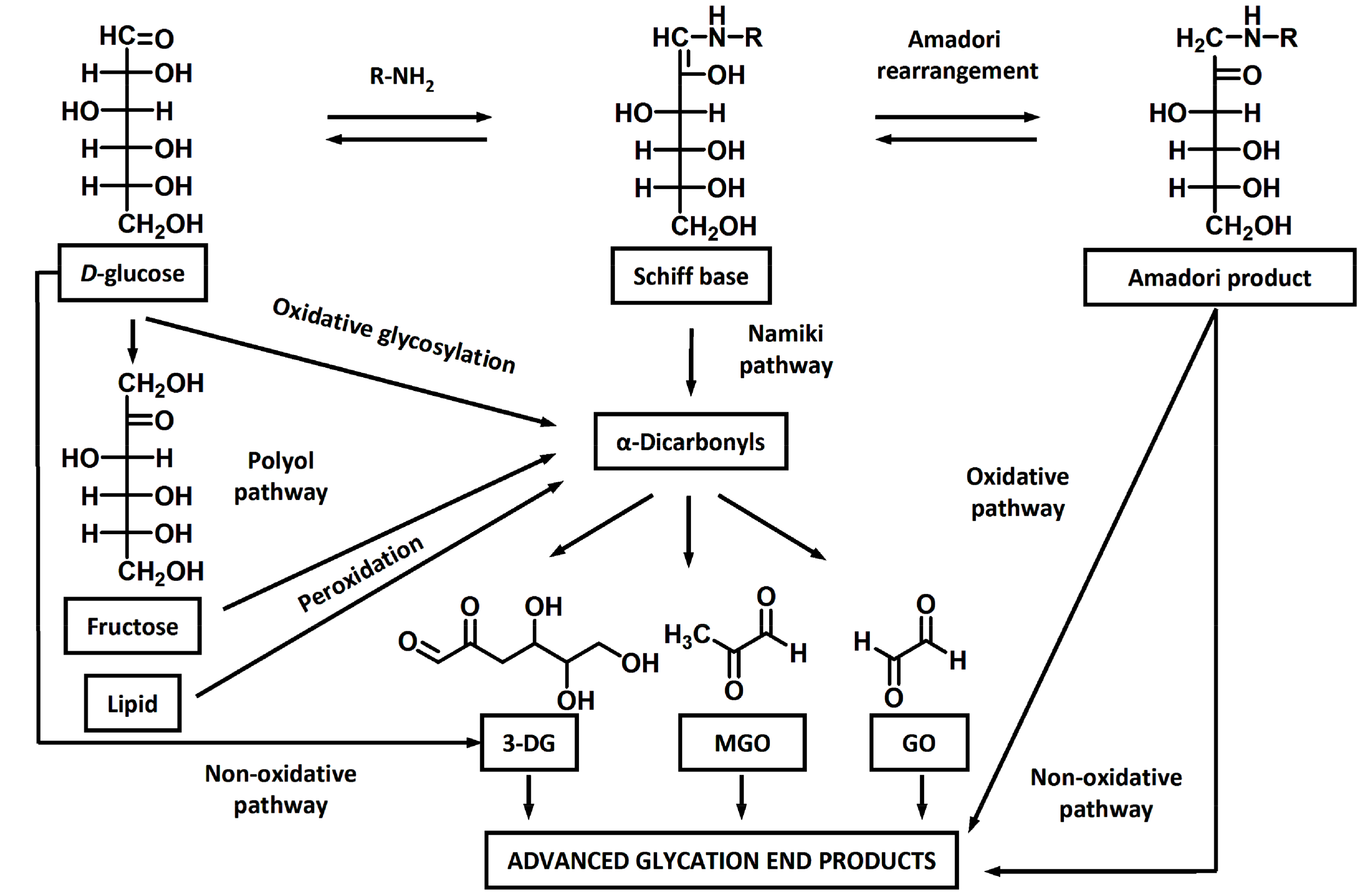
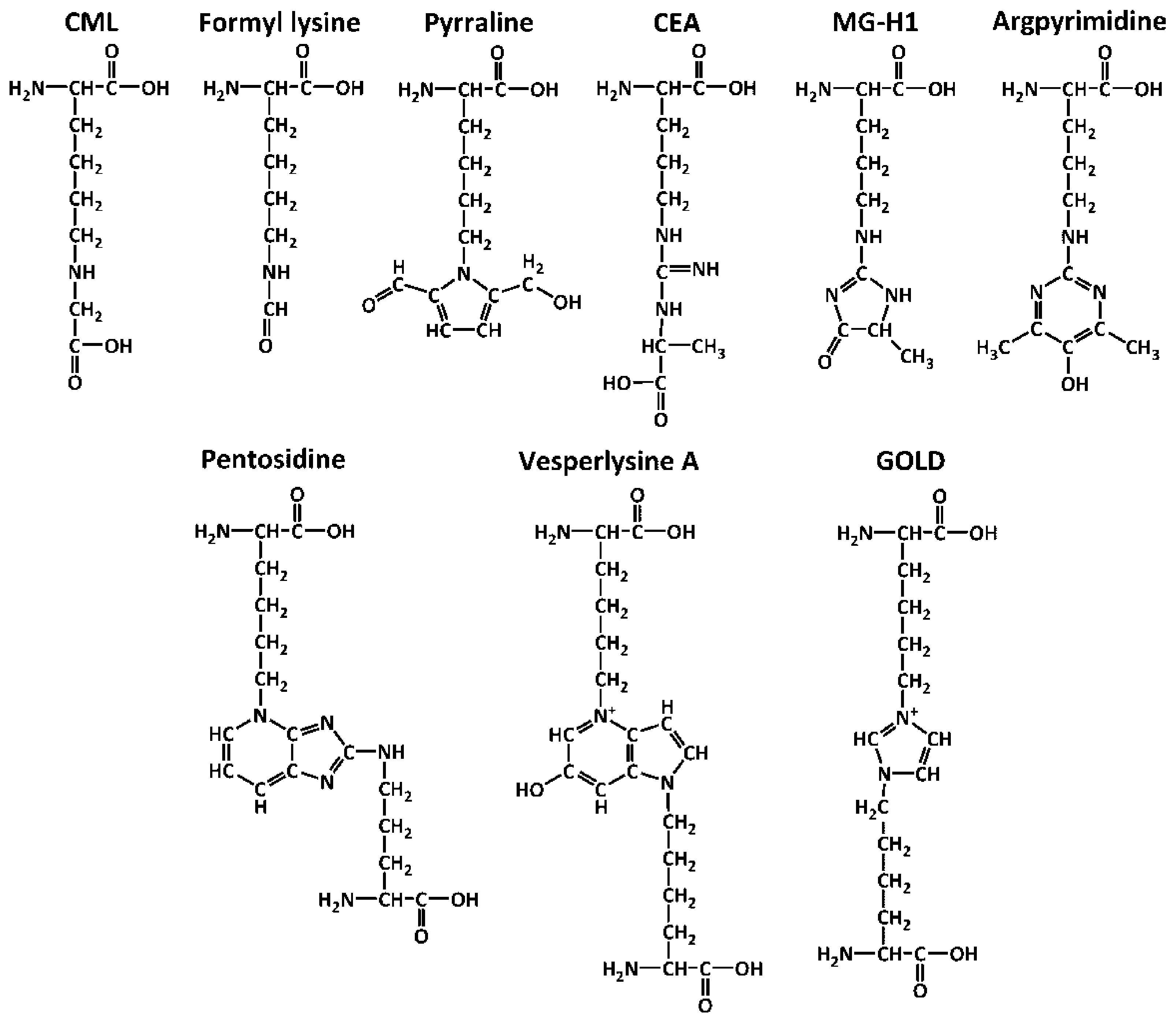
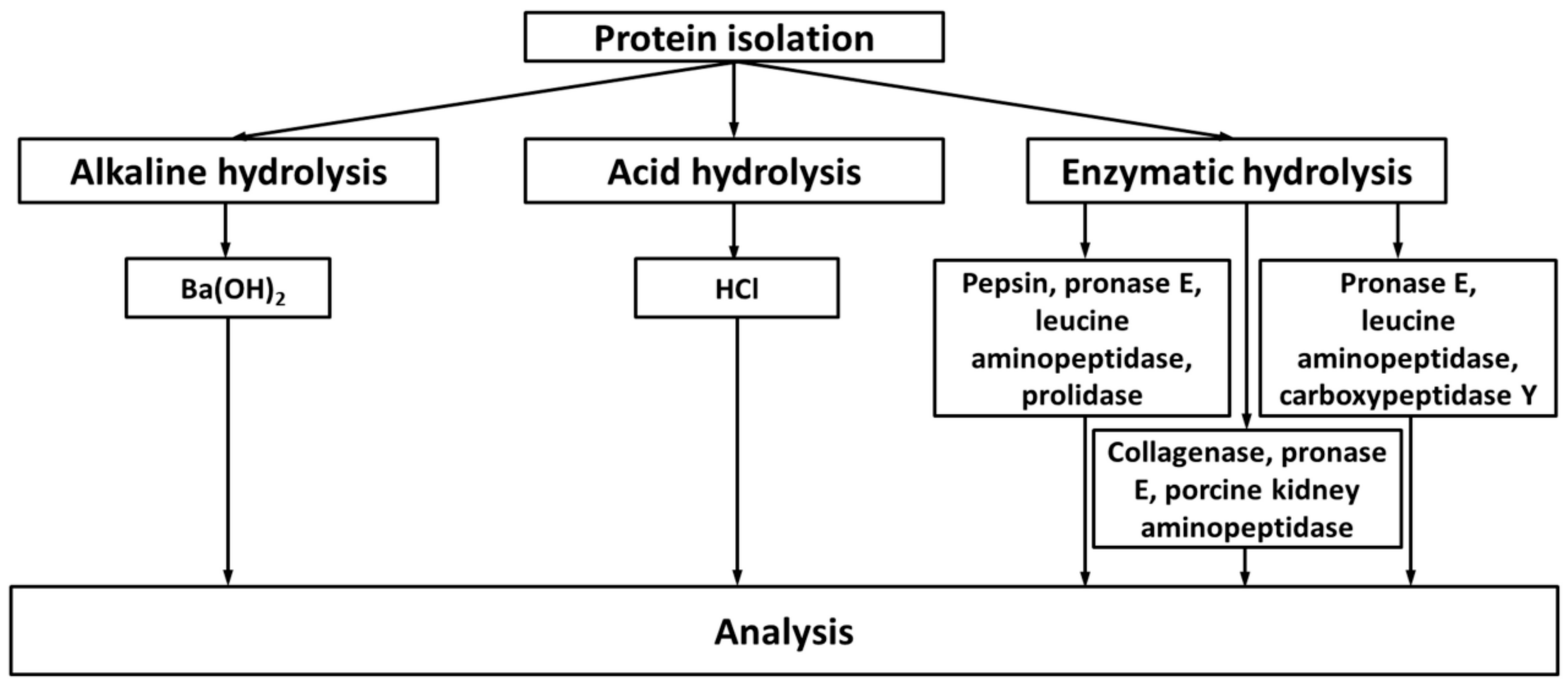
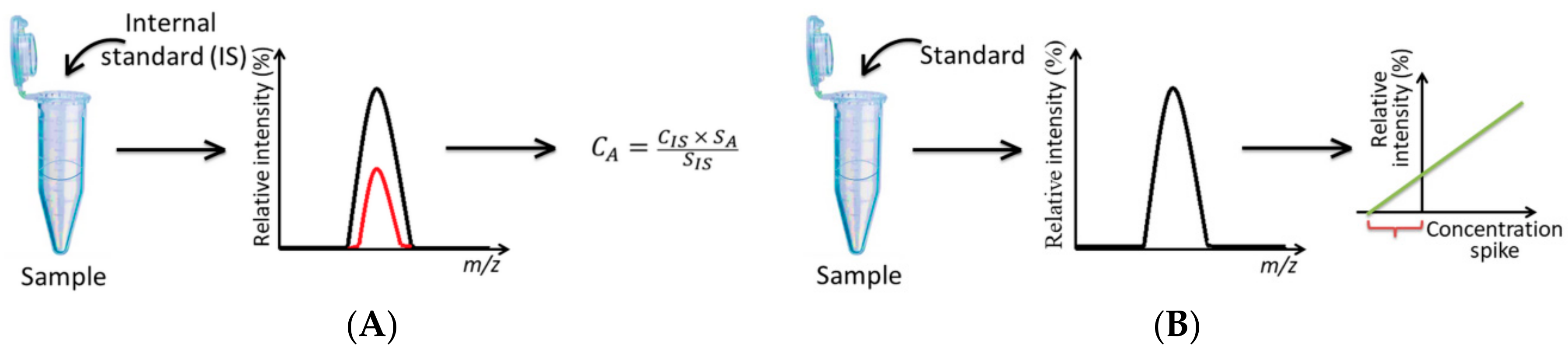
| # | Object | Analyzed Adducts | Methodology | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Technique | Protein Isolation | Protein Hydrolysis | Derivatization (Reagents) | Separation | Detection | Standardization | ||||
| 1 | FFL | CML | GC-MS | - | - | Acetylation (Ac2O) | 7%-cyanopropyl/7%-phenylmethyl coated capillary column | EI-SF, SIM | external | [41] |
| 2 | lense proteins | CML | GC-MS | cold water extraction, dialysis | acid | Acetylation (Ac2O) | SPB-1 capillary column (poly(dimethyl siloxane)), SE-30 capillary column (dimethylpolysiloxane) carrier helium | FID | external | [90] |
| 3 | collagen | CML, CML-OH, FL | GC-MS | CCl4/MeOH extraction | acid | esterification (HCL, MeOH, CH2Cl2, C4F6O3) | DB-5 capillary column ((5%-phenyl)-methylpolysiloxane) | EI-Q-MS, SIM | external | [91] |
| 4 | hemo-globin | CM-Ala, CM-Val, CM-Leu, CM-Ile, CM-Phe, CM-Gly, α-CML, ε-CML, bis-CML | GC-MS | - | acid | acetylation/esterification (Ac2O, i-But-OH), pentafluoro- propionic anhydride | DB-5 capillary column ((5%-phenyl)- methylpolysiloxane), DB-1701capillary column (14%-cyanopropyl- phenyl)- methylpolysiloxane) carrier helium | PICI, EI-QqQ, CAD | - | [92] |
| 5 | BSA | AGEs, poly-l-lysine | Py-GC-MS | - | pyrolysis | - | DBl capillary column (100% dimethylpolysiloxane) | EI-IT-MS | - | [95] |
| 6 | HSA | CML, CEL, MG-H, Glarg, 3-DG-H, THP, FL, pentosidine, CEL, AP, GOLD, MOLD, pyrraline | off-line HPLC-MALDI-TOF | - | enzymatic 1, acid | AQC | RP, analytical column NOVAPAK4 ODS (C18), NOVAPAK4 ODS (C18) Sentry guard column A: NaAc 140 mmol/L, TEA 17 mmol/L, pH 5.05, B: ACN, C: water | MALDI-TOF | internal, external | [49] |
| 7 | BSA | Nε-(1-deoxy-d-fructos-1-yl)-l-lysine | HPLC-MS | - | enzymatic 1 | - | RP, Nucleosil 100-5 NH2 column (aminopropyl modified silica), A: water, B: MeOH | ESI-IT-MS | internal | [96] |
| 8 | BSA | GODIC, MODIC | HPLC-MS | - | enzymatic 1 | - | RP, YMC- Pack Pro C 18 column, A: 10 mmol/L phosph. buffer (pH 4.0) B: MeOH, gradient | ESI-Q-MS MCA | external | [97] |
| 9 | β-lacto-globulin | Maillard reaction products | HPLC-MS | desalting and dialysis | enzymatic 2 | - | RP, Nucleosil 300-5 C18 column, A: 0.115% aq. TFA B: 80% ACN/0.1% aq. TFA | Ex/Em: 210/330 ESI-QqQ | - | [98] |
| 10 | BSA, HSA | Pyrraline | HPLC-UV | - | alkaline | - | RP-HPLC, Vydac C18 analytical column, A: 0.1% aq. TFA, B: 50% ACN; A: 0.16% aq. HFBA, B: 0.16% aq. HFBA/50% ACN | UV, 298 nm | external | [99] |
| 11 | food samples | CML | HPLC-Fluo | - | acid | OPA | RP, Spherisorb 5 C18 column, A: NaAc buffer (pH 6.7, 0.05 mol/L)/4% MeOH B: MeOH | Fluo Ex/Em: 340/455 | external | [100] |
| 12 | FFL | CML | HPLC-Fluo | - | - | OPA | CXC; A: 0.2 mol/L sodium citrate, pH 3.2 B: 0.2 mol/L sodium citrate, 1 mol/L NaCl pH 7.0 | Fluo | - | [41] |
| 13 | lense proteins | AGEs | HPLC-Fluo | dialysis | acid, enzymatic 3 | OPA | RP, column packed with RP-18 material A: 0.12% aq. HFBA B: 0.12% aq. HFBA/30% MeOH | Fluo Ex/Em: 340/455 | external | [19] |
| 14 | lense proteins | GALA, GOLA, GOLD, CML, CMPM | HPLC-MS | dialysis | acid, enzymatic 4 | OPA | RP, VYDAC column Knauer Eurospher 100 column RP18 A: 0.12% aq. HFBA B: 0.12% aq. HFBA/30% MeOH | ESI-Q-MS | external | [46] |
| 15 | lense proteins | AGEs | HPLC-MS/MS | dialysis | acid, enzymatic 5 | - | RP-C18 A: 0.12% aq. HFBA B: 0.12% aq. HFBA/30% MeOH | ESI-QqQ-MS/MS, CAD, MRM | external | [19] |
| 16 | beer proteins | FL, ML, pyrraline, formyline, maltosine, MG-H1, AP | HPLC-MS/MS | dialysis | acid, enzymatic 1 | - | RP, Zorbax 100 SB-C18 A: 10 mmol/L aq. NFPA B: 10 mmol/L aq. NFPA/ACN | ESI-QqQ-MS/MS, CAD MRM | external | [101] |
| 17 | serum | CML | LC-MS/MS | - | acid | - | HILIC (ZIC) A: 0.1% FA/ACN B: 0.1% aq. FA | ESI-QqQ-MS/MS, MRM | internal | [102] |
| 18 | food samples | α-fructosyl-amino acids | HPLC-MS | filtration | - | - | IP-RP, Kinetex core-shell C18 column A: 5 mmol/L aq, NFPA B: 5 mmol/L aq. NFPA/ACN | HESI-Orbitrap | external, internal | [103] |
| 19 | cellular and extra-cellular proteins | CML, CEL, pentosidine, GOLD, MOLD, DOLD, FL, AP, pyrraline, MG-H, 3-DG-H | HPLC-MS/MS | - | enzymatic 1 | - | RP, Hypercarb™ columns (carbon) A: 26 mmol/L aq. AM (pH 3.8) B: 26 mmol/L aq. AM (pH 3.8)/ACN | ESI-QqQ-MS/MS CAD MRM | internal | [65] |
| # | Disease | Object | Analyzed Adducts | Main Results | Methodology | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Technique | Protein Isolation | Protein Hydrolysis | Derivatization | Separation | Detection | Standardization | ||||||
| 1 | T1DM | serum | CML, pentosidine | increase of AGE levels | HPLC-MS/MS | serum treatment | acid | - | Kinetex HILIC/PFP (CML/pentosidine) A: 5 mmol/L aq. AM B: 100% ACN | ESI-QqQ-MS/MS CAD MRM | internal | [186] |
| 2 | DM | rabbit blastocyst cavity fluid | CML | increase of CML levels | HPLC-MS/MS | analysis of free adducts | - | - | RP, C18 A: 0.12% aq. HFBA B: 0.12% aq. HFBA/30% MeOH | ESI-QqQ-MS/MS CAD MRM | internal | [130] |
| 3 | DM | rat plasma protein | CML, CEL, Glarg, MG-H1 | increase of AGE levels | HPLC-MS/MS | Ultrafiltra-tion (12 kDa cut-off) | enzymatic 1 | - | RP, carbon Hypercarb™ A: 26 mmol/L AM, pH 3.8, B: ACN | ESI-QqQ-MS/MS CAD MRM | internal | [65] |
| 4 | diabetic nephro-pathy | blood from normoalbuminuric subjects (NHDNS) | CML, CEL, MG-HI | increase of AGE levels | HPLC-MS/MS | filtration (10 KDa cutoff) | - | - | RP, C18 Synergy 80 A A: 0.29% aq. HFBA, B: 0.29% aq. HFBA/MeOH | ESI-QqQ-MS/MS CAD MRM | internal | [187] |
| 5 | fibrosis | human aged lens capsules | CML, NFL, CMA, NAL, CEA, MG-H1, Pyrraline Glucosepane,MODIC | AGEs in the lens capsule promote fibrosis of lens epithelial cells | HPLC-MS/MS | enzymatic 2 | - | RP, C18 A: 0.12% aq. HFBA B: 0.12% aq. HFBA/30% MeOH | ESI-QqQ-MS/MS CAD MRM | internal | [128] | |
| 6 | cataract | lense proteins | CML, MG-HI | increase of CML levels | HPLC-MS/MS | phosphate-buffered Saline/EDTA dialysis | acid, enzymatic 3 | OPA | RP, C18, A: 0.12% aq. HFBA B: 0.12% aq. HFBA/30% MeOH | ESI-QqQ-MS/MS, CAD MRM | external | [19] |
| 7 | prion disease | Creutzfeldt- Jakob/brain scrapie/Syrian hamsters | CML, CEL | elevated AGE levels in plaques | GS-MS | CHCl3- CH3OH extraction | acid | esterify-cation (HCL, MeOH, CH2Cl2, C4F6O3) | HP-5MS column | EI-Q-MS | internal | [185] |
| 8 | schizophrenia | plasma/schizophrenia | pentosidine | elevated level of AGEs | IP-RP- HPLC-Fluo | - | acid | - | RP, C18 A: 0.1% aq.HFBA B: 0.1% aq.HFBA/ACN | Fluo Ex/Em: 335/385 nm | external | [184] |
| 9 | schizophrenia | plasma/schizophrenia | pentosidine | elevated level of AGEs | IP-RP- HPLC-Fluo | - | acid | - | RP, C18 A: 0.1% aq. HFBA B: 0.1% aq. HFBA/ACN | Fluo Ex/Em 335/385 nm | external | [188] |
| # | Type of Food | Analyzed Adducts | Methodology | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Technique | Protein Isolation | Protein Hydrolysis | Derivatization | Separation | Detection | Standardization | ||||
| 1 | milk products | CML | RP-HPLC -Fluo | Direct hydrolysis after reduction with 1 mol/L NaBH4 | acid | OPA | RP, C18 SpheriChROM RP-18 ODS, A: sodium acetate buffer (pH 6.50, 0.048 mol/L)/4% MeOH B: MeOH | Fluo Ex/Em: 340/455 nm | standard addition external | [205] |
| 2 | milk products | CML, CEL, MG-H, FL, Argpyrimidine, 3DG-H, DOLD, Glarg, GOLD, MOLD | HPLC- MS/MS | Ultrafiltration (12 kDa cutoff) delipidation | enzymatic 1 | - | Hypercarb™ A: 26 mmol/L AM (pH 3.8) B: ACN | ESI-QqQ- MS/MS CAD MRM | internal | [161] |
| 3 | milk products | CML | GS-MS | extraction: C2H6O-CH2Cl2 | acid | MeOH/TFAA | DB5-MS capillary column carrier helium | EI-IT-MS | internal, external, isotope dilution | [81] |
| 4 | milk products | CML, furosine, CEL | HPLC–MS/MS | Hydrolyzed without protein isolation | acid | - | RP, C18 core shell Kinetex A: 5 mmol/L PFPA B: 5 mmol/L aq. PFBA/ACN | ESI-QqQ- MS/MS CAD MRM | internal | [114] |
| 5 | milk products | CML | UHPLC–MS/MS | precipitation: TCA/extraction: CHCl3-MeOH | acid | - | RP, C18 Acquity UPLC™ BEC C18 column A: 0.13% aq. NFPA or 0.1% aq. TFA B: ACN | ESI-QqQ-MS/MS MRM | internal | [201] |
| 6 | bakery products | CML, furosine, CEL | HPLC– MS/MS | - | acid | - | RP, C18 core shell Kinetex A: 5 mmol/L aq. PFPA B: 5 mmol/L aq. PFBA/ACN | ESI-QqQ- MS/MS CAD MRM | internal | [114] |
| 7 | bakery products | CML | GS-MS | extraction: CHCl3-MeOH | acid | MeOH/TFAA | DB5-MS capillary carrier helium | EI-IT-MS | internal, external, isotope dilution | [81] |
| 8 | bakery products | CML | HPLC–MS/MS | precipitation: TCA/extraction: CHCl3- MeOH | acid | - | RP, C18 Acquity BEH C18 column A: 0.13% aq NFPA or 0.1% aq. TFA B: ACN | ESI-QqQ- MS/MS CAD MRM | internal | [201] |
| 9 | meat | CML | GS-MS | extraction: CHCl3-MeOH | acid | esterification by MeOH/acylation by TFAA | DB5-MS capillary carrier helium | EI-IT-MS | internal, external, isotope dilution | [81] |
| 10 | meat | CML | HPLC–MS/MS | precipitation: TCA/extraction: CHCl3-MeOH | acid | - | RP, C18 Acquity BEH C18 col. A: 0.13% aq. NFPA or aq. 0.1% TFA B: ACN | ESI-QqQ- MS/MS CAD MRM | internal | [201] |
| 11 | fish | CML | GS-MS | extraction: CHCl3-MeOH | acid | esterification by MeOH/acylation by TFAA | DB5-MS capillary carrier helium | EI-IT-MS | internal, external, isotope dilution | [81] |
| 12 | coffee | melanoidins | Off line LC-MALDI-TOF-MS | Hot water extraction delipidation | - | - | GFC Sephadex G-25 | MALDI- TOF-MS | external | [206] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soboleva, A.; Vikhnina, M.; Grishina, T.; Frolov, A. Probing Protein Glycation by Chromatography and Mass Spectrometry: Analysis of Glycation Adducts. Int. J. Mol. Sci. 2017, 18, 2557. https://doi.org/10.3390/ijms18122557
Soboleva A, Vikhnina M, Grishina T, Frolov A. Probing Protein Glycation by Chromatography and Mass Spectrometry: Analysis of Glycation Adducts. International Journal of Molecular Sciences. 2017; 18(12):2557. https://doi.org/10.3390/ijms18122557
Chicago/Turabian StyleSoboleva, Alena, Maria Vikhnina, Tatiana Grishina, and Andrej Frolov. 2017. "Probing Protein Glycation by Chromatography and Mass Spectrometry: Analysis of Glycation Adducts" International Journal of Molecular Sciences 18, no. 12: 2557. https://doi.org/10.3390/ijms18122557







