Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses
Abstract
:1. Introduction
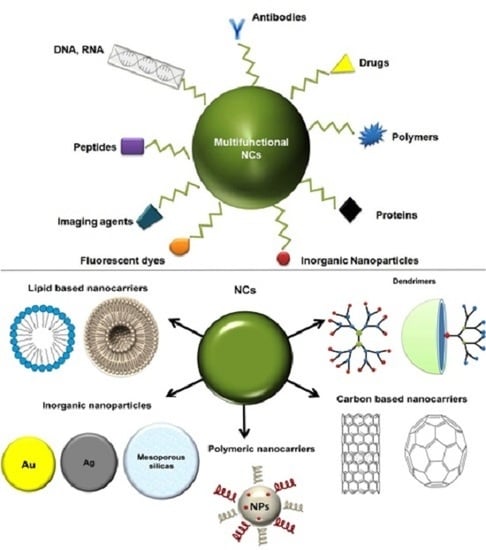
2. Types of Nanocarriers
2.1. Carbon-Based Nanoparticles
2.1.1. Carbon Nanotubes (CNTs)
2.1.2. Graphene (GF) and Graphene Oxide (GO)
2.1.3. Nanodiamonds (NDs)
2.2. Inorganic Nanoparticles
2.2.1. Gold Nanoparticles (GNPs, AuNPs)
2.2.2. Silver Nanoparticles (AgNPs)
2.2.3. Iron Oxide Nanoparticles (IONPs)
2.3. Mesoporous Nanoparticles (MSN or MSNPs)
2.4. Lipid-Based Nanoparticles (L-NPs)
2.4.1. Solid Lipid Nanoparticles (SLNs)
2.4.2. Nanostructured Lipid Carriers (NLCs)
2.4.3. Multifunctional SLNs and NLCs
2.4.4. Liposomes
2.5. Dendrimers
2.6. Polymeric NPs-NCs
2.6.1. NCs Derived from Natural Polymers
2.6.2. Chitosan NCs
2.6.3. Hyaluronic Acid (HA)
2.6.4. Alginic Acid and Its Salts
2.6.5. NCs of Synthetic Polymers
3. Future Aspects
4. Conclusions
Author Contributions
Conflicts of Interest
References
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Siafaka, P.; Betsiou, M.; Tsolou, A.; Angelou, E.; Agianian, B.; Koffa, M.; Chaitidou, S.; Karavas, E.; Avgoustakis, K.; Bikiaris, D. Synthesis of folate-pegylated polyester nanoparticles encapsulating ixabepilone for targeting folate receptor overexpressing breast cancer cells. J. Mater. Sci. Mater. Med. 2015, 26, 275. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticles—A historical perspective. Int. J. Pharm. 2007, 331, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.; Zhang, X.; Nadeau, J. Nanoparticle drug formulations for cancer diagnosis and treatment. Crit. Rev. Oncog. 2014, 19, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Estanqueiro, M.; Amaral, M.H.; Conceição, J.; Sousa Lobo, J.M. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surf. B Biointerfaces 2015, 126, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachari, Y.; Geary, S.M.; Lemke, C.D.; Salem, A.K. Nanoparticle delivery systems in cancer vaccines. Pharm. Res. 2011, 28, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Sahdev, P.; Ochyl, L.J.; Moon, J.J. Biomaterials for nanoparticle vaccine delivery systems. Pharm. Res. 2014, 31, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Kaminski, G.A.T.; Sierakowski, M.R.; Pontarolo, R.; Santos, L.A.; De Freitas, R.A. Layer-by-layer polysaccharide-coated liposomes for sustained delivery of epidermal growth factor. Carbohydr. Polym. 2016, 140, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z.; Tziveleka, L.-A. Drug delivery using multifunctional dendrimers and hyperbranched polymers. Expert Opin. Drug Deliv. 2010, 7, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Carrow, J.K.; Gaharwar, A.K. Nanomaterials for engineering stem cell responses. Adv. Healthc. Mater. 2015, 4, 1600–1627. [Google Scholar] [CrossRef] [PubMed]
- Mekaru, H.; Lu, J.; Tamanoi, F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015, 95, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Karimi, M.; Mirshekari, H.; Aliakbari, M.; Sahandi-Zangabad, P.; Hamblin, M.R. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnol. Rev. 2016, 5, 195–207. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 302–315. [Google Scholar] [CrossRef]
- Filippousi, M.; Siafaka, P.I.; Amanatiadou, E.P.; Nanaki, S.G.; Neratzaki, M.; Bikiaris, D.N.; Vizirianakis, I.S.; van Tendeloo, G. Modified chitosan coated mesoporous strontium hydroxyapatite nanorods as drug carriers. J. Mater. Chem. B 2015, 3, 5991–6000. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Jawahar, N.; Surendra, E.; Radha, K. A review on carbon nanotubes: A novel drug carrier for targeting to cancer cells. J. Pharm. Sci. Res. 2015, 7, 141–154. [Google Scholar]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Andujar, P.; Kermanizadeh, A.; Boczkowski, J. Determinants of carbon nanotube toxicity. Adv. Drug Deliv. Rev. 2013, 65, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- McShan, D.; Yu, H. DNA damage in human skin keratinocytes caused by multiwalled carbon nanotubes with carboxylate functionalization. Toxicol. Ind. Health 2012, 30, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.; Zeng, L.; Zhao, Z.; Chen, T. Functionalized multiwalled carbon nanotubes as carriers of ruthenium complexes to antagonize cancer multidrug resistance and radioresistance. ACS Appl. Mater. Interfaces 2015, 7, 14933–14945. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.M.; Arulselvan, P.; Fakurazi, S.; Ithnin, H.; Hussein, M.Z. A review on characterizations and biocompatibility of functionalized carbon nanotubes in drug delivery design. J. Nanomater. 2014, 2014, e917024. [Google Scholar] [CrossRef]
- Du, J.; Wang, S.; You, H.; Zhao, X. Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: A review. Environ. Toxicol. Pharmacol. 2013, 36, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Dinan, N.M.; Atyabi, F.; Rouini, M.R.; Amini, M.; Golabchifar, A.A.; Dinarvand, R. Doxorubicin loaded folate-targeted carbon nanotubes: Preparation, cellular internalization, in vitro cytotoxicity and disposition kinetic study in the isolated perfused rat liver. Mater. Sci. Eng. C 2014, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.W.; Fabbro, C.; Venturelli, E.; Mnard-Moyon, C.; Chaloin, O.; Da Ros, T.; Methven, L.; Nunes, A.; Sosabowski, J.K.; Mather, S.J.; et al. The relationship between the diameter of chemically-functionalized multi-walled carbon nanotubes and their organ biodistribution profiles in vivo. Biomaterials 2014, 35, 9517–9528. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.-W.; Rubio, N.; Kafa, H.; Venturelli, E.; Fabbro, C.; Ménard-Moyon, C.; Da Ros, T.; Sosabowski, J.K.; Lawson, A.D.; Robinson, M.K.; et al. Kinetics of functionalised carbon nanotube distribution in mouse brain after systemic injection: Spatial to ultra-structural analyses. J. Control. Release 2016, 224, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Koromilas, N.D.; Lainioti, G.C.; Gialeli, C.; Barbouri, D.; Kouravelou, K.B.; Karamanos, N.K.; Voyiatzis, G.A.; Kallitsis, J.K. Preparation and toxicological assessment of functionalized carbon nanotube-polymer hybrids. PLoS ONE 2014, 9, e107029. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.K.; Jain, N.K. Multifunctional hybrid-carbon nanotubes: New horizon in drug delivery and targeting. J. Drug Target. 2016, 24, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Modification strategies for carbon nanotubes as a drug delivery system. Ind. Eng. Chem. Res. 2013, 52, 13517–13527. [Google Scholar] [CrossRef]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.; Hosseini, S.H.; Adeli, M.; Pourjavadi, A. Polymer-functionalized carbon nanotubes in cancer therapy: A review. Iran. Polym. J. 2014, 23, 387–403. [Google Scholar] [CrossRef]
- Nagaraju, K.; Reddy, R.; Reddy, N. A review on protein functionalized carbon nanotubes. J. Appl. Biomater. Funct. Mater. 2015, 13, e301–e312. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Mazzaglia, A.; Scala, A.; Pistone, A.; Galvagno, S.; Lanza, M.; Riccucci, C.; Ingo, G.M.; Colao, I.; Sciortino, M.T.; et al. β-Cyclodextrin-grafted on multiwalled carbon nanotubes as versatile nanoplatform for entrapment of guanine-based drugs. Colloids Surf. B Biointerfaces 2014, 123, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.M.; Karthivashan, G.; Arulselvan, P.; Fakurazi, S.; Hussein, M.Z. Characterization and in vitro studies of the anticancer effect of oxidized carbon nanotubes functionalized with betulinic acid. Drug Des. Devel. Ther. 2014, 8, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholtz, C.D.; Hadimani, M.; King, S.B.; Torti, S.V.; Singh, R. Targeting breast cancer with sugar-coated carbon nanotubes. Nanomedicine 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Salmasi, Z.; Hashemi, M.; Mosaffa, F.; Abnous, K.; Ramezani, M. Single-walled carbon nanotubes functionalized with aptamer and piperazine-polyethylenimine derivative for targeted siRNA delivery into breast cancer cells. Int. J. Pharm. 2015, 485, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Foillard, S.; Lammers, T.; Schiffelers, R.M.; Doris, E.; Hennink, W.E.; Storm, G. SiRNA delivery with functionalized carbon nanotubes. Int. J. Pharm. 2011, 416, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.S.; Chen, D.; Zheng, X.; Zhang, X.; Johnston, N.; Liu, Y.; Yuan, K.; Koropatnick, J.; Gillies, E.R.; Min, W.P. Non-covalently functionalized single-walled carbon nanotube for topical sirna delivery into melanoma. Biomaterials 2014, 35, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tao, L.; Wen, S.; Hou, W.; Shi, X. Hyaluronic acid-modified multiwalled carbon nanotubes for targeted delivery of doxorubicin into cancer cells. Carbohydr. Res. 2015, 405, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shi, H.; Zhang, H.; Wang, X.; Yang, Y.; Yu, C.; Hao, C.; Du, J.; Hu, H.; Yang, S. Prostate stem cell antigen antibody-conjugated multiwalled carbon nanotubes for targeted ultrasound imaging and drug delivery. Biomaterials 2014, 35, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Grimes, S.W.; Lewis, R.L.; Alexis, F. Multilayered polymer-coated carbon nanotubes to deliver dasatinib. Mol. Pharm. 2014, 11, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Apartsin, E.K.; Buyanova, M.Y.; Novopashina, D.S.; Ryabchikova, E.I.; Filatov, A.V.; Zenkova, M.A.; Venyaminova, A.G. Novel multifunctional hybrids of single-walled carbon nanotubes with nucleic acids: Synthesis and interactions with living cells. ACS Appl. Mater. Interfaces 2014, 6, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hasumura, T.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Accelerated killing of cancer cells using a multifunctional single-walled carbon nanotube-based system for targeted drug delivery in combination with photothermal therapy. Int. J. Nanomed. 2013, 8, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Liu, H.; Cai, H.; Shen, M.; Shi, X. Targeted and pH-responsive delivery of doxorubicin to cancer cells using multifunctional dendrimer-modified multi-walled carbon nanotubes. Adv. Healthc. Mater. 2013, 2, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, J.; Bi, Y.; Xu, X.; Zhou, H.; Gao, J.; Hu, Y.; Zhao, Y.; Chai, Z. Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.J.; Sun, L.; Liu, Y.; Jiang, S.; Pu, Y.; Li, J.; Zhang, Y. Monodistearoylphosphatidylethanolamine-hyaluronic acid functionalization of single-walled carbon nanotubes for targeting intracellular drug delivery to overcome multidrug resistance of cancer cells. Carbon 2016, 96, 362–376. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, L.; Yang, X.; Ren, J.; Wang, Y.; Zhang, H.; Feng, Q.; Shi, Y.; Shan, X.; Yuan, Y. A novel redox-sensitive system based on single-walled carbon nanotubes for chemo-photothermal therapy and magnetic resonance imaging. Int. J. Nanomed. 2016, 11, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Duran, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahlinder, L.; Henych, J.; Lindström, S.W.; Ekstrand-Hammarström, B.; Stengl, V.; Österlund, L. Graphene oxide nanoparticle attachment and its toxicity on living lung epithelial cells. RSC Adv. 2015, 5, 59447–59457. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, F.; Li, S.; Hu, Z.; Zhao, Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Ibrahim, I.; Ioannides, N.; Trzebicka, B.; Hampel, S.; Rümmeli, M.H. Graphene oxide-based drug delivery vehicles: Functionalization, characterization, and cytotoxicity evaluation. J. Nanopart. Res. 2013, 15, 2099. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. The advancing uses of nano-graphene in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Grüner, G.; Zhao, Y. Recent advancements of graphene in biomedicine. J. Mater. Chem. B 2013, 1, 2542–2567. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ma, X.Q.; Li, C.M. Highly efficient nuclear delivery of anti-cancer drugs using a bio-functionalized reduced graphene oxide. J. Colloid Interface Sci. 2016, 467, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Tao, L.; Annie Bligh, S.W.; Yang, H.; Pan, Q.; Zhu, L. Targeted delivery and controlled release of doxorubicin into cancer cells using a multifunctional graphene oxide. Mater. Sci. Eng. C 2016, 59, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, F.; Han, H.; Yang, L.; Zhang, G.; Fan, Z. Functionalized graphene oxide as a drug carrier for loading pirfenidone in treatment of subarachnoid hemorrhage. Colloids Surf. B Biointerfaces 2015, 129, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Sahu, A.; Jang, C.; Tae, G. The effect of ligand density on in vivo tumor targeting of nanographene oxide. J. Control. Release 2015, 209, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, K.; Luo, Z.; Duan, Y. Preparation and tumor cell model based biobehavioral evaluation of the nanocarrier system using partially reduced graphene oxide functionalized by surfactant. Int. J. Nanomed. 2015, 10, 4605–4620. [Google Scholar]
- Feng, L.; Zhang, S.; Liu, Z. Graphene based gene transfection. Nanoscale 2011, 3, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.M.; Chen, Y.; Zhao, D.; Chen, J.T.; Liu, Y. Construction of a graphene oxide based noncovalent multiple nanosupramolecular assembly as a scaffold for drug delivery. Chem. A Eur. J. 2012, 18, 4208–4215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, L.; Li, X.; Jia, X.; Liu, L.; Zeng, J.; Guo, J.; Liu, P. Functionalized graphene oxide nanoparticles for cancer cell-specific delivery of antitumor drug. Bioconjug. Chem. 2015, 26, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Liu, L.; Li, X.R.; Zeng, J.; Jia, X.; Liu, P. Biocompatible graphene oxide nanoparticle-based drug delivery platform for tumor microenvironment-responsive triggered release of doxorubicin. Langmuir 2014, 30, 10419–10429. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Dong, H.; Jia, X.; Guo, W.; Lu, H.; Yang, Y.; Ju, H.; Zhang, X.; Hu, Y. Functionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitro. PLoS ONE 2013, 8, e60034. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Feng, Q.; Wang, Y.; Yang, X.; Ren, J.; Shi, Y.; Shan, X.; Yuan, Y.; Wang, Y.; Zhang, Z. Multifunctional hyaluronic acid modified graphene oxide loaded with mitoxantrone for overcoming drug resistance in cancer. Nanotechnology 2016, 27, 015701. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cai, X.; Li, H.; Lin, Y.; Du, D. Hyaluronic acid-modified multifunctional Q-graphene for targeted killing of drug-resistant lung cancer cells. ACS Appl. Mater. Interfaces 2016, 8, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Lao, J.; He, H.; Cheng, T.; Wang, M.; Wang, S.; Huang, F. Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surf. B Biointerfaces 2014, 122, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Tehrani, Z.M.; Jokar, S. Functionalized mesoporous silica-coated magnetic graphene oxide by polyglycerol-G-polycaprolactone with pH-responsive behavior: Designed for targeted and controlled doxorubicin delivery. J. Ind. Eng. Chem. 2015, 28, 45–53. [Google Scholar] [CrossRef]
- Wang, C.; Ravi, S.; Garapati, U.S.; Das, M.; Howell, M.; MallelaMallela, J.; Alwarapan, S.; Mohapatra, S.S.; Mohapatra, S. Multifunctional chitosan magnetic-graphene (CMG) nanoparticles: A theranostic platform for tumor-targeted co-delivery of drugs, genes and MRI contrast agents. J. Mater. Chem. B. Mater. Biol. Med. 2013, 1, 4396–4405. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, Q.; Yu, N.; Li, J.; Yang, L.; Yang, R.; Wang, C. Aptamer–conjugated graphene oxide-gold nanocomposites for targeted chemo-photothermal therapy of cancer cells. J. Mater. Chem. B 2015, 3, 4036–4042. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Lin, Y.C.; Jani, M.; Cheng, C.L. Biomedical applications of nanodiamonds in imaging and therapy. Nanomedicine 2013, 8, 2041–2060. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Badea, I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int. J. Nanomed. 2013, 8, 203–220. [Google Scholar]
- Vaijayanthimala, V.; Lee, D.K.; Kim, S.V.; Yen, A.; Tsai, N.; Ho, D.; Chang, H.-C.; Shenderova, O. Nanodiamond-mediated drug delivery and imaging: Challenges and opportunities. Expert Opin. Drug Deliv. 2015, 12, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Barnard, A.S. Functionalized nanodiamonds for biological and medical applications. J. Nanosci. Nanotechnol. 2015, 15, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Passeri, D.; Rinaldi, F.; Ingallina, C.; Carafa, M.; Rossi, M.; Terranova, M.L.; Marianecci, C. Biomedical applications of nanodiamonds: An overview. J. Nanosci. Nanotechnol. 2015, 15, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, Y.H.; Akasaka, T.; Abe, S.; Komatsu, N.; Watari, F.; Chen, X. Polyglycerol-coated nanodiamond as a macrophage-evading platform for selective drug delivery in cancer cells. Biomaterials 2014, 35, 5393–5406. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, Y.; Zhang, J.; Kong, H.; Tang, X.; Pan, L.; Xia, K.; Aldalbahi, A.; Li, A.; Tai, R.; Fan, C.; Zhu, Y. Sodium alginate-functionalized nanodiamonds as sustained chemotherapeutic drug-release vectors. Carbon 2016, 97, 78–86. [Google Scholar] [CrossRef]
- Badea, I.; Alwani, S.; Kaur, R.; Michel, D.; Chitanda, J.M.; Verrall, R.; Karunakaran, C. Lysine-functionalized nanodiamonds as gene carriers: Development of stable colloidal dispersion for in vitro cellular uptake studies and siRNA delivery application. Int. J. Nanomed. 2016, 11, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tong, Y.; Li, Y.; Tian, Z.; Cao, R.; Yang, B. PEGylated nanodiamond for chemotherapeutic drug delivery. Diam. Relat. Mater. 2013, 36, 26–34. [Google Scholar] [CrossRef]
- Man, H.B.; Kim, H.; Kim, H.J.; Robinson, E.; Liu, W.K.; Chow, E.K.H.; Ho, D. Synthesis of nanodiamond-daunorubicin conjugates to overcome multidrug chemoresistance in leukemia. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 359–369. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Al-Daihan, S. On the toxicity of therapeutically used nanoparticles: An overview. J. Toxicol. 2009, 2009, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Vigderman, L.; Zubarev, E.R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv. Drug Deliv. Rev. 2013, 65, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Kodiha, M.; Wang, Y.M.; Hutter, E.; Maysinger, D.; Stochaj, U. Off to the organelles—Killing cancer cells with targeted gold nanoparticles. Theranostics 2015, 5, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 1401, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Jalalpure, S.; Jadhav, K. Doxorubicin functionalized gold nanoparticles: Characterization and activity against human cancer cell lines. Process. Biochem. 2015, 50, 2298–2306. [Google Scholar] [CrossRef]
- Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Gover Antoniraj, M.; Ruckmani, K. Hyaluronic acid co-functionalized gold nanoparticle complex for the targeted delivery of metformin in the treatment of liver cancer (HepG2 cells). Carbohydr. Polym. 2015, 128, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, C.; Das, A.; Chakraborty, A. Controlled release of a sparingly water-soluble anticancer drug through pH-responsive functionalized gold-nanoparticle-decorated liposomes. ChemPhysChem 2015, 16, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Adokoh, C.K.; Quan, S.; Hitt, M.; Darkwa, J.; Kumar, P.; Narain, R. Synthesis and evaluation of glycopolymeric decorated gold nanoparticles functionalized with gold-triphenyl phosphine as anti-cancer agents. Biomacromolecules 2014, 15, 3802–3810. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Almeida, G.M.; Pereira, M.C.; Santos-Silva, F.; Coelho, M.A.N. Functionalized gold nanoparticles improve afatinib delivery into cancer cells. Expert Opin. Drug Deliv. 2016, 13, 133–141. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.J.; Johnson, J.A. PEGylated N-heterocyclic carbene anchors designed to stabilize gold nanoparticles in biologically relevant media. J. Am. Chem. Soc. 2015, 137, 7974–7977. [Google Scholar] [CrossRef] [PubMed]
- Stellacci, F.; Carney, R.P.; Van Lehn, R.C.; Irvine, D.J.; Alexander-Katz, A.; Yang, Y.-S.; Atukorale, P.U. Effect of particle diameter and surface composition on the spontaneous fusion of monolayer-protected gold nanoparticles with lipid bilayers. Nano Lett. 2013, 13, 4060–4067. [Google Scholar]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Wei, S.C.; Chang, H.T.; Lin, H.J.; Huang, C.C. Gold nanoparticles modified with self-assembled hybrid monolayer of triblock aptamers as a photoreversible anticoagulant. J. Control. Release 2016, 221, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, Z.; Wang, L.; Wang, B.; Xu, L.; Hou, L.; Zhang, Z. A tumor-specific cleavable nanosystem of PEG-modified C60@Au hybrid aggregates for radio frequency-controlled release, hyperthermia, photodynamic therapy and X-ray imaging. Acta Biomater. 2016, 29, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799. [Google Scholar] [CrossRef]
- Gerber, A.; Bundschuh, M.; Klingelhofer, D.; Groneberg, D.A. Gold nanoparticles: Recent aspects for human toxicology. J. Occup. Med. Toxicol. 2013, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Bozich, J.; Lohse, S.; Torelli, M. Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environ. Sci. Nano 2014, 1, 260–270. [Google Scholar] [CrossRef]
- Singh, M.; Harris-Birtill, D.C.C.; Markar, S.R.; Hanna, G.B.; Elson, D.S. Application of gold nanoparticles for gastrointestinal cancer theranostics: A systematic review. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2083–2098. [Google Scholar] [CrossRef] [PubMed]
- Coradeghini, R.; Gioria, S.; García, C.P.; Nativo, P.; Franchini, F.; Gilliland, D.; Ponti, J.; Rossi, F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 2013, 217, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Haider, A.; Kang, I.-K. Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive review. Adv. Mater. Sci. Eng. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F.R. Carbohydrate nanocarriers in biomedical applications: Functionalization and construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible hydrogel nanocomposite with covalently embedded silver nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; Orts-Gil, G.; Lai, C.-H.; Müller, L.; Haase, A.; Luch, A.; Seeberger, P.H. Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake. J. Nanobiotechnol. 2014, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Benyettou, F.; Rezgui, R.; Ravaux, F.; Jaber, T.; Blumer, K.; Jouiad, M.; Motte, L.; Olsen, J.-C.; Platas-Iglesias, C.; Magzoub, M.; Trabolsi, A. Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J. Mater. Chem. B 2015, 3, 7237–7245. [Google Scholar] [CrossRef]
- Lima-Tenorio, M.K.; Gomez Pineda, E.A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.K.; Padmanabhan, P.; Selvan, S.T. Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics 2013, 3, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dähring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.D.; Kadu, B.S.; Mansara, P.; Gupta, P.; Deore, A.V.; Chikate, R.C.; Poddar, P.; Dhole, S.D.; Kaul-Ghanekar, R. Synthesis, characterization and in vitro study of biocompatible cinnamaldehyde functionalized magnetite nanoparticles (CPGF Nps) for hyperthermia and drug delivery applications in breast cancer. PLoS ONE 2014, 9, e107315. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Wiench, J.W.; Trewyn, B.G.; Song, S.; Pruski, M.; Lin, V.S.-Y. Tuning of particle morphology and pore properties in mesoporous silicas with multiple organic functional groupselectronic supplementary information (ESI) available: Experimental details, SEM images, N2 adsorption isotherms, Pore size distributions, TEM Im. Chem. Commun. 2003, 18, 2364. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Gai, S.; Yang, P.; Li, C.; Ansari, M.B.; Lin, J. Stimuli responsive drug delivery application of polymer and silica in biomedicine. J. Mater. Chem. B 2015, 3, 8599–8622. [Google Scholar] [CrossRef]
- Lee, C.-H.; Cheng, S.-H.; Huang, I.-P.; Souris, J.S.; Yang, C.-S.; Mou, C.-Y.; Lo, L.-W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed. Engl. 2010, 49, 8214–8219. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.-Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Cui, Y.; Dong, H.; Cai, X.; Wang, D.; Li, Y. Mesoporous silica nanoparticles capped with disulfide-linked peg gatekeepers for glutathione-mediated controlled release. ACS Appl. Mater. Interfaces 2012, 4, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, F.; Nguyen, K.T.; Ma, X.; Wang, X.; Xing, B.; Zhao, Y. Multifunctional mesoporous silica nanoparticles for cancer-targeted and controlled drug delivery. Adv. Funct. Mater. 2012, 22, 5144–5156. [Google Scholar] [CrossRef]
- Aznar, E.; Coll, C.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P.; Cano, J.; Ruiz, E. Borate-driven gatelike scaffolding using mesoporous materials functionalised with saccharides. Chem. A Eur. J. 2009, 15, 6877–6888. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, B.; Luo, Z.; Ding, X.; Li, J.; Dai, L.; Zhou, J.; Zhao, X.; Ye, J.; Cai, K. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale 2015, 7, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Mondragón, L.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Barat, J.M.; Pérez-Payá, E.; Guillem, C.; et al. Enzyme-responsive intracellular controlled release using nanometric silica mesoporous supports capped with “saccharides”. ACS Nano 2010, 4, 6353–6368. [Google Scholar] [CrossRef] [PubMed]
- Mondragón, L.; Mas, N.; Ferragud, V.; de la Torre, C.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F.; Amorós, P.; Pérez-Payá, E.; Orzáez, M. Enzyme-responsive intracellular-controlled release using silica mesoporous nanoparticles capped with ε-poly-l-lysine. Chem. A Eur. J. 2014, 20, 5271–5281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Luo, G.F.; Zhu, J.Y.; Xu, X.D.; Zeng, X.; Cheng, D.B.; Li, Y.M.; Wu, Y.; Zhang, X.Z.; Zhuo, R.X.; et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, U.; Natarajan, T.K.; Wagner, H.N.; Johns, T.; Medical, H. Albumin for study of the reticuloendothelial system. J. Nucl. Med. 1972, 13, 498–503. [Google Scholar] [PubMed]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and “self-microemulsifying” drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, 93–98. [Google Scholar] [CrossRef]
- Shukla, D.; Chakraborty, S.; Singh, S.; Mishra, B. Lipid-based oral multiparticulate formulations—Advantages, technological advances and industrial applications. Expert Opin. Drug Deliv. 2011, 8, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Holm, R.; Mullertz, A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Rege, B.D.; Kao, J.P.Y.; Polli, J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Hauss, D.J. Oral lipid-based formulations. Adv. Drug Deliv. Rev. 2007, 59, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Andreani, T.; Macedo, A.S.; Fangueiro, J.F.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J. Drug Deliv. 2012, 2012, 750891. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.K.; Jain, A.; Singh, S. Studies on binary lipid matrix based solid lipid nanoparticles of repaglinide: In vitro and in vivo evaluation. J. Pharm. Sci. 2011, 100, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, R.; Li, F.; He, H.; Tang, X. The influence of lipid characteristics on the formation, in vitro release, and in vivo absorption of protein-loaded SLN prepared by the double emulsion process. Drug Dev. Ind. Pharm. 2011, 37, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Cristina Bonferoni, M.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012, 7, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, E.; Gokce, E.H.; Ozer, O. Development and evaluation of coenzyme Q10 loaded solid lipid nanoparticle hydrogel for enhanced dermal delivery. Acta Pharm. 2013, 63, 517–529. [Google Scholar]
- Jawahar, N.; Meyyanathan, S.N.; Reddy, G.; Sood, S. Solid lipid nanoparticles for oral delivery of poorly soluble drugs. J. Pharm. Sci. Res. 2012, 4, 1848–1855. [Google Scholar]
- Harde, H.; Das, M.; Jain, S. Solid lipid nanoparticles: An oral bioavailability enhancer vehicle. Expert Opin. Drug Deliv. 2011, 8, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, P.; Singhal, G.B.; Patel, R.P.; Prajapati, B.G.; Patel, N.A.; Feb, I. Solid lipid nanoparticles and nano lipid carriers: As novel solid lipid based drug carrier. Indian J. Pharm. Sci. 2011, 2, 40–52. [Google Scholar]
- Yadav, N.; Khatak, S.; Vir, U.; Sara, S. Solid lipid nanoparticles—A review. Int. J. Appl. Pharm. 2013, 5, 8–18. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Müller, R.H. Drug Delivery; Schäfer-Korting, M., Ed.; Springer Berlin Heidelberg: Berlin & Heidelberg, Gremany, 2010; pp. 115–141. [Google Scholar]
- Üner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspective. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavonoids loaded in nanocarriers: An opportunity to increase oral bioavailability and bioefficacy. Food Nutr. Sci. 2014, 5, 1212–1227. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Üstündağ-Okur, N.; Gökçe, E.H.; Bozbiyik, D.I.; Eǧrilmez, S.; Özer, Ö.; Ertan, G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur. J. Pharm. Sci. 2014, 63, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.W.; Rodriguez, B.L.; Li, X.; Hursting, S.D.; Williams, R.O.; Cui, Z. Solid lipid nanoparticle formulations of docetaxel prepared with high melting point triglycerides: In vitro and in vivo evaluation. Mol. Pharm. 2014, 11, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Rühl, D.; Runge, S.; Schulze-Forster, K.; Mehnert, W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharm. Res. 1997, 14, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.; Garse, H.; Kadam, V. Solid lipid nanoparticles and nanostructured lipid carriers: A review. Curr. Drug Ther. 2011, 6, 240–250. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, J.; Zhang, X.; Pan, W. Nanostructured lipid carrier (NLC) coated with chitosan oligosaccharides and its potential use in ocular drug delivery system. Int. J. Pharm. 2011, 403, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Li, V.H.; Wood, R.W.; Kreuter, J.; Harmia, T.; Robinson, J.R. Ocular drug delivery of progesterone using nanoparticles. J. Microencapsul. 1986, 3, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ-Okur, N.; Gökçe, E.H.; Bozbıyık, D.İ.; Eğrilmez, S.; Ertan, G.; Özer, Ö. Novel nanostructured lipid carrier-based inserts for controlled ocular drug delivery: Evaluation of corneal bioavailability and treatment efficacy in bacterial keratitis. Expert Opin. Drug Deliv. 2015, 12, 1791–1807. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, W.M.; Schwabe, K.; Müller, R.H.; Keck, C.M. Preservation of nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2010, 76, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.B.; Gonzalez-Gonzalez, E.; Spitler, R.; Shinde, R.; Leake, D.; Kaspar, R.L.; Contag, C.H.; Zare, R.N. Biodegradable nanoparticles with sustained release of functional siRNA in skin. J. Pharm. Sci. 2010, 99, 4261–4266. [Google Scholar] [CrossRef] [PubMed]
- Bondì, M.L.; Craparo, E.F.; Giammona, G.; Drago, F. Brain-targeted solid lipid nanoparticles containing riluzole: Preparation, characterization and biodistribution. Nanomedicine 2010, 5, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, E.; Kawano, K.; Hattori, Y.; Fukushima, M.; Hayashi, K.; Maitani, Y. Long-circulating liposome-encapsulated ganciclovir enhances the efficacy of HSV-TK suicide gene therapy. J. Control. Release 2007, 120, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Han, H.D.; Lee, A.; Hwang, T.; Song, C.K.; Seong, H.; Hyun, J.; Shin, B.C. Enhanced circulation time and antitumor activity of doxorubicin by comblike polymer-incorporated liposomes. J. Control. Release 2007, 120, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.; Giasson, S.; Khalid, M.N.; Delmas, P.; Allen, C.; Leroux, J.C. Long-circulating poly(ethylene glycol)-coated emulsions to target solid tumors. Eur. J. Pharm. Biopharm. 2007, 67, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; You, J.; Sun, Y.; Zhang, X.G.; Cui, F.D.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Studies on PEG-modified SLNs loading vinorelbine bitartrate (I): Preparation and evaluation in vitro. Int. J. Pharm. 2008, 359, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, D.; Li, Z.; Duan, C.; Wang, Y.; Feng, F.; Wang, F.; Liu, Y.; Zhang, Q. Nanostructured lipid carriers for parenteral delivery of silybin: Biodistribution and pharmacokinetic studies. Colloids Surf. B Biointerfaces 2010, 80, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Li, Y.X.; Li, M.; Zhang, L.; Feng, L.X.; Zhang, N. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013, 334, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Zheng, F.; Yang, X.; Yu, A.; Zhai, G. Nanostructured lipid carriers for oral delivery of baicalin: In vitro and in vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 154–159. [Google Scholar] [CrossRef]
- Esposito, E.; Boschi, A.; Ravani, L.; Cortesi, R.; Drechsler, M.; Mariani, P.; Moscatelli, S.; Contado, C.; di Domenico, G.; Nastruzzi, C.; et al. Biodistribution of nanostructured lipid carriers: A tomographic study. Eur. J. Pharm. Biopharm. 2015, 89, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Torres, D.; Martin-Pastor, M.; Alonso, M.J. Application of NMR spectroscopy to the characterization of PEG-stabilized lipid nanoparticles. Langmuir 2004, 20, 8839–8845. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ-Okur, N.; Yurdasiper, A.; Gündoğdu, E.; Homan Gökçe, E. Modification of solid lipid nanoparticles loaded with nebivolol hydrochloride for improvement of oral bioavailability in treatment of hypertension: Polyethylene glycol versus chitosan oligosaccharide lactate. J. Microencapsul. 2016, 33, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Niu, J.; Xiao, Y.; Ping, Q.; Sun, M.; Huang, A.; You, W.; Sang, X.; Yuan, D. Effect of octreotide-polyethylene glycol(100) monostearate modification on the pharmacokinetics and cellular uptake of nanostructured lipid carrier loaded with hydroxycamptothecine. Mol. Pharm. 2011, 8, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.-Q.; Zhang, J.-L.; Li, B.-A.; Wang, X.-Y.; Han, P. Biotinylated epidermal growth factor surface modified lipid nanoparticles to enhance the targeting efficiency in liver cancer therapy. J. Biomater. Tissue Eng. 2015, 5, 135–141. [Google Scholar] [CrossRef]
- Fan, T.; Chen, C.; Guo, H.; Xu, J.; Zhang, J.; Zhu, X.; Yang, Y.; Zhou, Z.; Li, L.; Huang, Y. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur. J. Pharm. Biopharm. 2014, 88, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Amiji, M. Gene delivery and transfection in human pancreatic cancer cells using epidermal growth factor receptor-targeted gelatin-based engineered nanovectors. J. Vis. Exp. 2011, 3, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, J.W.; Yoon, I.S.; Kim, D.D. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: Enhanced intestinal absorption and lymphatic uptake. Int. J. Nanomed. 2014, 9, 495–504. [Google Scholar]
- Jiang, W.; Wang, J.; Yang, L.; Jiang, X.; Bai, Z.; Wang, Z.; He, Y.; Wang, D. Nanostructured lipid carriers modified with PEGylated carboxymethylcellulose polymers for effective delivery of docetaxel. RSC Adv. 2015, 5, 90386–90395. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, W.; Li, X.; Ye, T.; Chen, F.; Yu, S.; Chen, J.; Yang, X.; Yang, N.; Zhang, J.; Liu, J.; Kong, J. Nanostructured lipid carrier surface modified with eudragit RS 100 and its potential ophthalmic functions. Int. J. Nanomed. 2014, 9, 4305–4315. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Desai, P.R.; Channer, D.; Singh, M. Enhanced skin permeation using polyarginine modified nanostructured lipid carriers. J. Control. Release 2012, 161, 735–745. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Vabbilisetty, P.; Sun, X.-L. Liposome surface functionalization based on different anchoring lipids via staudinger ligation. Org. Biomol. Chem. 2014, 12, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Belletti, D.; Vandelli, M.A.; Tonelli, M.; Zapparoli, M.; Forni, F.; Tosi, G.; Ruozi, B. Functionalization of liposomes: Microscopical methods for preformulative screening. J. Liposome Res. 2015, 25, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Oude Blenke, E.; Klaasse, G.; Merten, H.; Plückthun, A.; Mastrobattista, E.; Martin, N.I. Liposome functionalization with copper-free “click chemistry”. J. Control. Release 2015, 202, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Weingart, J.J.; Vabbilisetty, P.; Sun, X.-L. Glyco-functionalized liposomes. In Carbohydrate Nanotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 211–232. [Google Scholar]
- Immordino, M.L.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2016, 1, 297–315. [Google Scholar]
- Wolfram, J.; Suri, K.; Yang, Y.; Shen, J.; Celia, C.; Fresta, M.; Zhao, Y.; Shen, H.; Ferrari, M. Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf. B Biointerfaces 2014, 114, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Dzieciuch, M.; Rissanen, S.; Szydłowska, N.; Bunker, A.; Kumorek, M.; Jamróz, D.; Vattulainen, I.; Nowakowska, M.; Róg, T.; Kepczynski, M. PEGylated liposomes as carriers of hydrophobic porphyrins. J. Phys. Chem. B 2015, 119, 6646–6657. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Park, J.; Lee, E.; Ahn, S.; Lee, J.; Kim, H.; Kim, J.; Choi, M.; Farokhzad, O.C.; Jon, S. Effect of PEG pairing on the efficiency of cancer-targeting liposomes. Theranostics 2015, 5, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Asai, T.; Oyama, D.; Agato, Y.; Yasuda, N.; Fukuta, T.; Shimizu, K.; Minamino, T.; Oku, N. Treatment of cerebral ischemia-reperfusion injury with PEGylated liposomes encapsulating FK506. FASEB J. 2013, 27, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Lee, C.K.; Lee, Y.-B. Preparation and evaluation of PEGylated and folate-PEGylated liposomes containing paclitaxel for lymphatic delivery. J. Nanomater. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Garnier, B.; Bouter, A.; Gounou, C.; Petry, K.G.; Brisson, A.R. Annexin A5-functionalized liposomes for targeting phosphatidylserine-exposing membranes. Bioconjug. Chem. 2009, 20, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Bana, L.; Minniti, S.; Salvati, E.; Sesana, S.; Zambelli, V.; Cagnotto, A.; Orlando, A.; Cazzaniga, E.; Zwart, R.; Scheper, W.; Masserini, M.; Re, F. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood–brain-barrier: Implications for therapy of Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Salvati, E.; Re, F.; Sesana, S.; Cambianica, I.; Sancini, G.; Masserini, M.; Gregori, M. Liposomes functionalized to overcome the blood brain barrier and to target amyloid-β peptide: The chemical design affects the permeability across an in vitro model. Int. J. Nanomed. 2013, 8, 1749–1758. [Google Scholar]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood–brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Reijerkerk, A.; Appeldoorn, C.C.M.; Rip, J.; de Boer, M.; Gaillard, P.J. Systemic treatment with glutathione PEGylated liposomal methylprednisolone (2B3–201) improves therapeutic efficacy in a model of ocular inflammation. Investig. Opthalmol. Vis. Sci. 2014, 55, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Andriyanov, A.V.; Koren, E.; Barenholz, Y.; Goldberg, S.N. Therapeutic efficacy of combining PEGylated liposomal doxorubicin and radiofrequency (RF) ablation: Comparison between slow-drug-releasing, non-thermosensitive and fast-drug-releasing, thermosensitive nano-liposomes. PLoS ONE 2014, 9, e92555. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, X.; Tu, L.; Zou, Q.; Li, Q.; Tang, C.; Chen, B.; Wu, C.; Wei, M. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 2015, 10, 5123–5137. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, W.; Han, Q.; Wang, Z.; Jia, Y.; Hu, Z. Micromixer based preparation of functionalized liposomes and targeting drug delivery. ACS Med. Chem. Lett. 2016, 7, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; He, L.; Qiu, S.; Li, Y.; Liao, Y.; Li, X.; Xie, D.; Peng, Y. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Cancer 2014, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.R.; Paliwal, R.; Agrawal, G.P.; Vyas, S.P. Hyaluronic acid modified pH-sensitive liposomes for targeted intracellular delivery of doxorubicin. J. Liposome Res. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Liu, Y.; Gao, H.; Kuang, Q.; Zhang, Q.; Tang, J.; Fu, H.; Zhang, Z.; He, Q. PEGylated hyaluronic acid-modified liposomal delivery system with anti-γ-Glutamylcyclotransferase siRNA for drug-resistant MCF-7 breast cancer therapy. J. Pharm. Sci. 2015, 104, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, L.; Zhang, L.; Shi, K.; Cun, X.; Yang, Y.; Liu, Y.; Gao, H.; He, Q. Dual-functionalized liposomal delivery system for solid tumors based on RGD and a pH-responsive antimicrobial peptide. Sci. Rep. 2016, 6, 19800. [Google Scholar] [CrossRef] [PubMed]
- Szymański, P.; Markowicz, M.; Mikiciuk-Olasik, E. Nanotechnology in pharmaceutical and biomedical applications: Dendrimers. Nano 2011, 6, 509–539. [Google Scholar] [CrossRef]
- Fu, F.; Wu, Y.; Zhu, J.; Wen, S.; Shen, M.; Shi, X. Multifunctional Lactobionic acid-modified dendrimers for targeted drug delivery to liver cancer cells: Investigating the role played by peg spacer. ACS Appl. Mater. Interfaces 2014, 6, 16416–16425. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, S.; Wang, L.; Feng, H. Use of multifunctional phosphorylated PAMAM dendrimers for dentin biomimetic remineralization and dentinal tubule occlusion. RSC Adv. 2015, 5, 11136–11144. [Google Scholar] [CrossRef]
- Lim, J.; Simanek, E.E. Synthesis of water-soluble dendrimers based on melamine bearing 16 paclitaxel groups. Org. Lett. 2008, 10, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lo, S.-T.; Lim, J.; da Costa, V.C.P.; Ramezani, S.; Öz, O.K.; Pavan, G.M.; Annunziata, O.; Sun, X.; Simanek, E.E. Design, synthesis and biological assessment of a triazine dendrimer with approximately 16 paclitaxel groups and 8 PEG groups. Mol. Pharm. 2013, 10, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- Restani, R.B.; Conde, J.; Pires, R.F.; Martins, P.; Fernandes, A.R.; Baptista, P.V.; Bonifácio, V.D.B.; Aguiar-Ricardo, A. POxylated polyurea dendrimers: Smart core-shell vectors with IC 50 lowering capacity. Macromol. Biosci. 2015, 15, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, R.; Luo, Y.; Wu, Y.; Guo, R.; Shi, X. Dendrimer-functionalized laponite nanodisks as a platform for anticancer drug delivery. Nanomaterials 2015, 5, 1716–1731. [Google Scholar] [CrossRef]
- Zong, H.; Thomas, T.P.; Lee, K.-H.; Desai, A.M.; Li, M.; Kotlyar, A.; Zhang, Y.; Leroueil, P.R.; Gam, J.J.; Banaszak Holl, M.M.; et al. Bifunctional PAMAM dendrimer conjugates of folic acid and methotrexate with defined ratio. Biomacromolecules 2012, 13, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, M.A.; Silpe, J.E.; Dougherty, C.A.; Kanduluru, A.K.; Choi, S.K.; Orr, B.G.; Low, P.S.; Banaszak Holl, M.M. Avidity mechanism of dendrimer-folic acid conjugates. Mol. Pharm. 2014, 11, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; van Dongen, M.A.; Merzel, R.L.; Dougherty, C.A.; Orr, B.G.; Kandaluru, A.K.; Holl, M.M.B. Substrate-triggered exosite binding: Synergistic dendrimer/folic acid action for achieving specific, tight-binding to folate binding protein. Biomacromolecules 2016, 17, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yao, F.; Sun, F.; Tan, Z.; Tian, L.; Xie, L.; Song, Q. Antibacterial action mode of quaternized carboxymethyl chitosan/poly(amidoamine) dendrimer core-shell nanoparticles against Escherichia coli correlated with molecular chain conformation. Mater. Sci. Eng. C 2015, 48, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, Y.; Li, J.; Lu, F.; Deng, J.; Zhang, J.; Fang, P.; Zhou, S.-F. A study on the hemocompatibility of dendronized chitosan derivatives in red blood cells. Drug Des. Devel. Ther. 2015, 9, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Yeo, Y. Zwitterionic chitosan—Polyamidoamine dendrimer complex nanoparticles as a pH-sensitive drug carrier. Mol. Pharm. 2013, 10, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Fan, Y.; He, H.; Wu, Z. Hyaluronic acid-grafted polyamidoamine dendrimers enable long circulation and active tumor targeting simultaneously. Carbohydr. Polym. 2015, 126, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Xie, L.; Banerjee, S.; Mao, G.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf. B Biointerfaces 2015, 136, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Chen, W.; Liu, Y.; Tsukamoto, Y.; Inoue, Y. Cytocompatible and multifunctional polymeric nanoparticles for transportation of bioactive molecules into and within cells. Sci. Technol. Adv. Mater. 2016, 17, 300–312. [Google Scholar] [CrossRef]
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. Natural polymer biomaterials: Advanced applications. In New Functional Biomaterials for Medicine and Healthcare; Woodhead Publishing Limited: Cambridge, UK, 2014; pp. 32–70. [Google Scholar]
- Jana, S.; Gandhi, A.; Sen, K.K.; Basu, S.K. Natural polymers and their application in drug delivery and biomedical field. J. PharmaSciTech 2011, 1, 16–27. [Google Scholar]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.; Üstündağ Okur, N.; Mone, M.; Giannakopoulou, S.; Er, S.; Pavlidou, E.; Karavas, E.; Bikiaris, D. Two different approaches for oral administration of voriconazole loaded formulations: Electrospun fibers versus β-cyclodextrin complexes. Int. J. Mol. Sci. 2016, 17, 282. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Zisi, A.P.; Exindari, M.K.; Karantas, I.D.; Bikiaris, D.N. Porous dressings of modified chitosan with poly(2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr. Polym. 2016, 143, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.-W.; Jia, Y.-Y.; Wan, N.; Luo, M.; Huan, M.-L.; Kang, T.-B.; Zhou, S.-Y.; Zhang, B.-L. Design and evaluation of novel pH-sensitive ureido-conjugated chitosan/TPP nanoparticles targeted to Helicobacter pylori. Biomaterials 2016, 84, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Nasti, A.; Zaki, N.M.; de Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, V.M.; Costa, E.C.; Queiroz, J.A.; Pichon, C.; Sousa, F.; Correia, I.J. Folate-targeted multifunctional amino acid-chitosan nanoparticles for improved cancer therapy. Pharm. Res. 2015, 32, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, D.; Odabas, S.; Guven, E.; Demirbilek, M.; Denkbas, E.B. Bleomycin loaded magnetic chitosan nanoparticles as multifunctional nanocarriers. J. Bioact. Compat. Polym. 2010, 25, 305–318. [Google Scholar] [CrossRef]
- Li, J.; Jiang, C.; Lang, X.; Kong, M.; Cheng, X.; Liu, Y.; Feng, C.; Chen, X. Multilayer sodium alginate beads with porous core containing chitosan based nanoparticles for oral delivery of anticancer drug. Int. J. Biol. Macromol. 2016, 85, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Min, K.H.; Lee, S.C.; Park, J.H.; Park, K.; Jeong, S.Y.; Choi, K.; Kwon, I.C.; Kim, K. Enhanced drug-loading and therapeutic efficacy of hydrotropic oligomer-conjugated glycol chitosan nanoparticles for tumor-targeted paclitaxel delivery. J. Control. Release 2013, 172, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tomaro-Duchesneau, C.; Prakash, S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials 2013, 34, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Martens, T.F.; Remaut, K.; Deschout, H.; Engbersen, J.F.J.; Hennink, W.E.; van Steenbergen, M.J.; Demeester, J.; de Smedt, S.C.; Braeckmans, K. Coating nanocarriers with hyaluronic acid facilitates intravitreal drug delivery for retinal gene therapy. J. Control. Release 2015, 202, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, F.; Qi, H.; Li, F.; Xin, T.; Xu, J.; Ye, T.; Sheng, N.; Yang, X.; Pan, W. Multifunctional tumor-targeting nanocarriers based on hyaluronic acid-mediated and pH-sensitive properties for efficient delivery of docetaxel. Pharm. Res. 2014, 31, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qi, H.; Xu, J.; Guo, P.; Chen, F.; Li, F.; Yang, X.; Sheng, N.; Wu, Y.; Pan, W. Hyaluronan-based nanocarriers with CD44-overexpressed cancer cell targeting. Pharm. Res. 2014, 31, 2988–3005. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, G.; Ren, J.; Zhang, B.; Yan, J.; Li, W.; Khashab, N.M. Enzymatically triggered multifunctional delivery system based on hyaluronic acid micelles. RSC Adv. 2012, 2, 12909–12914. [Google Scholar] [CrossRef]
- Liang, D.; Wang, A.; Yang, Z.; Liu, Y.; Qi, X. Enhance cancer cell recognition and overcome drug resistance using hyaluronic acid and α-tocopheryl succinate based multifunctional nanoparticles. Mol. Pharm. 2015, 12, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, Y.; Sun, W.; Luo, Y.; Cai, H.; Pan, Y.; Shen, M.; Xia, J.; Shi, X. Hyaluronic acid-modified hydrothermally synthesized iron oxide nanoparticles for targeted tumor MR imaging. Biomaterials 2014, 35, 3666–3677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Sun, W.; Hu, Y.; Zhang, G.; Shen, M.; Shi, X. Hyaluronic acid-modified magnetic iron oxide nanoparticles for MR imaging of surgically induced endometriosis model in rats. PLoS ONE 2014, 9, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Jiao, J.; Cui, Y.; Guo, J.; Han, N.; Di, D.; Chang, D.; Wang, P.; Jiang, T.; Wang, S. Hyaluronic acid modified mesoporous carbon nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanotechnology 2016, 27, 135102. [Google Scholar] [CrossRef] [PubMed]
- Borges, O.; Cordeiro-da-Silva, A.; Romeijn, S.G.; Amidi, M.; de Sousa, A.; Borchard, G.; Junginger, H.E. Uptake studies in rat peyer’s patches, cytotoxicity and release studies of alginate coated chitosan nanoparticles for mucosal vaccination. J. Control. Release 2006, 114, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, D.; Liu, S.; Gong, J.; Wang, D.; Xiong, M.; Chen, X.; Xiang, R.; Tan, X. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: Effect against breast cancer in mice. PLoS ONE 2013, 8, e60190. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Singh, D.; Singh, D.; Choi, S.M.; Zo, S.M.; Park, S.J.; Han, S.S. Novel alginate-gelatin hybrid nanoparticle for drug delivery and tissue engineering applications. J. Nanomater. 2014, 2014, 124236. [Google Scholar] [CrossRef]
- Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic polyesters for medical imaging and theranostic applications. Eur. J. Pharm. Biopharm. 2015, 97, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Palacio, J.; Agudelo, N.A.; Lopez, B.L. PEGylation of PLA nanoparticles to improve mucus-penetration and colloidal stability for oral delivery systems. Curr. Opin. Chem. Eng. 2016, 11, 14–19. [Google Scholar] [CrossRef]
- Ferrari, R.; Yu, Y.; Lattuada, M.; Storti, G.; Morbidelli, M.; Moscatelli, D. Controlled PEGylation of PLA-based nanoparticles. Macromol. Chem. Phys. 2012, 213, 2012–2018. [Google Scholar] [CrossRef]
- Betancourt, T.; Byrne, J.D.; Sunaryo, N.; Crowder, S.W.; Kadapakkam, M.; Patel, S.; Casciato, S.; Brannon-Peppas, L. PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J. Biomed. Mater. Res. Part A 2009, 91, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Xie, L.; Ding, D.; Hu, Y.; Qian, X.; Yu, L.; Ding, Y.; Jiang, X.; Liu, B. Preparation and evaluation of PEG-PCL nanoparticles for local tetradrine delivery. Int. J. Pharm. 2009, 379, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Varshney, R.; Shukla, J.; Ganeshpurkar, A.; Hazari, P.P.; Bandopadhaya, G.P.; Mishra, A.K.; Trivedi, P. Synthesis and evaluation of biodegradable PCL/PEG nanoparticles for neuroendocrine tumor targeted delivery of somatostatin analog. Drug Deliv. 2012, 19, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Xia, H.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; et al. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials 2013, 34, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Lupi, M.; Colombo, C.; Frapolli, R.; Ferrari, R.; Sitia, L.; Dragoni, L.; Bello, E.; Licandro, S.A.; Falcetta, F.; Ubezio, P.; et al. A biodistribution study of PEGylated PCL-based nanoparticles in C57BL/6 mice bearing B16/F10 melanoma. Nanotechnology 2014, 25, 335706. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, B.; Jiang, W.; Yan, H.; Zhang, X.; Yang, L.; Deng, L.; Singh, G.K. Novel PEG-graft-PLA nanoparticles with the potential for encapsulation and controlled release of hydrophobic and hydrophilic medications in aqueous medium. Int. J. Nanomed. 2011, 6, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; He, B.; Li, D.; Lai, Y.; Tang, J.Z.; Gu, Z. Stabilization of pH-sensitive mPEG-PH-PLA nanoparticles by stereocomplexation between enantiomeric polylactides. Macromol. Rapid Commun. 2012, 33, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.T.; Lim, C.; Lee, K.S.; Hoang, N.H.; Sim, T.H.; Youn, Y.S.; Lee, E.S. Development of a robust pH-sensitive polyelectrolyte ionomer complex for anticancer nanocarriers. Int. J. Nanomed. 2016, 11, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Inchaurraga, L.; Martín-Arbella, N.; Zabaleta, V.; Quincoces, G.; Peñuelas, I.; Irache, J.M. In vivo study of the mucus-permeating properties of peg-coated nanoparticles following oral administration. Eur. J. Pharm. Biopharm. 2015, 97, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Palacio, J.; Agudelo, N.A.; Lopez, B.L. PLA/pluronic® nanoparticles as potential oral delivery systems: Preparation, colloidal and chemical stability, and loading capacity. J. Appl. Polym. Sci. 2016, 133, 43828. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Huang, R.-J.; Xu, F.; Chen, Y.-S. pH-Sensitive biodegradable PMAA2-b-PLA-b-PMAA2 H-Type multiblock copolymer micelles: Synthesis, characterization, and drug release applications. J. Mater. Sci. 2014, 49, 7730–7741. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Lin, Z.; Li, X.; Li, J. Tumor-acidity activated surface charge-conversion of polymeric nanocarriers for enhanced cell adhesion and targeted drug release. Macromol. Rapid Commun. 2014, 35, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Lale, S.V.; Aswathy, R.G.; Aravind, A.; Kumar, D.S.; Koul, V. AS1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA-PCL-pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapy. Biomacromolecules 2014, 15, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, L.; Liu, T.; Zhang, L.; Yao, Y.; Yu, D.; Wang, L.; Zhang, N. Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 2014, 6, 3231. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, S. PLA-PEG coated multifunctional imaging probe for targeted drug delivery. Mol. Pharm. 2015, 12, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Hami, Z.; Amini, M.; Ghazi-Khansari, M.; Rezayat, S.M.; Gilani, K. Synthesis and in vitro evaluation of a pH-sensitive PLA-PEG-folate based polymeric micelle for controlled delivery of docetaxel. Colloids Surf. B Biointerfaces 2014, 116, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ouahab, A.; Cheraga, N.; Onoja, V.; Shen, Y.; Tu, J. Novel pH-sensitive charge-reversal cell penetrating peptide conjugated PEG-PLA micelles for docetaxel delivery: In vitro study. Int. J. Pharm. 2014, 466, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Cai, S.; Zhang, R.; Liu, P.; Chen, H.; Zheng, Y.; Sun, L. Paclitaxel-loaded nanoparticles of star-shaped cholic acid-core PLA-TPGS copolymer for breast cancer treatment. Nanoscale Res. Lett. 2013, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tao, W.; Mei, L.; Huang, L.; Tan, C.; Feng, S.-S. Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials 2013, 34, 6058–6067. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.P.; Nam, N.H.; Quang, B.T.; Son, H.A.; Toan, N.L.; Quang, D.T. In vitro and in vivo targeting effect of folate decorated paclitaxel loaded PLA-TPGS nanoparticles. Saudi Pharm. J. 2015, 23, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Man, D.K.W.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F.; Sze, S.C.W.; Leung, G.P.H.; Lam, J.K.W.; Kwok, P.C.L. Oleanolic acid loaded PEGylated PLA and PLGA nanoparticles with enhanced cytotoxic activity against cancer cells. Mol. Pharm. 2015, 12, 2112–2125. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhu, M.; Song, N.; Du, X.; Yang, Y. Reverse micelles based on biocompatible β-cyclodextrin conjugated polyethylene glycol block polylactide for protein delivery. J. Mater. Chem. B Mater. Biol. Med. 2014, 3, 316–322. [Google Scholar] [CrossRef]
- Vangara, K.K.; Liu, J.L.; Palakurthi, S. Hyaluronic acid-decorated PLGA-PEG nanoparticles for targeted delivery of SN-38 to ovarian cancer. Anticancer Res. 2013, 33, 2425–2434. [Google Scholar] [PubMed]
- Yadav, A.K.; Mishra, P.; Jain, S.; Mishra, P.; Mishra, A.K.; Agrawal, G.P. Preparation and characterization of HA-PEG-PCL intelligent core-corona nanoparticles for delivery of doxorubicin. J. Drug Target. 2008, 16, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agarwal, P.; Zhao, S.; Xu, R.X.; Yu, J.; Lu, X.; He, X. Hyaluronic acid-decorated dual responsive nanoparticles of pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells. Biomaterials 2015, 72, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Nie, S.Y.; Chen, Q.; Qian, Y.; Wen, X.F.; Zhang, L.J. Structure-property relationship of pH-sensitive (PCL)2(PDEA-b-PPEGMA)2 micelles: Experiment and DPD simulation. AIChE J. 2014, 60, 3634–3646. [Google Scholar] [CrossRef]
- Laouini, A.; Koutroumanis, K.P.; Charcosset, C.; Georgiadou, S.; Fessi, H.; Holdich, R.G.; Vladisavljević, G.T. pH-Sensitive micelles for targeted drug delivery prepared using a novel membrane contactor method. ACS Appl. Mater. Interfaces 2013, 5, 8939–8947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; He, X.; Su, H.; Zhou, D.; Song, H.; Wang, L.; Jiang, X. Poly(ethylene glycol)-block-poly(ε-caprolactone)- and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery. Int. J. Nanomed. 2015, 10, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, L.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Suen, W.-L.L.; Chau, Y. Size-dependent internalisation of folate-decorated nanoparticles via the pathways of clathrin and caveolae-mediated endocytosis in ARPE-19 cells. J. Pharm. Pharmacol. 2014, 66, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, D.; Li, D.; Lu, J.; Wei, Q. Folate modified nanoparticles for targeted co-delivery chemotherapeutic drugs and imaging probes for ovarian cancer. Biomed. Phys. Eng. Express 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Bernabeu, E.; Helguera, G.; Legaspi, M.J.; Gonzalez, L.; Hocht, C.; Taira, C.; Chiappetta, D.A. Paclitaxel-loaded PCL–TPGS nanoparticles: In vitro and in vivo performance compared with abraxane®. Colloids Surf. B Biointerfaces 2014, 113, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, V.M.; Gonçalves, C.; de Melo-Diogo, D.; Costa, E.C.; Queiroz, J.A.; Pichon, C.; Sousa, F.; Correia, I.J. Poly(2-ethyl-2-oxazoline)-PLA-g-PEI amphiphilic triblock micelles for co-delivery of minicircle DNA and chemotherapeutics. J. Control. Release 2014, 189, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, X.; Liu, L.; Zhang, Q. Thiolated chitosan-modified PLA-PCL-TPGS nanoparticles for oral chemotherapy of lung cancer. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.A.; Papadimitriou, S.A.; Bikiaris, D.N.; Mattheolabakis, G.; Avgoustakis, K. Facile synthesis of polyester-PEG triblock copolymers and preparation of amphiphilic nanoparticles as drug carriers. J. Control. Release 2010, 148, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, S.; Mattheolabakis, G.; Papadimitriou, S.; Karavas, E.; Bikiaris, D.; Avgoustakis, K. PPSu-PEG copolymers and their application in the preparation of cisplatin-loaded nanoparticles. Curr. Nanosci. 2011, 7, 503–509. [Google Scholar] [CrossRef]
- Gromadzki, D.; Rychter, P.; Uchman, M.; Momekova, D.; Marcinkowski, A.; Koseva, N.S.; El Fray, M.; Marić, M. Multifunctional amphiphilic nanoparticles featuring (bio)degradable core and dual-responsive shell as biomedical platforms for controlled release. Macromol. Chem. Phys. 2015, 216, 2287–2301. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Gao, H.; Sun, Y.; Sun, Y.; Yang, Y.-W.; Wu, G.; Wang, Y.; Fan, Y.; Ma, J. Temperature- and pH-responsive nanoparticles of biocompatible polyurethanes for doxorubicin delivery. Int. J. Pharm. 2013, 441, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Bellucci, D.; Sola, A.; Mattu, C.; Cannillo, V.; Ciardelli, G. Composite scaffolds for controlled drug release: Role of the polyurethane nanoparticles on the physical properties and cell behaviour. J. Mech. Behav. Biomed. Mater. 2015, 44, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mattu, C.; Silvestri, A.; Wang, T.R.; Boffito, M.; Ranzato, E.; Cassino, C.; Ciofani, G.; Ciardelli, G. Surface-functionalized polyurethane nanoparticles for targeted cancer therapy. Polym. Int. 2016, 65, 770–779. [Google Scholar] [CrossRef]

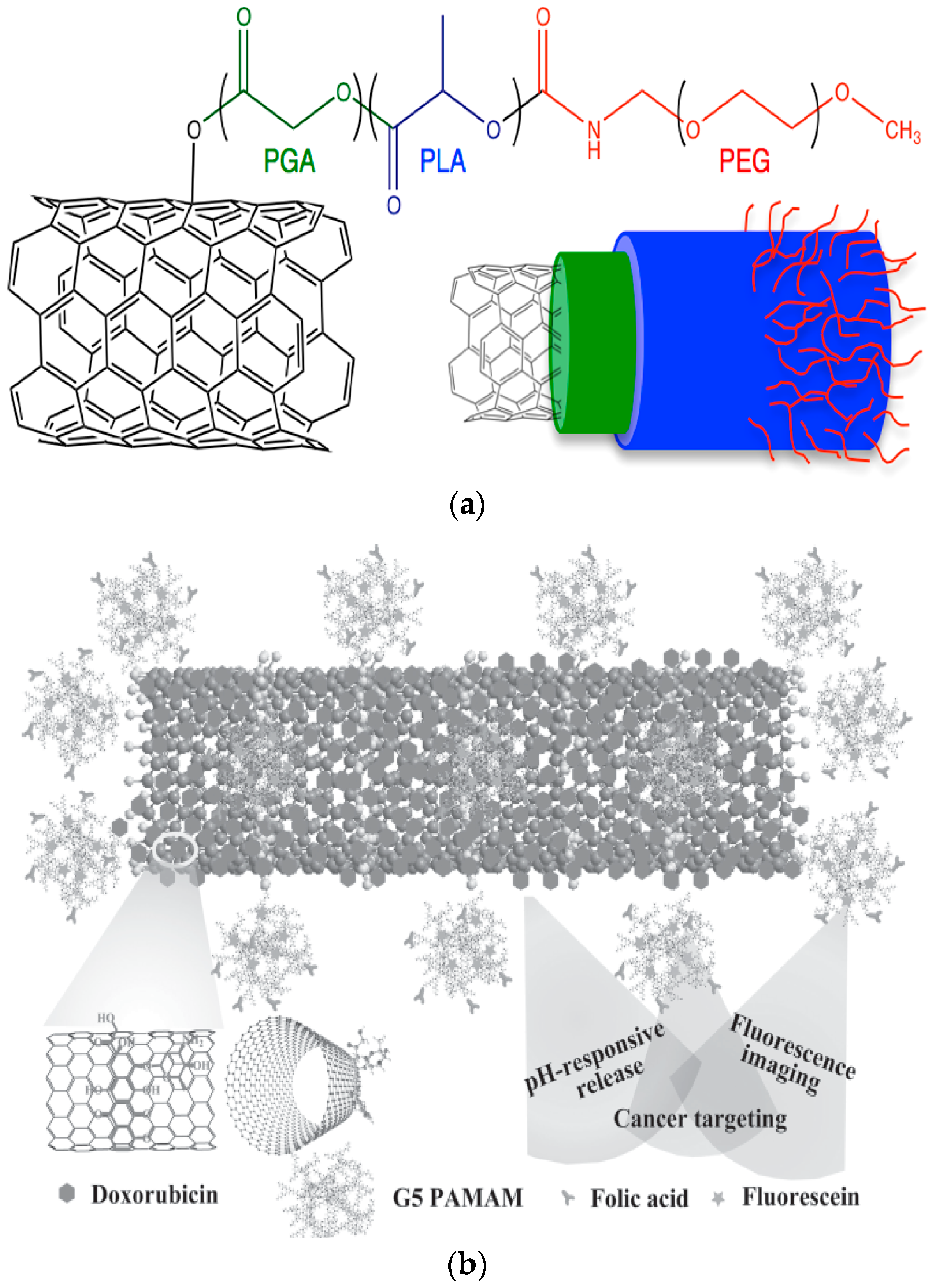
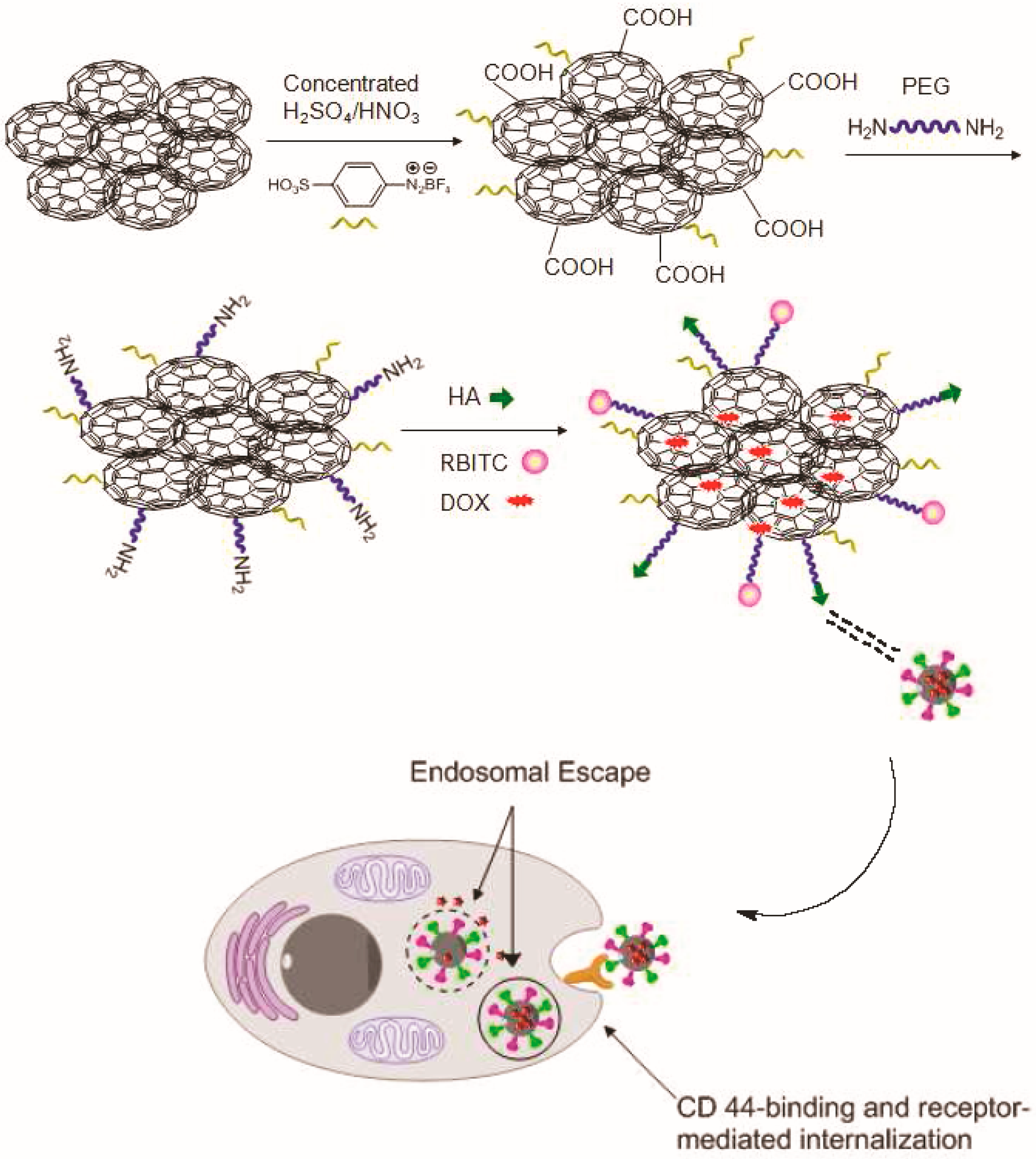

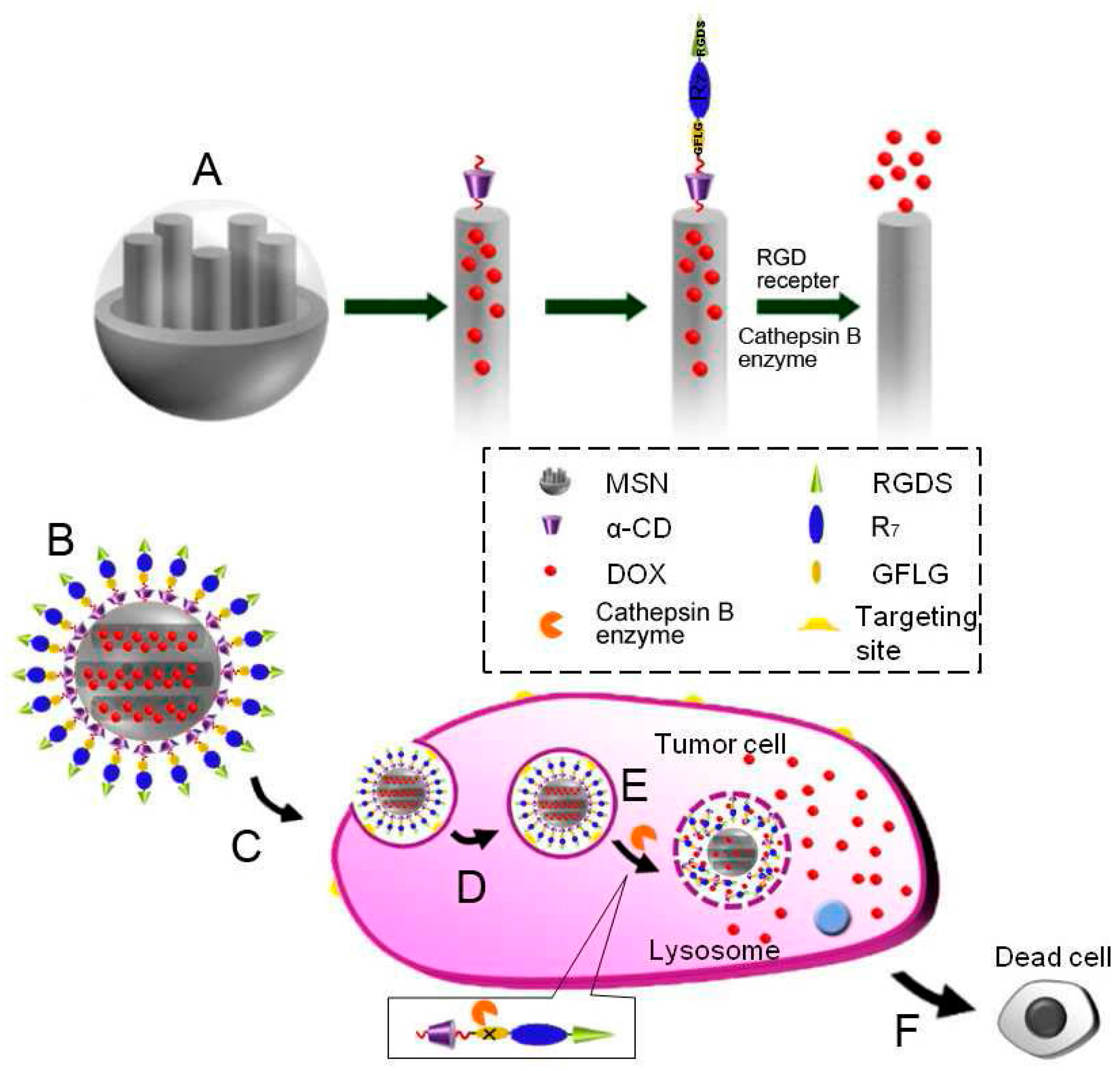
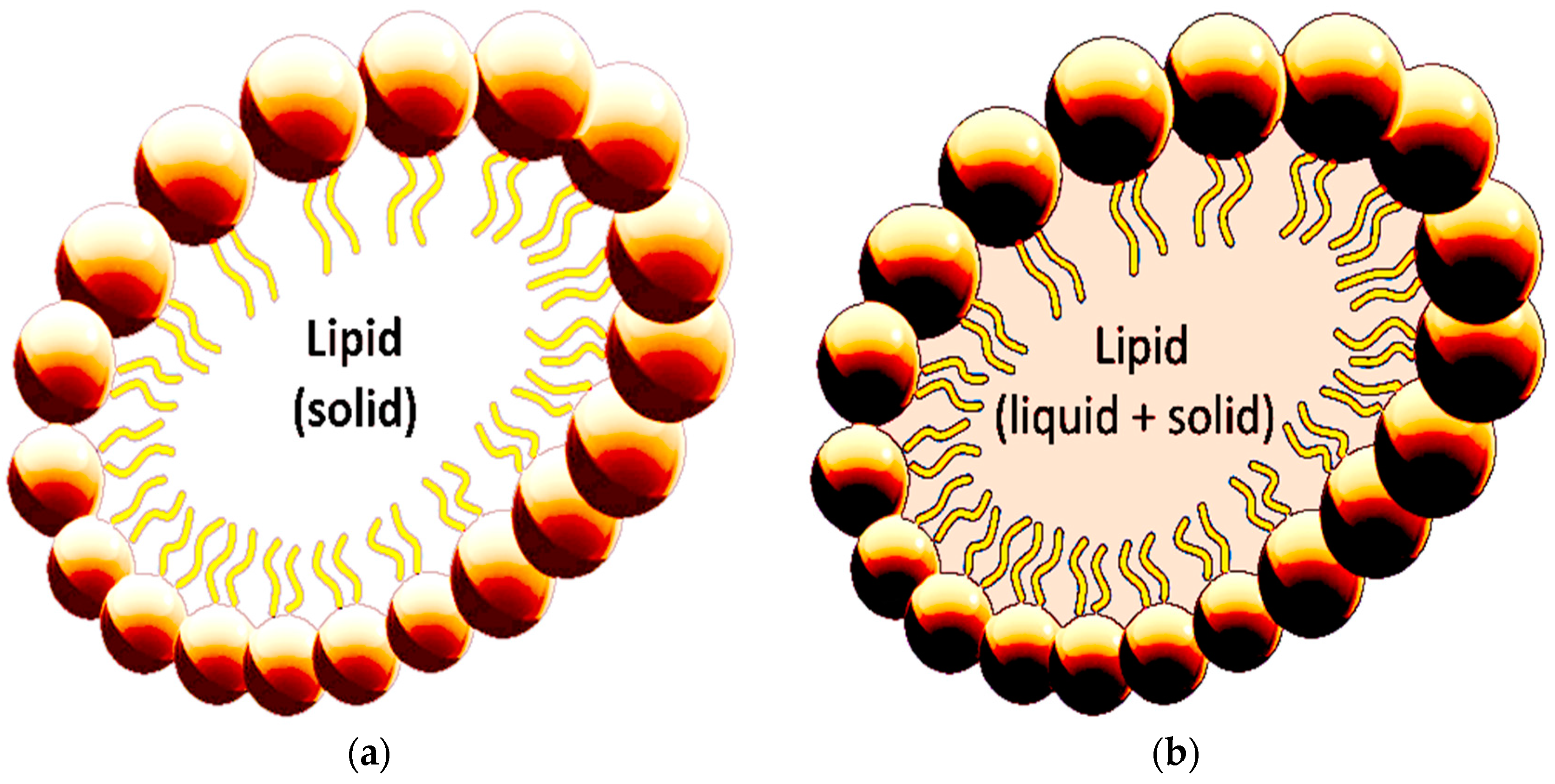
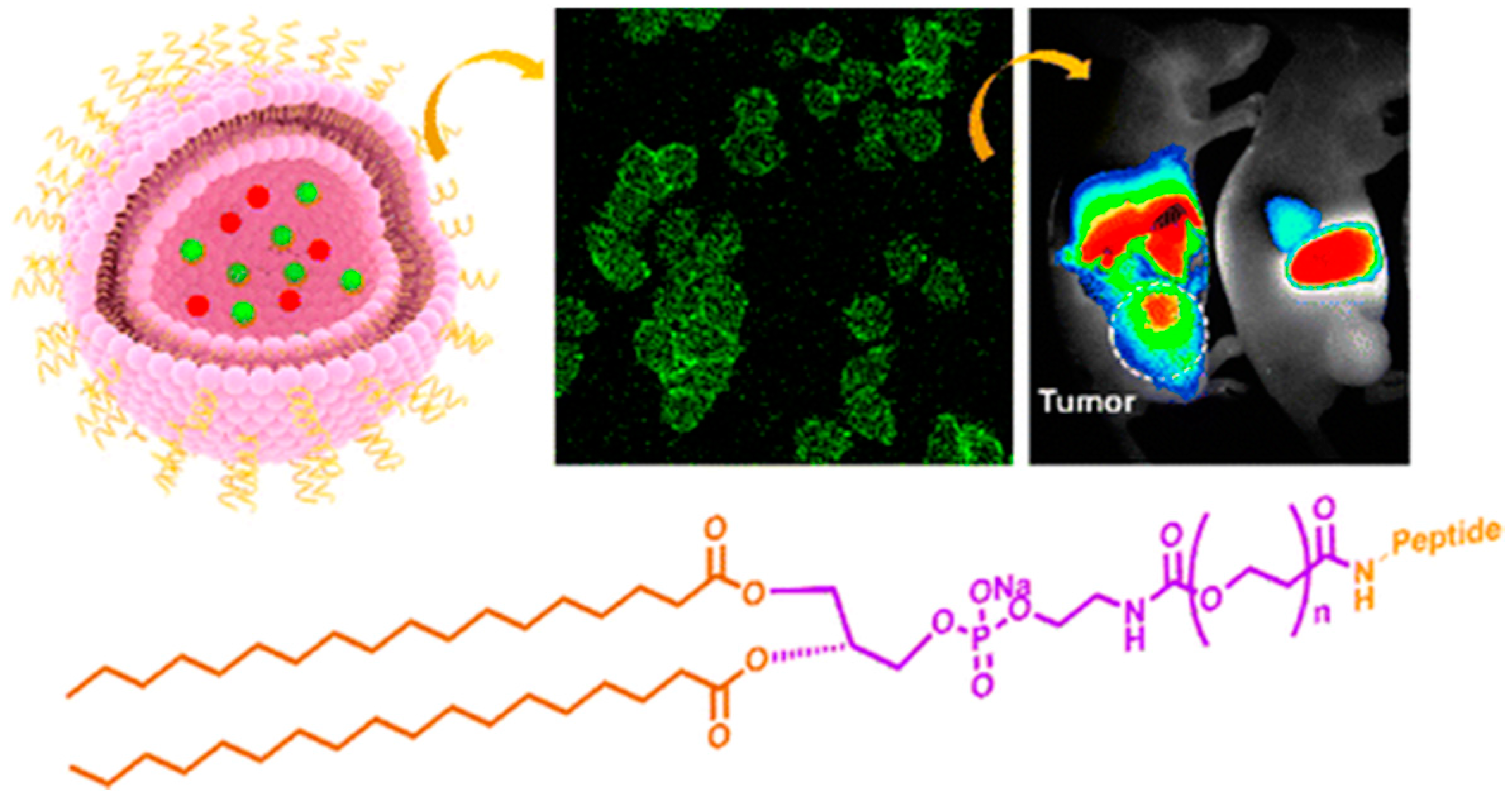
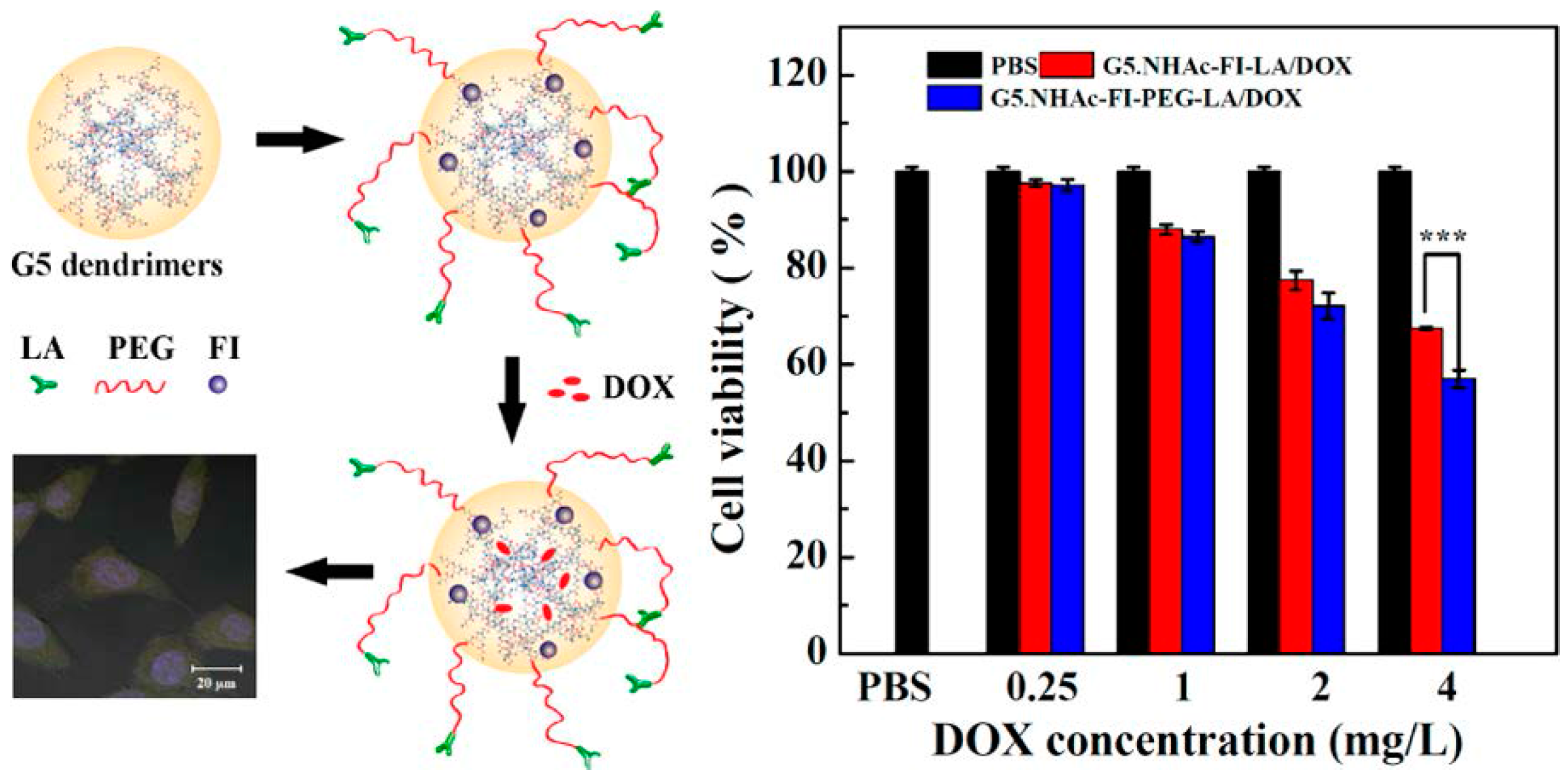
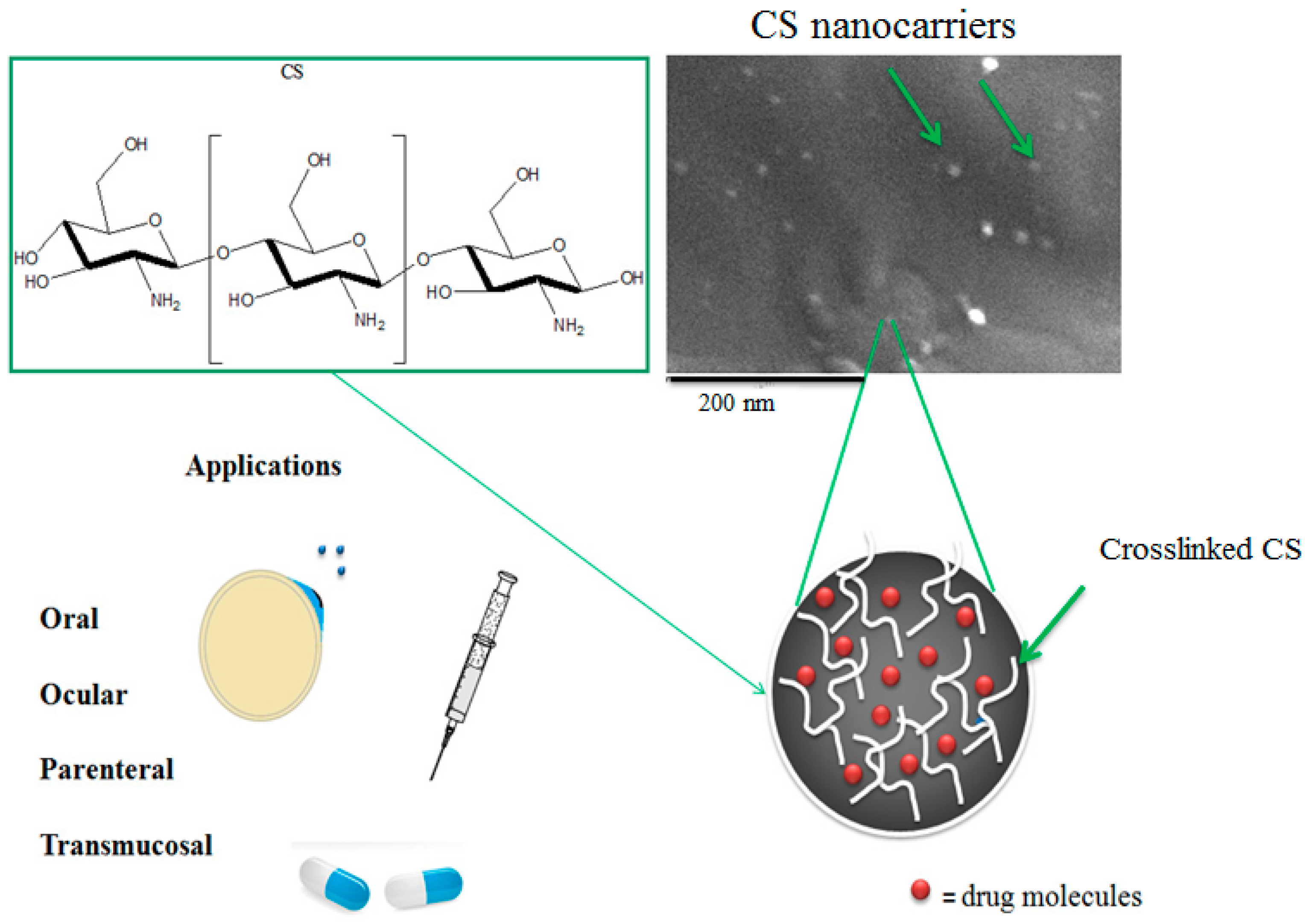

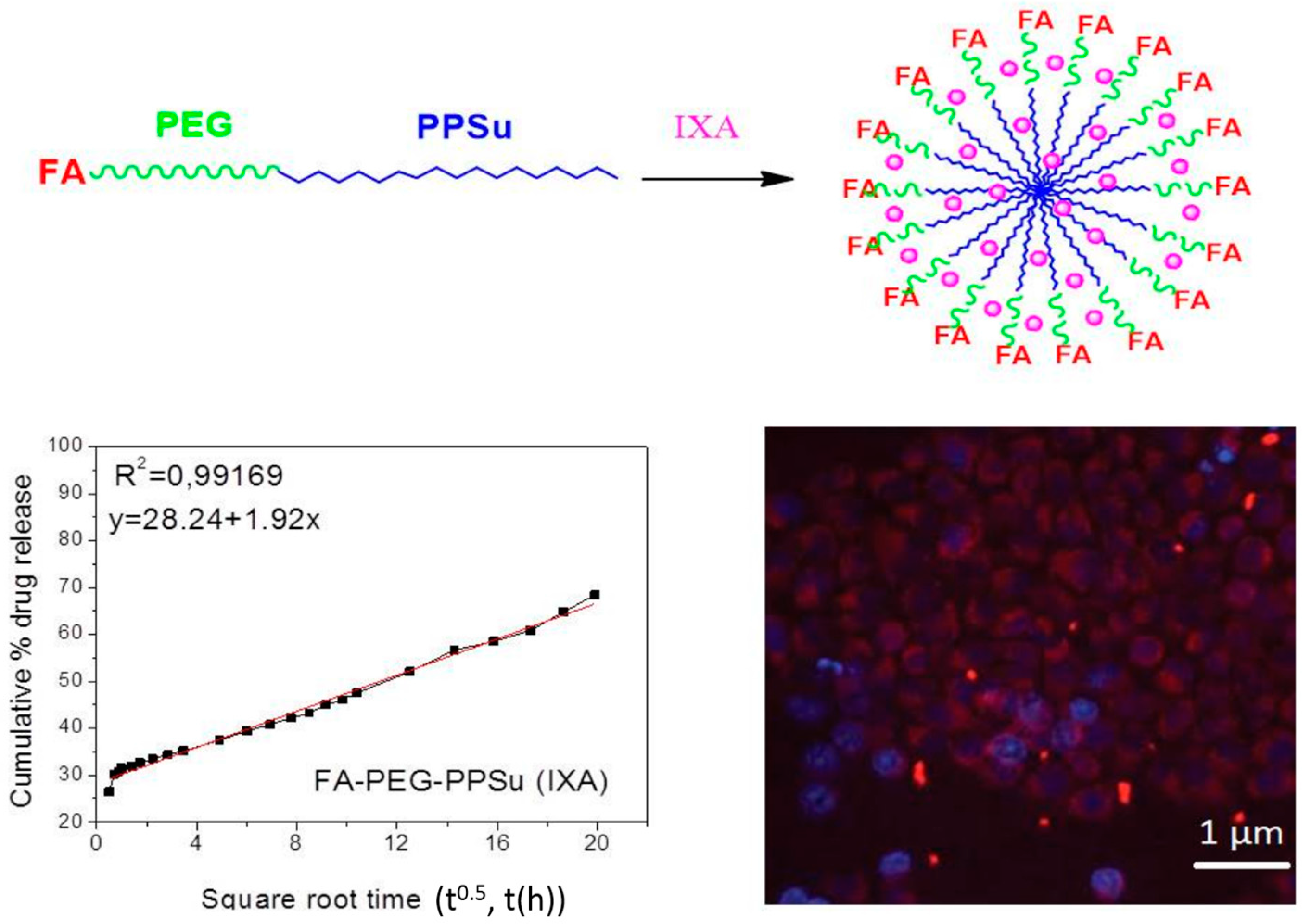
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siafaka, P.I.; Üstündağ Okur, N.; Karavas, E.; Bikiaris, D.N. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. Int. J. Mol. Sci. 2016, 17, 1440. https://doi.org/10.3390/ijms17091440
Siafaka PI, Üstündağ Okur N, Karavas E, Bikiaris DN. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. International Journal of Molecular Sciences. 2016; 17(9):1440. https://doi.org/10.3390/ijms17091440
Chicago/Turabian StyleSiafaka, Panoraia I., Neslihan Üstündağ Okur, Evangelos Karavas, and Dimitrios N. Bikiaris. 2016. "Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses" International Journal of Molecular Sciences 17, no. 9: 1440. https://doi.org/10.3390/ijms17091440