Protective Effects of Liquiritigenin against Citrinin-Triggered, Oxidative-Stress-Mediated Apoptosis and Disruption of Embryonic Development in Mouse Blastocysts
Abstract
:1. Introduction
2. Results
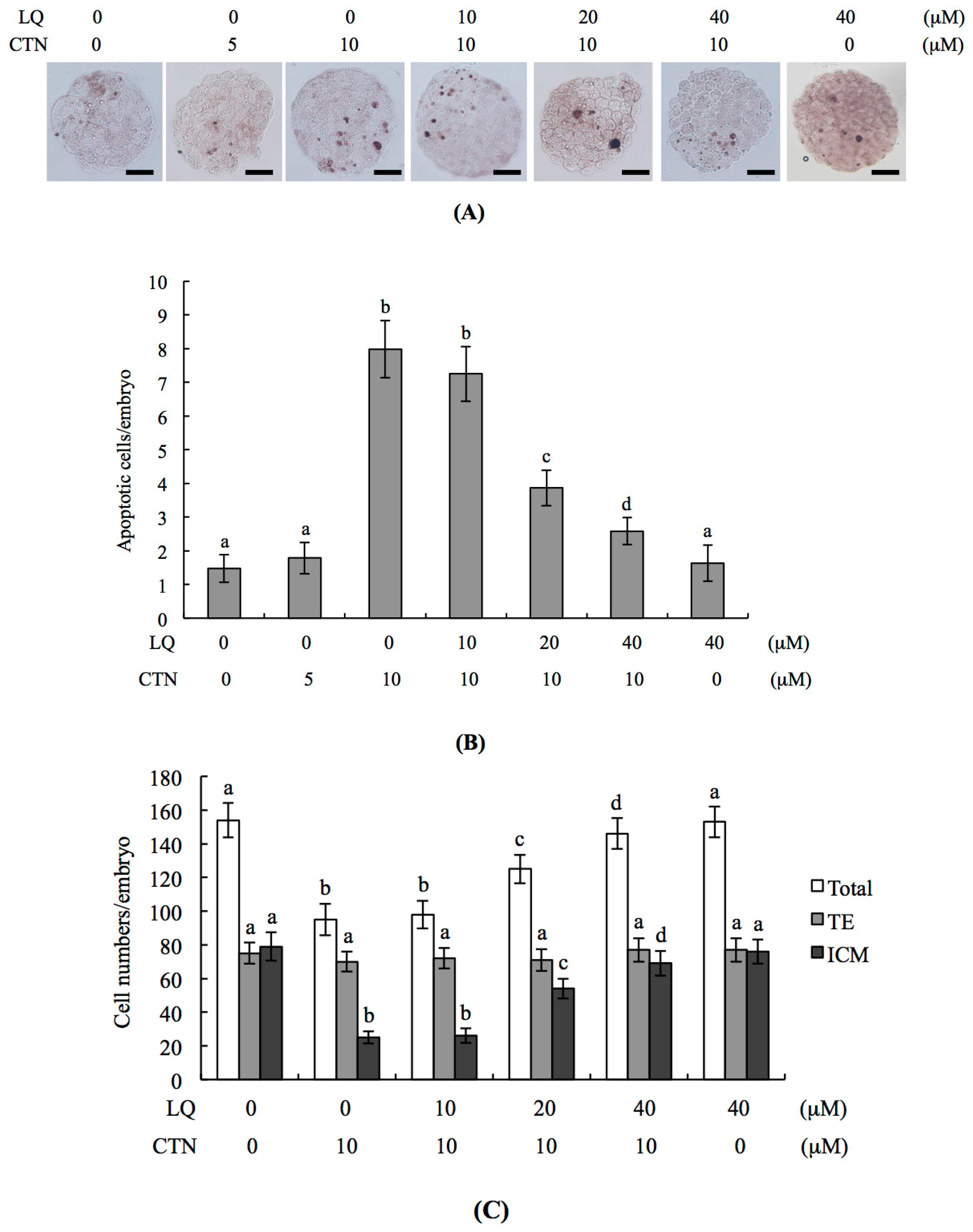
2.1. Protective Effects of LQ against CTN-Induced Injury to Blastocysts
2.2. CTN-Triggered Disruption of Blastocyst Development Is Prevented by LQ In Vitro
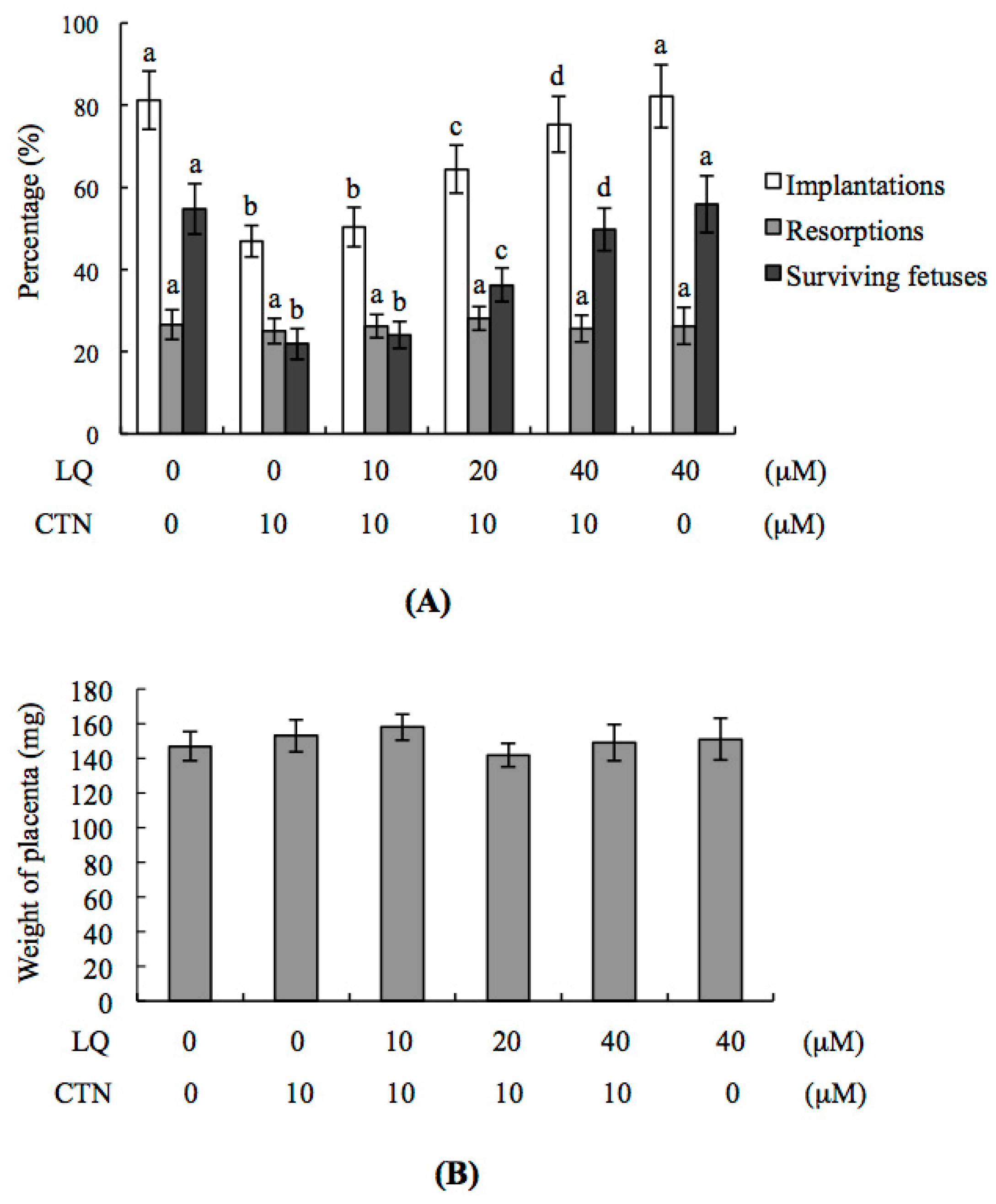
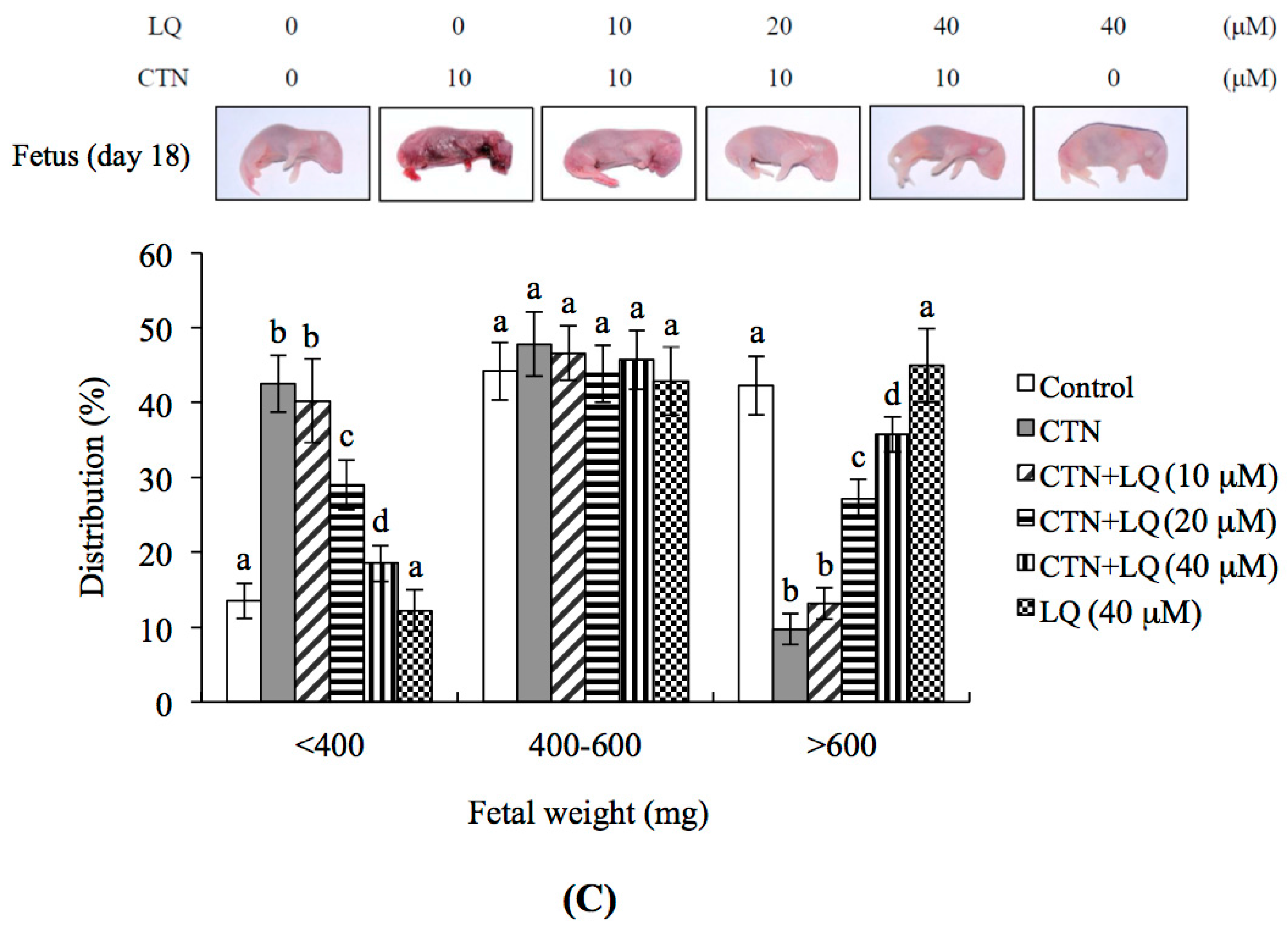
2.3. Effect of LQ on the Disruption of Blastocyst Development by CTN in the Embryo Transfer Assay
2.4. Mechanism of LQ Activity against MG-Induced Development Injury in Mouse Blastocysts
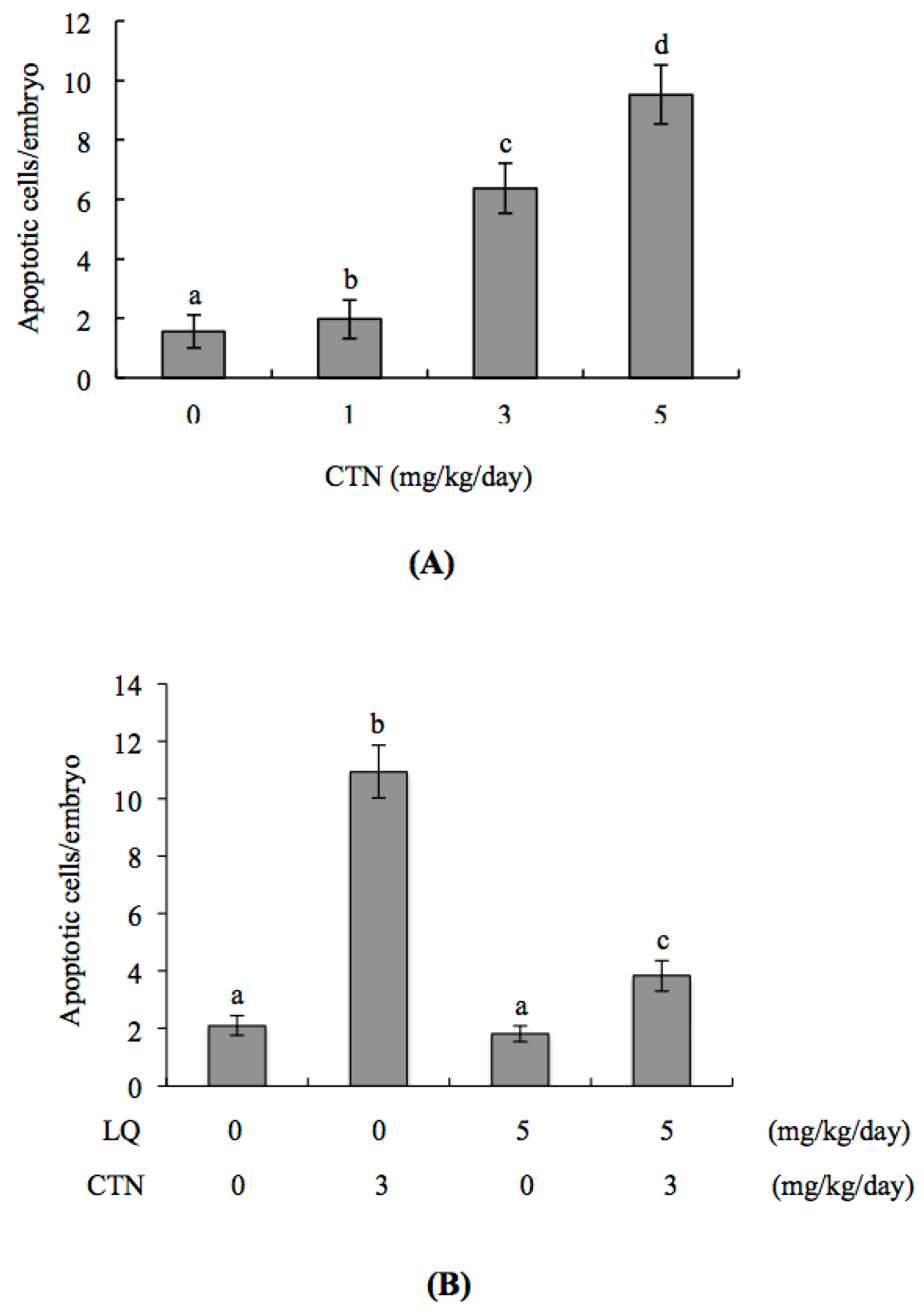
2.5. Effect of LQ on CTN-Induced Blastocyst Development Disruption in an Animal Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection of Mouse Morulae and Blastocysts
4.3. Analysis of Blastocysts Developing from Morulae
4.4. TUNEL Assay of Blastocysts
4.5. Measurement of Embryo Cell Numbers
4.6. In Vitro Embryonic Development Analysis
4.7. Blastocyst Development Following Embryo Transfer
4.8. Intravenous Injection of Female Mice with LQ and CTN
4.9. Measurement of ROS in Mouse Blastocysts
4.10. Detection of Mitochondrial Membrane Potential (MMP) in Mouse Blastocysts
4.11. Measurement of Caspase Activity
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blanc, P.J.; Laussac, J.P.; Le Bars, J.; Le Bars, P.; Loret, M.O.; Pareilleux, A.; Prome, D.; Prome, J.C.; Santerre, A.L.; Goma, G. Characterization of monascidin A from Monascus as citrinin. Int. J. Food Microbiol. 1995, 27, 201–213. [Google Scholar] [CrossRef]
- Council of Agricultural Science and Technology. Mycotoxins: Risks in Plant, Animal, and Human Systems; Council for Agricultural Science and Technology: Ames, IA, USA, 2003; pp. 48–57. [Google Scholar]
- Wei, W.; Li, C.; Wang, Y.; Su, H.; Zhu, J.; Kritchevsky, D. Hypolipidemic and anti-atherogenic effects of long-term Cholestin (Monascus purpureus-fermented rice, red yeast rice) in cholesterol fed rabbits. J. Nutr. Biochem. 2003, 14, 314–318. [Google Scholar] [CrossRef]
- Liu, B.H.; Wu, T.S.; Su, M.C.; Chung, C.P.; Yu, F.Y. Evaluation of citrinin occurrence and cytotoxicity in Monascus fermentation products. J. Agric. Food Chem. 2005, 53, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Vilar, M.; Maas, R.F.; Fink-Gremmels, J. Mutagenicity of commercial Monascus fermentation products and the role of citrinin contamination. Mutat. Res. 1999, 444, 7–16. [Google Scholar] [CrossRef]
- Chan, W.H.; Shiao, N.H. Effect of citrinin on mouse embryonic development in vitro and in vivo. Reprod. Toxicol. 2007, 24, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H. Citrinin induces apoptosis via a mitochondria-dependent pathway and inhibition of survival signals in embryonic stem cells, and causes developmental injury in blastocysts. Biochem. J. 2007, 404, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H. Effects of citrinin on maturation of mouse oocytes, fertilization, and fetal development in vitro and in vivo. Toxicol. Lett. 2008, 180, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Katan, M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Kim, Y.W.; Zhao, R.J.; Park, S.J.; Lee, J.R.; Cho, I.J.; Yang, C.H.; Kim, S.G.; Kim, S.C. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharmacol. 2008, 154, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.W.; Bae, E.A.; Lee, B.; Lee, S.H.; Kim, J.A.; Kim, Y.S.; Kim, D.H. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Med. 2007, 73, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.A.; Ray, A.B. Antihyperlipidemic effect of flavonoids from Pterocarpus marsupium. J. Natl. Prod. 1993, 56, 989–994. [Google Scholar] [CrossRef]
- Kim, Y.W.; Ki, S.H.; Lee, J.R.; Lee, S.J.; Kim, C.W.; Kim, S.C.; Kim, S.G. Liquiritigenin, an aglycone of liquiritin in Glycyrrhizae radix, prevents acute liver injuries in rats induced by acetaminophen with or without buthionine sulfoximine. Chem. Biol. Interact. 2006, 161, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, J.; Liu, Y.; Meng, Q.; Xie, J.; Wang, Z.; Teng, L. Liquiritigenin induces tumor cell death through mitogen-activated protein kinase- (MPAKs-) mediated pathway in hepatocellular carcinoma cells. BioMed Res. Int. 2014, 2014, 965316. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Xie, S.; Zhou, Y.; Ren, X.; Li, X.; Cai, Y. Liquiritigenin induces mitochondria-mediated apoptosis via cytochrome c release and caspases activation in HeLa Cells. Phytother. Res. 2011, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Zhou, Y.J.; Liu, Y.; Cai, Y.Q. Effect of liquiritigenin, a flavanone existed from Radix glycyrrhizae on pro-apoptotic in SMMC-7721 cells. Food Chem. Toxicol. 2009, 47, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Chen, W.Z.; Taniyama, T.; Harada, E.; Hori, K.; Kobayashi, M.; Ren, J. [Quantitative determination of constituents in various licorice roots by means of high performance liquid chromatography]. Yakugaku Zasshi 1998, 118, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; van Breemen, R.B. New metabolic pathways for flavanones catalyzed by rat liver microsomes. Drug Metab. Dispos. Biol. Fate Chem. 2004, 32, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Park, G.H.; Song, K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology 2013, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Byun, S.H.; Yang, C.H.; Kim, C.Y.; Kim, J.W.; Kim, S.G. Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage). Toxicology 2004, 197, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Rhee, S.Y.; Kim, Y.S.; Choi, E.M. Protective effect of liquiritigenin against methylglyoxal cytotoxicity in osteoblastic MC3T3-E1 cells. Food Funct. 2014, 5, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Suh, K.S.; Lee, Y.S. Liquiritigenin restores osteoblast damage through regulating oxidative stress and mitochondrial dysfunction. Phytother. Res. 2014, 28, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Y.; Liao, Y.C.; Chang, C.H.; Liu, B.H. Citrinin induces apoptosis in HL-60 cells via activation of the mitochondrial pathway. Toxicol. Lett. 2006, 161, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Chan, W.H. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum. Reprod. 2006, 21, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H.; Shiao, N.H.; Lu, P.Z. CdSe quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial-dependent pathways and inhibition of survival signals. Toxicol. Lett. 2006, 167, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chan, W.H. Inhibition of citrinin-induced apoptotic biochemical signaling in human hepatoma G2 cells by resveratrol. Int. J. Mol. Sci. 2009, 10, 3338–3357. [Google Scholar] [CrossRef] [PubMed]
- Vrabcheva, T.; Usleber, E.; Dietrich, R.; Martlbauer, E. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J. Agric. Food Chem. 2000, 48, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kong, L.D.; Zhang, Y.; Cheng, C.H.; Tan, R.X. In vitro inhibition of rat monoamine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Acta Pharmacol. Sin. 2000, 21, 949–953. [Google Scholar] [PubMed]
- Xie, X.; Zhang, H.D.; Chen, Y.Z.; Cao, Y.; Wang, L.; Xu, Z. Chemical constituents and activities of total flavonoids from Yushen Tang. China J. Chin. Mater. Med. 2012, 37, 3585–3590. [Google Scholar] [CrossRef]
- Park, S.M.; Ki, S.H.; Han, N.R.; Cho, I.J.; Ku, S.K.; Kim, S.C.; Zhao, R.J.; Kim, Y.W. Tacrine, an oral acetylcholinesterase inhibitor, induced hepatic oxidative damage, which was blocked by liquiritigenin through GSK3-beta inhibition. Biol. Pharm. Bull. 2015, 38, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H. Citrinin induces apoptosis in mouse embryonic stem cells. IUBMB Life 2008, 60, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Jerry Teng, C.L.; Hung, P.S.; Cheng, C.C.; Hsu, S.L.; Hwang, G.Y.; Tzeng, Y.M. Ovatodiolide isolated from Anisomeles indica induces cell cycle G2/M arrest and apoptosis via a ROS-dependent ATM/ATR signaling pathways. Eur. J. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.G.; Tan, S.X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 2007, 39, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, M.; Woo, J.E.; Lee, T.; Jung, J.W.; Song, K.S. The comparison of neuroprotective effects of isoliquiritigenin and its Phase I metabolites against glutamate-induced HT22 cell death. Bioorg. Med. Chem. Lett. 2016, 26, 5639–5643. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.C.; Werb, Z.; Fisher, S.J. Implantation and the placenta: Key pieces of the development puzzle. Science 1994, 266, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Pampfer, S.; de Hertogh, R.; Vanderheyden, I.; Michiels, B.; Vercheval, M. Decreased inner cell mass proportion in blastocysts from diabetic rats. Diabetes 1990, 39, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Robaire, B.; Hales, B.F. Paternal cyclophosphamide treatment causes postimplantation loss via inner cell mass-specific cell death. Teratology 1992, 45, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H. Ginkgolides induce apoptosis and decrease cell numbers in mouse blastocysts. Biochem. Biophys. Res. Commun. 2005, 338, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Anggelia, M.R.; Chan, W.H. Impairment of preimplantation and postimplantation embryonic development through intrinsic apoptotic processes by ginsenoside Rg1 in vitro and in vivo. Environ. Toxicol. 2017, 32, 1937–1951. [Google Scholar] [CrossRef] [PubMed]
- Ratno Budiarto, B.; Chan, W.H. Oxidative stresses-mediated apoptotic effects of ginsenoside Rb1 on pre- and post-implantation mouse embryos in vitro and in vivo. Environ. Toxicol. 2017, 32, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Chan, W.H. Methylglyoxal has injurious effects on maturation of mouse oocytes, fertilization, and fetal development, via apoptosis. Toxicol. Lett. 2010, 193, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.C.; Chen, C.P.; Chan, W.H. Epicatechin gallate decreases the viability and subsequent embryonic development of mouse blastocysts. Taiwan J. Obstet. Gynecol. 2010, 49, 174–180. [Google Scholar] [CrossRef]
- Chan, W.H. Hazardous apoptotic effects of 2-bromopropane on maturation of mouse oocytes, fertilization, and fetal development. Int. J. Mol. Sci. 2010, 11, 4361–4380. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.S.; Shiao, N.H.; Chan, W.H. Cytotoxic effects of CdSe quantum dots on maturation of mouse oocytes, fertilization, and fetal development. Int. J. Mol. Sci. 2009, 10, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Kong, R.H.; Zhang, L.M.; Zhang, J.N. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br. J. Pharmacol. 2012, 167, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.; Handyside, A.H.; Winston, R.M. The human blastocyst: Cell number, death and allocation during late preimplantation development in vitro. Development 1989, 107, 597–604. [Google Scholar] [PubMed]
- Gardner, R.L.; Davies, T.J. Lack of coupling between onset of giant transformation and genome endoreduplication in the mural trophectoderm of the mouse blastocyst. J. Exp. Zool. 1993, 265, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.J.; Wu, T.C.; Tsai, M.Y. Effect of retinoic acid on implantation and post-implantation development of mouse embryos in vitro. Hum. Reprod. 2001, 16, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Witschi, E. Characterization of developmental stages. Part II. Rat. In Biology Data Book, 2nd ed.; Federation of American Societies of Experimental Biologies: Bethesda, MD, USA, 1972; pp. 178–180. [Google Scholar]
- Armant, D.R.; Kaplan, H.A.; Lennarz, W.J. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev. Biol. 1986, 116, 519–523. [Google Scholar] [CrossRef]
- Pampfer, S.; Wuu, Y.D.; Vanderheyden, I.; de Hertogh, R. In vitro study of the carry-over effect associated with early diabetic embryopathy in the rat. Diabetologia 1994, 37, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Shiao, N.H.; Hsuuw, Y.D.; Chan, W.H. Protective effects of resveratrol on ethanol-induced apoptosis in embryonic stem cells and disruption of embryonic development in mouse blastocysts. Toxicology 2007, 242, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.H.; Hester, J.M.; Stone, B.J.; Carrico, K.M.; Spear, B.T.; Fath-Goodin, A. Nonsurgical embryo transfer device compared with surgery for embryo transfer in mice. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 17–21. [Google Scholar] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Chan, W.-H. Protective Effects of Liquiritigenin against Citrinin-Triggered, Oxidative-Stress-Mediated Apoptosis and Disruption of Embryonic Development in Mouse Blastocysts. Int. J. Mol. Sci. 2017, 18, 2538. https://doi.org/10.3390/ijms18122538
Huang C-H, Chan W-H. Protective Effects of Liquiritigenin against Citrinin-Triggered, Oxidative-Stress-Mediated Apoptosis and Disruption of Embryonic Development in Mouse Blastocysts. International Journal of Molecular Sciences. 2017; 18(12):2538. https://doi.org/10.3390/ijms18122538
Chicago/Turabian StyleHuang, Chien-Hsun, and Wen-Hsiung Chan. 2017. "Protective Effects of Liquiritigenin against Citrinin-Triggered, Oxidative-Stress-Mediated Apoptosis and Disruption of Embryonic Development in Mouse Blastocysts" International Journal of Molecular Sciences 18, no. 12: 2538. https://doi.org/10.3390/ijms18122538



