Impaired Sleep, Circadian Rhythms and Neurogenesis in Diet-Induced Premature Aging
Abstract
:1. Introduction
2. Results
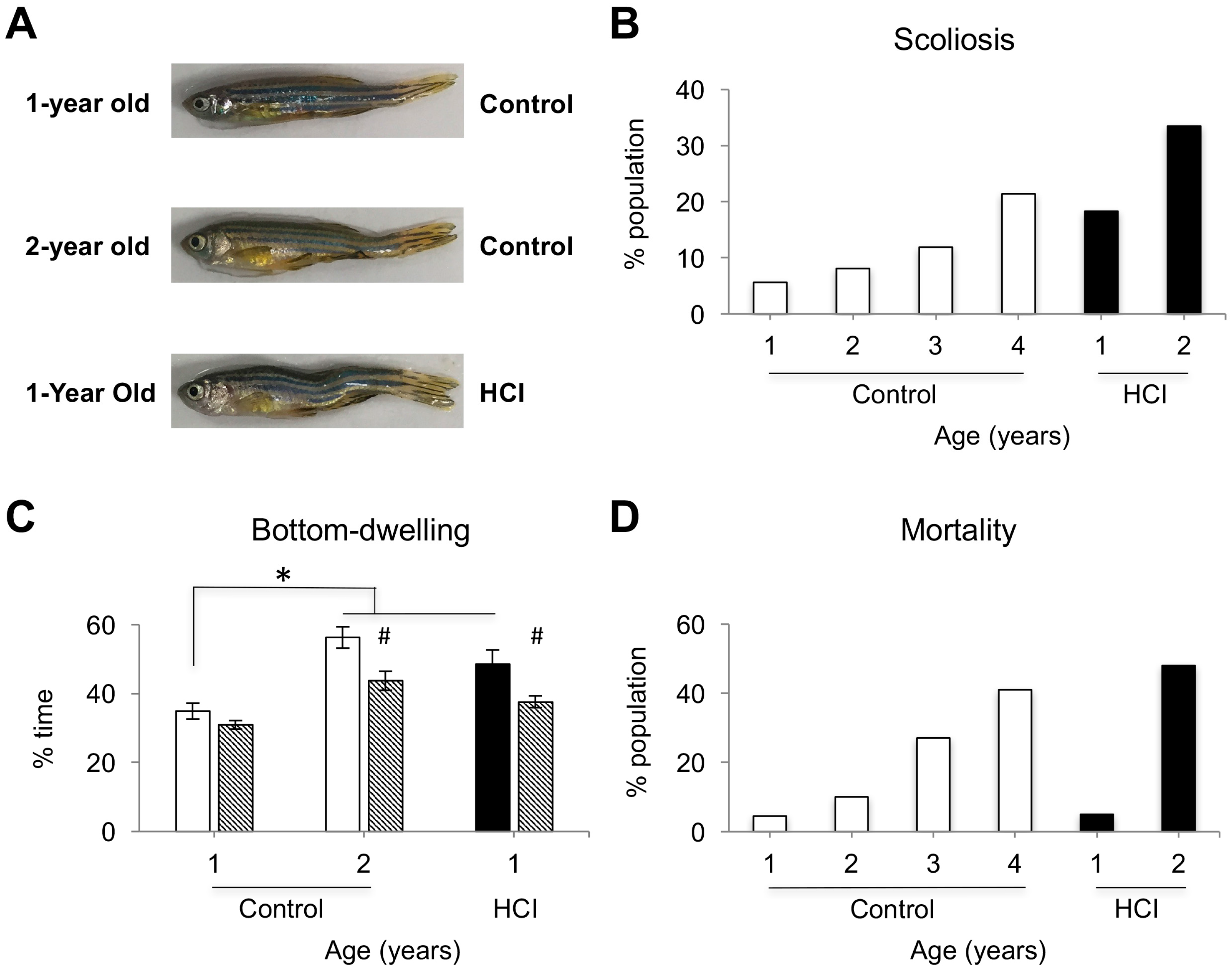
2.1. High Caloric Intake Leads to Increase in Brain Volume, Body Weight, Scoliosis, Anxiety-Like Behavior and High Mortality Rate
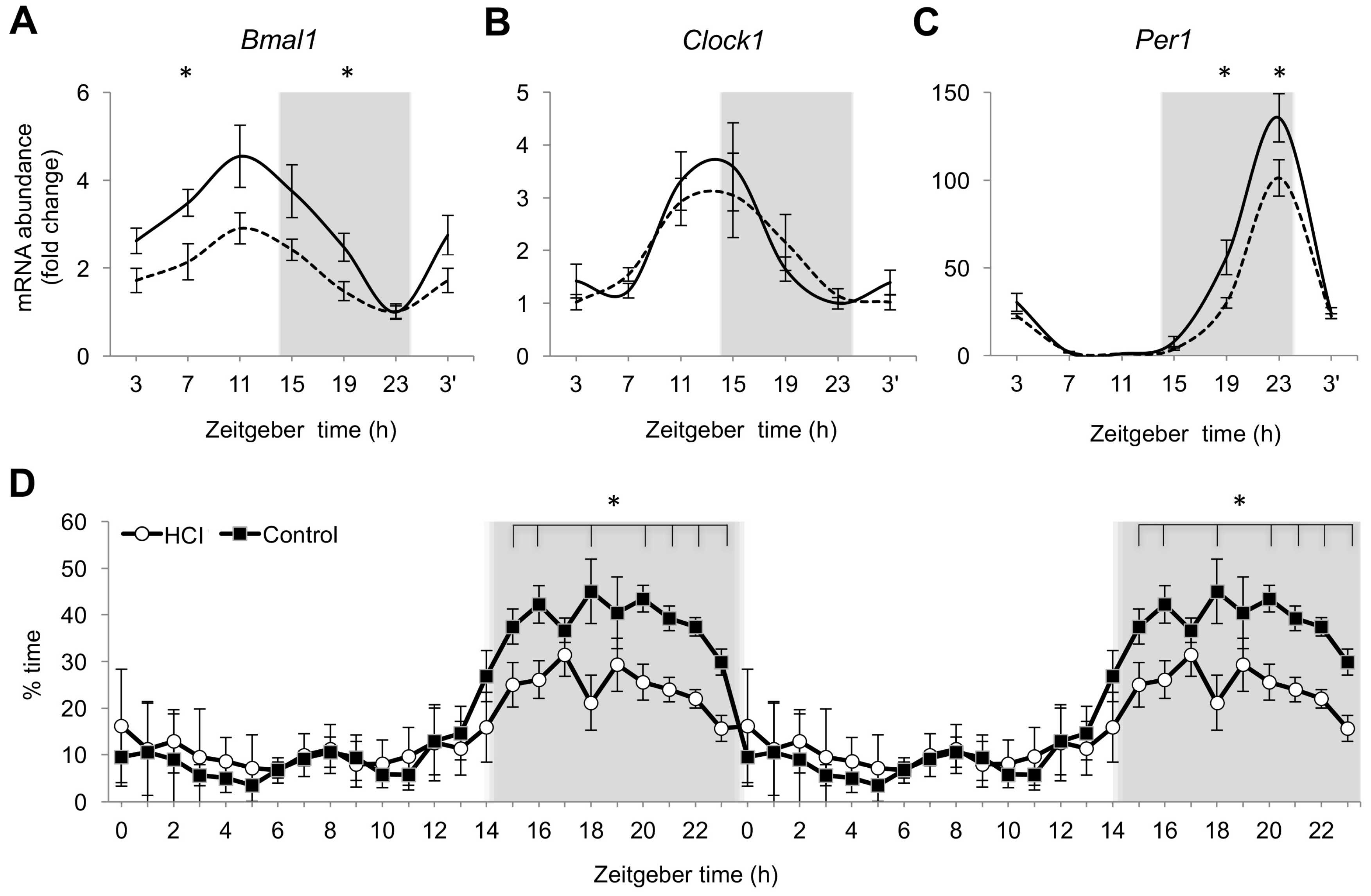
2.2. Chronic High Caloric Intake Alters Circadian Rhythms and Sleep
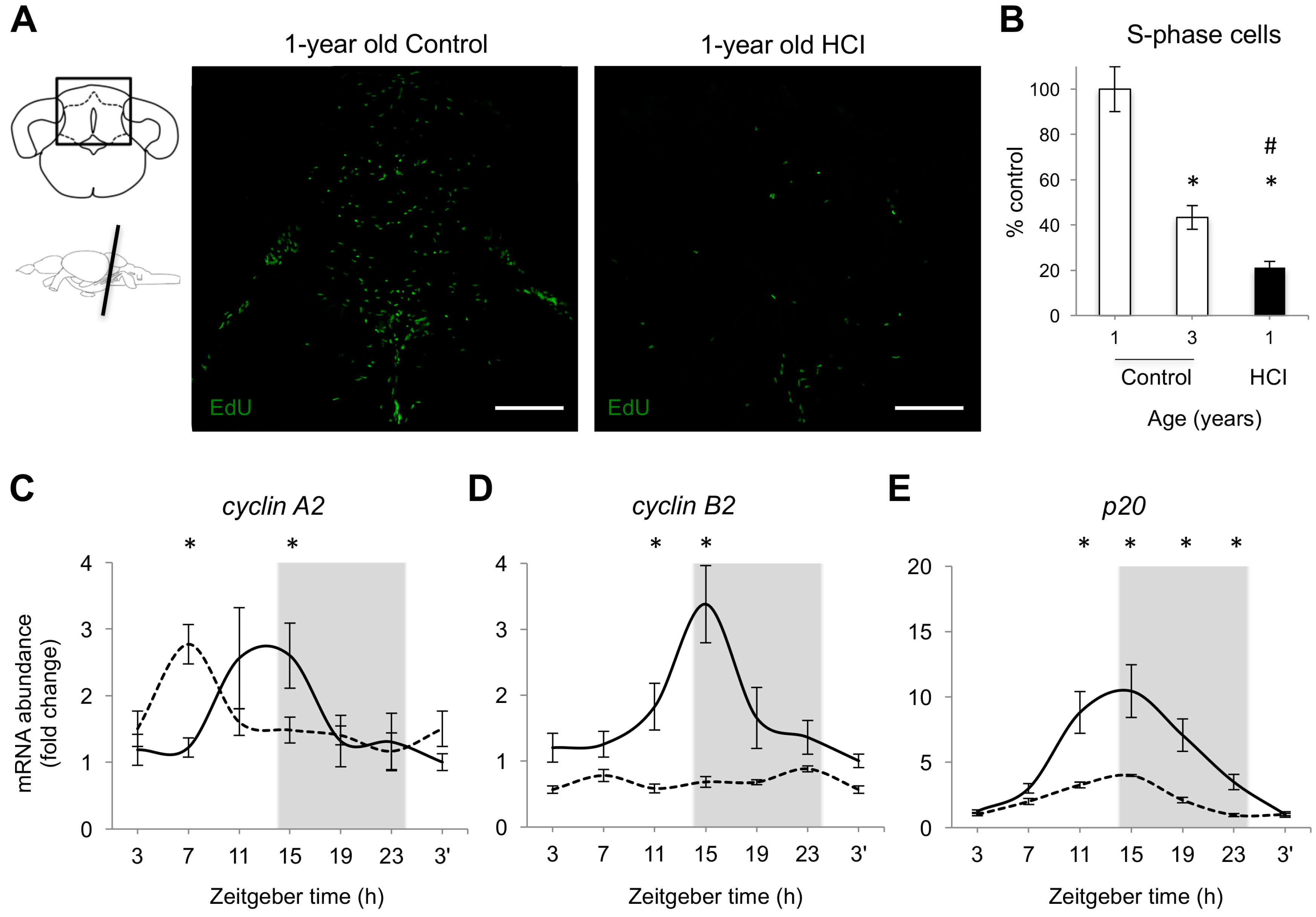
2.3. Adult Neurogenesis Is Attenuated in Premature Aging Induced by High Caloric Intake
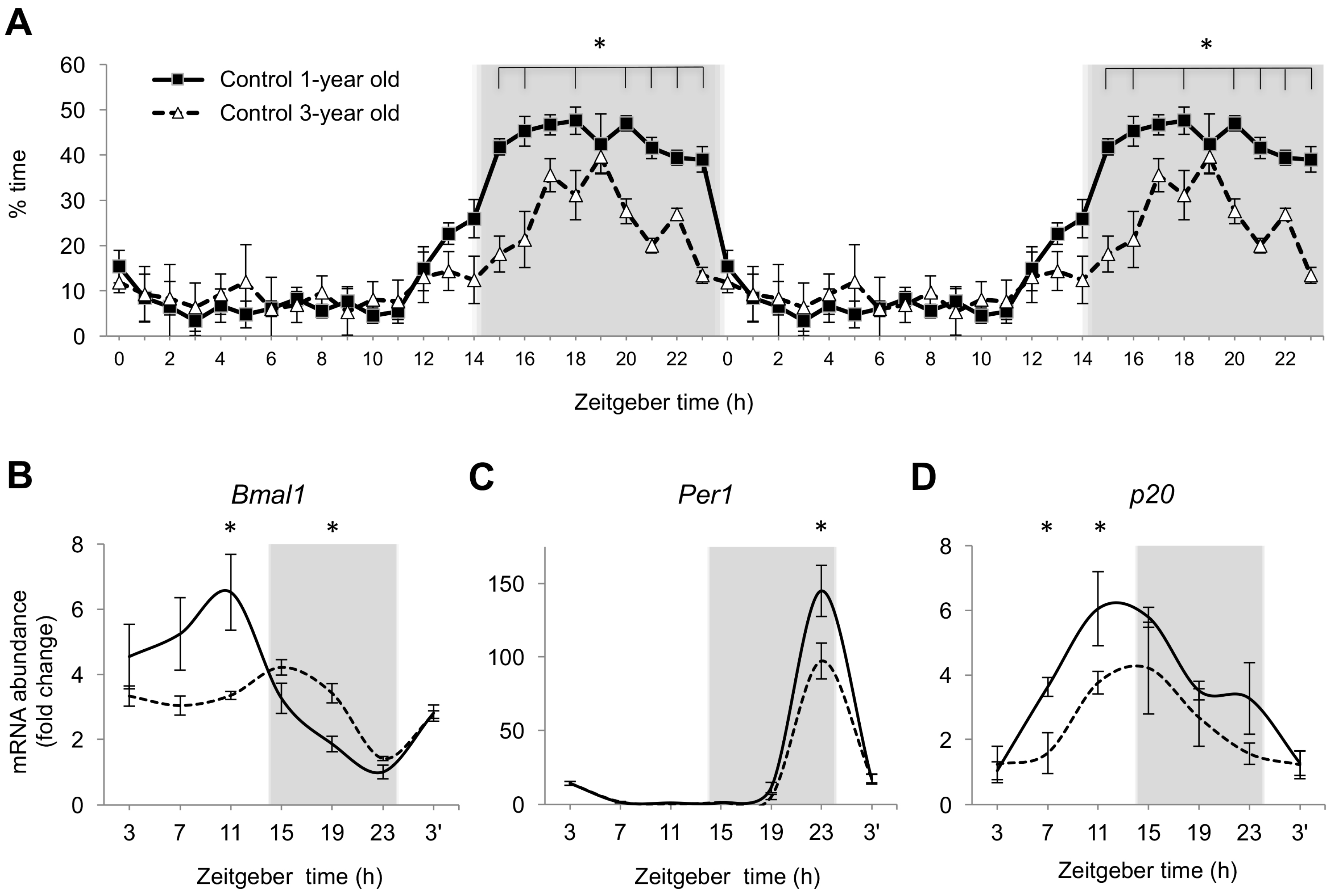
2.4. Age-Dependent Changes in Nighttime Sleep and Gene Expression in Zebrafish Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Feeding
4.3. Behavioral Activity Recordings
4.4. Treatments
4.4.1. 5-Ethynyl-2′-deoxyuridine (EdU) Treatment
4.4.2. Diazepam
4.5. EdU Staining
4.6. Microscopy, Analysis and Brain Volume Estimates
4.7. Real-Time Quantitative RT-PCR
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HCI | High caloric intake |
| DPF | Days post fertilization |
| EdU | 5-Ethynyl-2′-deoxyuridine |
| CDC | Cell division cycle |
| i.p. | Intraperitoneal |
| PBS | Phosphate-buffered saline |
| LD | Light-dark |
| ZT | Zeitgeber time |
| S-phase | Synthesis phase |
| SEM | Standard error of the mean |
References
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Yaturu, S. Obesity and type 2 diabetes. J. Diabetes Mellit. 2011, 1, 79–95. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, 102–138. [Google Scholar] [CrossRef] [PubMed]
- Akinnusi, M.E.; Saliba, R.; Porhomayon, J.; El-Solh, A.A. Sleep disorders in morbid obesity. Eur. J. Intern. Med. 2012, 23, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.A.; Guarente, L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 2007, 447, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Ergul, A.; Ozdemir, T.A.; Adams, M.M. Aging, Neurogenesis, and Caloric Restriction in Different Model Organisms. Aging Dis. 2013, 4, 221–232. [Google Scholar] [PubMed]
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obesity 2008, 16, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.-B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Després, J.P.; Bouchard, C.; Tremblay, A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia 2007, 50, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Scheer, F.A.J.L. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Knutson, K.; Leproult, R.; Tasali, E.; Van Cauter, E. Sleep loss: A novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. 2005, 99, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L. Sleep duration and cardiometabolic risk: A review of the epidemiologic evidence. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Keckeis, M.; Lattova, Z.; Maurovich-Horvat, E.; Beitinger, P.A.; Birkmann, S.; Lauer, C.J.; Wetter, T.C.; Wilde-Frenz, J.; Pollmächer, T. Impaired glucose tolerance in sleep disorders. PLoS ONE 2010, 5, e9444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutson, K.L.; Van Cauter, E.; Zee, P.; Liu, K.; Lauderdale, D.S. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: The Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care 2011, 34, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Rudic, R.D.; McNamara, P.; Curtis, A.M.; Boston, R.C.; Panda, S.; Hogenesch, J.B.; Fitzgerald, G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004, 2, e377. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Carter, A.M.; Grant, P.J. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 2008, 32, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Gemma, C.; Gianotti, T.F.; Burgueño, A.; Castaño, G.; Pirola, C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008, 87, 1606–1615. [Google Scholar] [PubMed]
- Uemura, H.; Katsuura-Kamano, S.; Yamaguchi, M.; Arisawa, K.; Hamajima, N.; Hishida, A.; Kawai, S.; Oze, I.; Shinchi, K.; Takashima, N.; et al. A variant of the CLOCK gene and related haplotypes are associated with the prevalence of type 2 diabetes in the Japanese population. J. Diabetes 2015, 8, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Asensio, E.M.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Lapetra, J.; et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovasculardiseases in type-2 diabetic subjects: Dietary modulation in the PREDIMED randomized trial. Cardiovasc. Diabetol. 2016, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Smith, C.E.; Gomez-Abellán, P.; Ordovás-Montañés, M.; Lee, Y.C.; Parnell, L.D.; Arnett, D.K.; Ordovás, J.M. REV-ERB-ALPHA circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol. Nutr. Food Res. 2014, 58, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Chaudhari, A.; Gupta, R.; Velingkaar, N.; Kondratov, R.V. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 2016, 30, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Akagi, K.; Bose, N.; Rakshit, K.; Camarella, T.; Zheng, X.; Hall, D.; Davis, S.; Nelson, C.S.; Brem, R.B.; et al. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab. 2016, 23, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Etienne, C. Minireview: Entrainment of the Suprachiasmatic Clockwork in Diurnal and Nocturnal Mammals. Endocrinology 2007, 148, 5648–5655. [Google Scholar]
- Kumar, J.P.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 2015, 418, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [PubMed]
- Whitmer, R.A.; Gustafson, D.R.; Barrett-Connor, E.; Haan, M.N.; Gunderson, E.P.; Yaffe, K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008, 72, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Harling, H.; Darzi, A.; Athanasiou, T. Neurodegenerative disease and obesity: What is the role of weight loss and bariatric interventions? Metab. Brain Dis. 2013, 28, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; de Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duan, W.; Long, J.M.; Ingram, D.K.; Mattson, M.P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 2000, 15, 99–108. [Google Scholar] [CrossRef]
- Lee, J.; Seroogy, K.B.; Mattson, M.P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 2000, 80, 539–547. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2000, 82, 1367–1375. [Google Scholar] [CrossRef]
- Bondolfi, L.; Ermini, F.; Long, J.M.; Ingram, D.K.; Jucker, M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging 2004, 25, 333–340. [Google Scholar] [CrossRef]
- Kumar, S.; Parkash, J.; Kataria, H.; Kaur, G. Interactive effect of excitotoxic injury and dietary restriction on neurogenesis and neurotrophic factors in adult male rat brain. Neurosci. Res. 2009, 65, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Stangl, D.; Thuret, S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009, 4, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, J.; Kaneko, M.; Cardone, L.; Cahill, G.; Sassone-Corsi, P. Analysis of circadian rhythms in zebrafish. Methods Enzymol. 2005, 393, 186–204. [Google Scholar] [PubMed]
- Zhdanova, I.V. Sleep in zebrafish. Zebrafish 2006, 3, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Slack, B.E.; Uchiyama, J.; Zhdanova, I.V. Zebrafish as a genetic model in biological and behavioral gerontology: Where development meets aging in vertebrates—A mini-review. Gerontology 2009, 55, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, G.S.; Kauffman, E.J.; Wang, X.; Stewart, R.; Moore, J.L.; Kasales, C.J.; Demidenko, E.; Cheng, K.C. Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio). Exp. Gerontol. 2002, 37, 1055–1068. [Google Scholar] [CrossRef]
- Tsai, S.B.; Tucci, V.; Uchiyama, J.; Fabian, N.J.; Lin, M.C.; Bayliss, P.E.; Neuberg, D.S.; Zhdanova, I.V.; Kishi, S. Differential effects of genotoxic stress on both concurrent body growth and gradual senescence in the adult zebrafish. Aging Cell 2007, 6, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Bayliss, P.E.; Uchiyama, J.; Koshimizu, E.; Qi, J.; Nanjappa, P.; Imamura, S.; Islam, A.; Neuberg, D.; Amsterdam, A.; et al. The identification of zebrafish mutants showing alterations in senescence-associated biomarkers. PLoS Genet. 2008, 4, e1000152. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, V.; Patel, N.A.; Riley, E.; Yu, L.; Yeh, J.R.; Zhdanova, I.V. Dopaminergic control of anxiety in young and aged zebrafish. Pharmacol. Biochem. Behav. 2017, 1, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tucci, V.; Kishi, S.; Zhdanova, I.V. Cognitive aging in zebrafish. PLoS ONE 2006, 1, e14. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.J.; Zerulla, T.C.; Tierney, K.B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, I.V.; Yu, L.; Lopez-Patino, M.; Shang, E.; Kishi, S.; Guelin, E. Aging of the circadian system in zebrafish and the effects of melatonin on sleep and cognitive performance. Brain Res. Bull. 2008, 75, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef] [PubMed]
- López-Patiño, M.A.; Yu, L.; Cabral, H.; Zhdanova, I.V. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol. Behav. 2008, 93, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Akle, V.; Stankiewicz, A.J.; Kharchenko, V.; Yu, L.; Kharchenko, P.V.; Zhdanova, I.V. Circadian Kinetics of Cell Cycle Progression in Adult Neurogenic Niches of a Diurnal Vertebrate. J. Neurosci. 2017, 37, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.; Catenacci, V.; Wyatt, H.R.; Hill, J.O. Obesity: Overview of an epidemic. Psychiatr. Clin. N. Am. 2011, 34, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.J.; Ferraro, K.F. Aging, Obesity, and Mortality Misplaced Concern about Obese Older People? Res. Aging 2004, 26, 108–129. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Yan, J.H.; Payne, V.G. The Impact of Obesity and Exercise on Cognitive Aging. Front. Aging Neurosci. 2013, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Halvorson, E.E.; Cohen, G.M.; Lazorick, S.; Skelton, J.A. Addressing Childhood Obesity: Opportunities for Prevention. Pediatr. Clin. N. Am. 2015, 62, 1241–1261. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, M.; Birken, C.; Hamilton, J. Childhood Obesity: Causes, Consequences, and Management. Pediatr. Clin. N. Am. 2015, 62, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Hong, H.K.; Bass, J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. 2014, 40, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Solanas, G.; Peixoto, F.O.; Bee, L.; Symeonidi, A.; Schmidt, M.; Brenner, C.; Masri, S.; Benitah, S.A.; Sassone-Corsi, P. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 2017, 170, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.; Amir, S. Neurodegeneration and the Circadian Clock. Front. Aging Neurosci. 2017, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Bass, J. Circadian Mechanisms in Bioenergetics and Cell Metabolism. In A Time for Metabolism and Hormones; Sassone-Corsi, P., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 132–144. ISBN 978-3-319-27069-2. [Google Scholar]
- Tamai, T.K.; Carr, A.J.; Whitmore, D. Zebrafish circadian clocks: Cells that see light. Biochem. Soc. Trans. 2005, 33, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, I.V.; Wang, S.Y.; Leclair, O.U.; Danilova, N.P. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001, 903, 263–268. [Google Scholar] [CrossRef]
- Levitas-Djerbi, T.; Appelbaum, L. Modeling sleep and neuropsychiatric disorders in zebrafish. Curr. Opin. Neurobiol. 2017, 44, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, T.; Marin, W.; Faraco, J.; Pézeron, G.; Appelbaum, L.; Zhang, J.; Rosa, F.; Mourrain, P.; Mignot, E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007, 5, e277. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, L.; Wang, G.X.; Maro, G.S.; Mori, R.; Tovin, A.; Marin, W.; Yokogawa, T.; Kawakami, K.; Smith, S.J.; Gothilf, Y.; et al. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 21942–21947. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, I.; Zada, D.; Tovin, A.; Braun, T.; Lerer-Goldshtein, T.; Wang, G.; Mourrain, P.; Appelbaum, L. Sleep-Dependent Structural Synaptic Plasticity of Inhibitory Synapses in the Dendrites of Hypocretin/Orexin Neurons. Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Singh, C.; Oikonomou, G.; Prober, D.A. Genetic Analysis of Histamine Signaling in Larval Zebrafish Sleep. eNeuro 2017. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Prober, D.A. Attacking sleep from a new angle: Contributions from zebrafish. Curr. Opin. Neurobiol. 2017, 44, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, I.; Levitas-Djerbi, T.; Appelbaum, L. The Hypocretin/Orexin Neuronal Networks in Zebrafish. Curr. Top. Behav. Neurosci. 2017, 33, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.; Hinsch, K.; Gage, F.H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005, 488, 290–319. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, D.; Foulkes, N.S.; Sassone-Corsi, P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature 2000, 404, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Pan, F.; Jones, L.A.; Lim, M.M.; Griffin, E.A.; Sheline, Y.I.; Mintun, M.A.; Holtzman, D.M.; Mach, R.H. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010, 1319, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Smthison, K.G.; Sieck, G.C. Application of the Cavalieri principle in volume estimation using laser confocal microscopy. Neuroimage 1994, 1, 325–333. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stankiewicz, A.J.; McGowan, E.M.; Yu, L.; Zhdanova, I.V. Impaired Sleep, Circadian Rhythms and Neurogenesis in Diet-Induced Premature Aging. Int. J. Mol. Sci. 2017, 18, 2243. https://doi.org/10.3390/ijms18112243
Stankiewicz AJ, McGowan EM, Yu L, Zhdanova IV. Impaired Sleep, Circadian Rhythms and Neurogenesis in Diet-Induced Premature Aging. International Journal of Molecular Sciences. 2017; 18(11):2243. https://doi.org/10.3390/ijms18112243
Chicago/Turabian StyleStankiewicz, Alexander J., Erin M. McGowan, Lili Yu, and Irina V. Zhdanova. 2017. "Impaired Sleep, Circadian Rhythms and Neurogenesis in Diet-Induced Premature Aging" International Journal of Molecular Sciences 18, no. 11: 2243. https://doi.org/10.3390/ijms18112243



