Multifaceted Roles of ALG-2 in Ca2+-Regulated Membrane Trafficking
Abstract
:1. Introduction
2. Structure of ALG-2
3. Ca2+-Binding Capacity
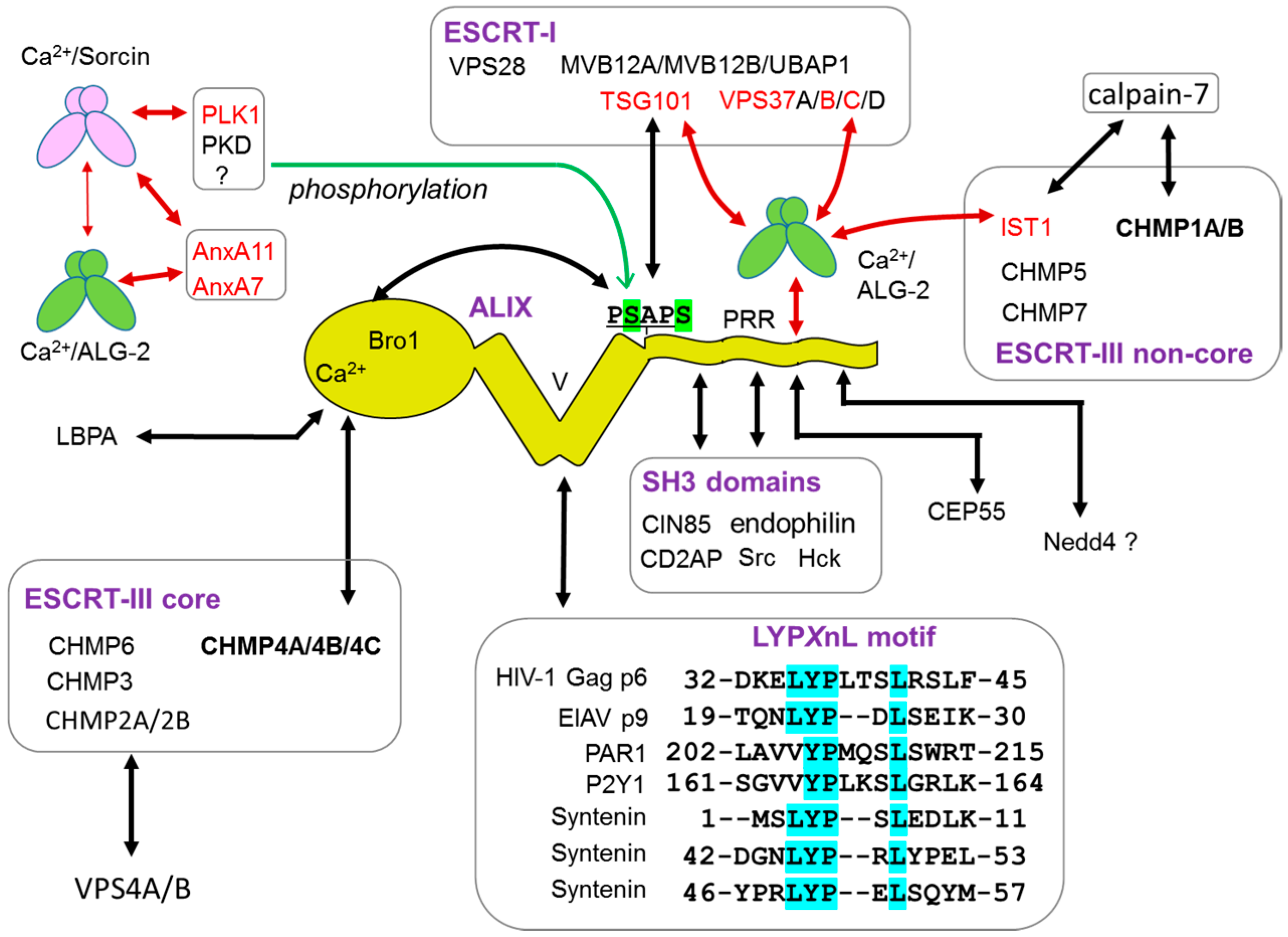
4. Interacting Proteins
5. Mode of Binding
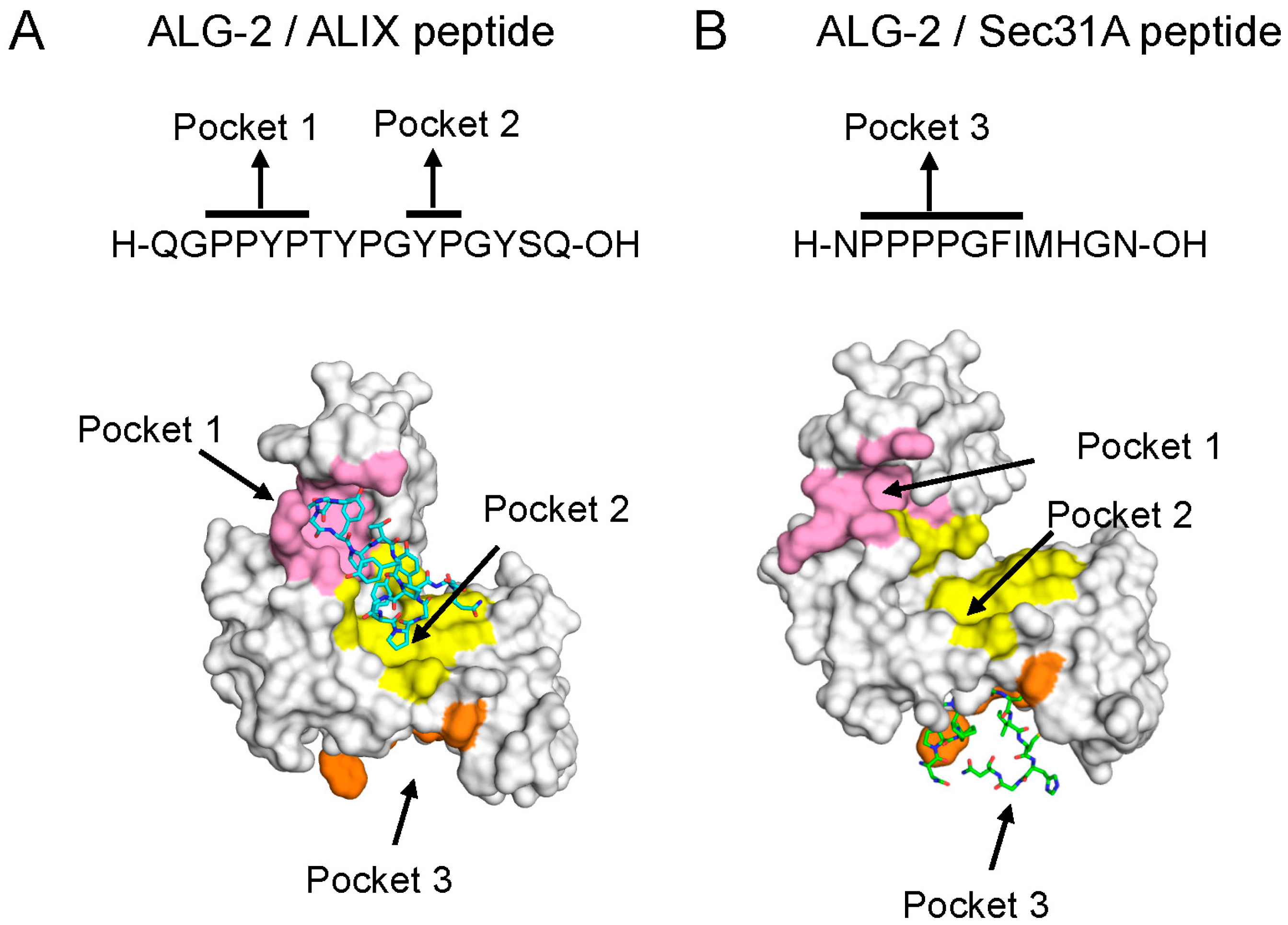
5.1. Binding Pockets in ALG-2
5.2. Mechanism of Binding
5.3. ALG-2-Binding Motifs (ABMs)
5.3.1. Type 1
5.3.2. Type 2
5.3.3. Type 3
5.3.4. Non-Pro-Based Motif
6. Interacting Protein Network of ALG-2 in Membrane Trafficking
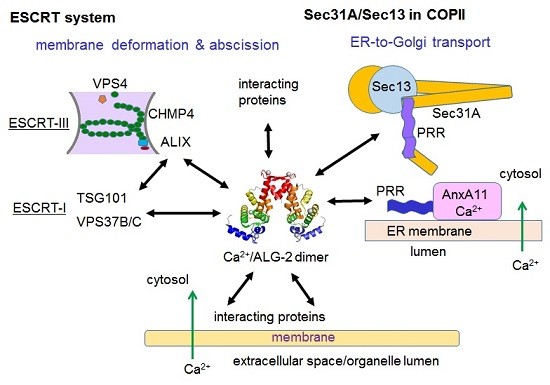
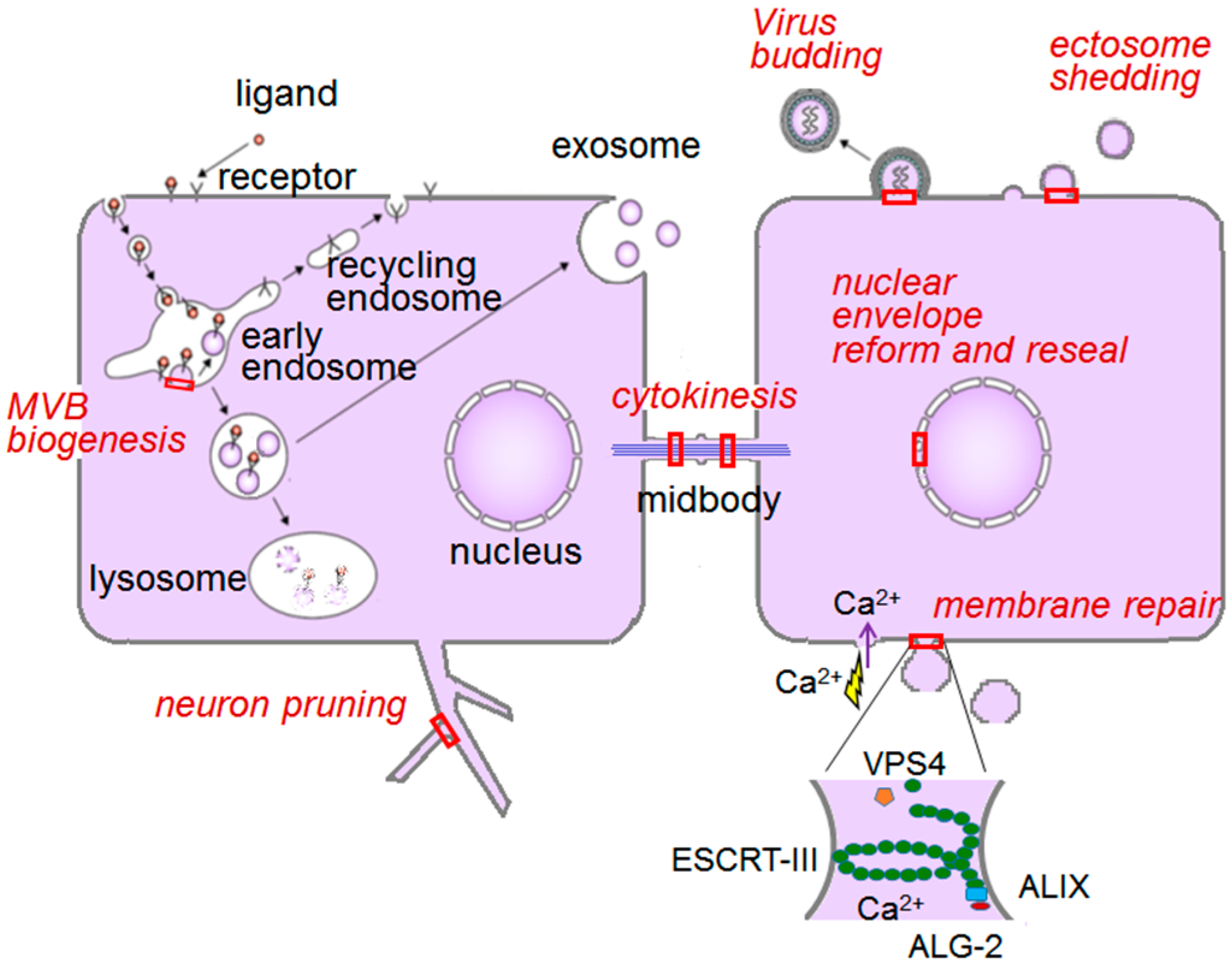
6.1. ESCRT System
6.1.1. Endosomal Sorting Pathway
6.1.2. Plasma Membrane Repair
6.2. ER-to-Golgi Vesicular Transport
6.3. Membrane Associated Proteins Interacting with ALG-2
6.3.1. Scotin
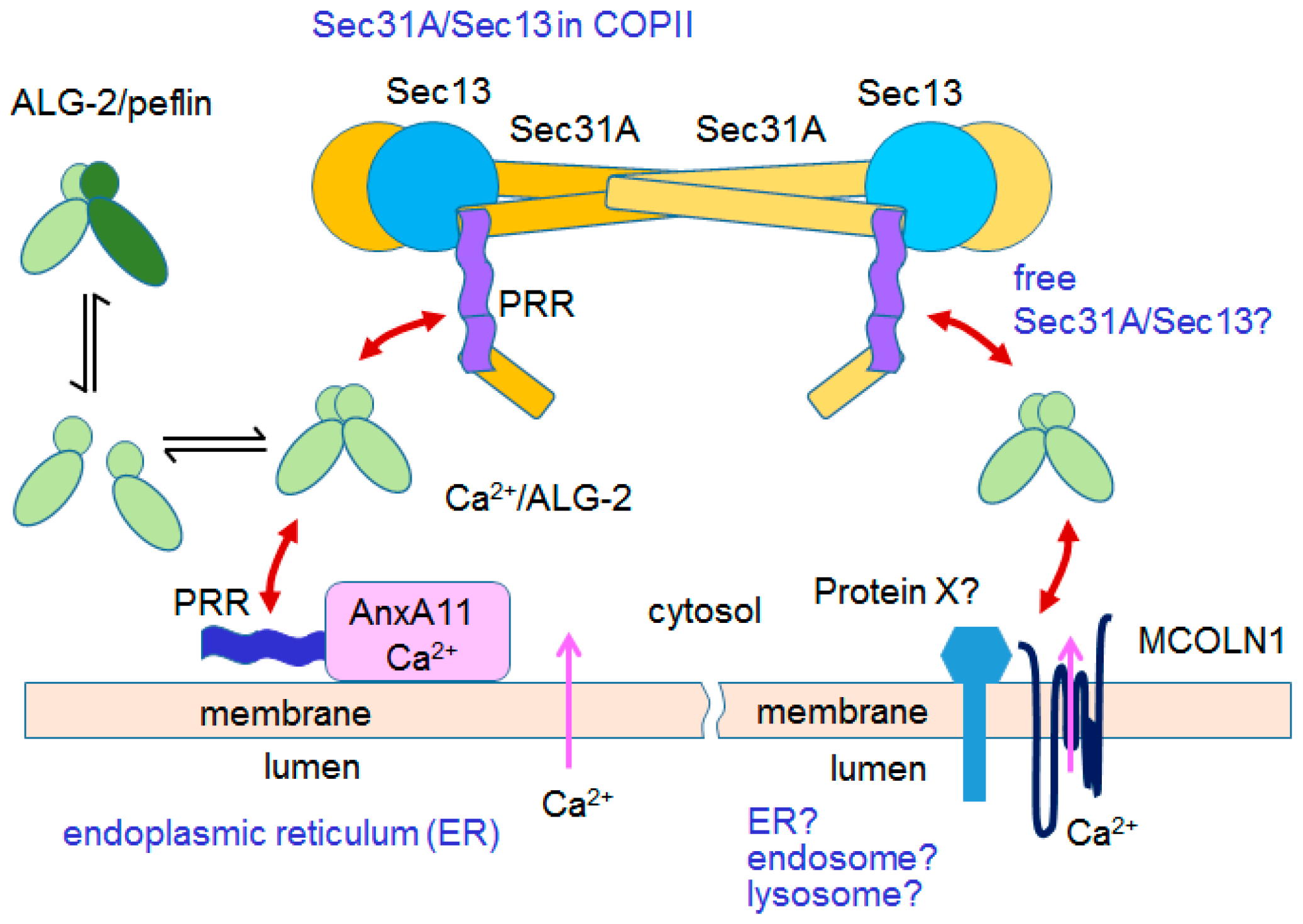
6.3.2. Mucolipin-1
6.3.3. PLSCR3
7. Interplay of PEF Proteins
7.1. Peflin
7.2. Sorcin
7.3. Calpains
8. Interaction of ALG-2 with Membrane Receptors and Signal Transducers
9. Association with the Nucleus
10. Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABM | ALG-2-binding motif |
| ALIX | ALG-2-interacting protein X |
| AnxA11 | annexin A11 |
| CEP55 | centrosomal protein of 55 kDa |
| CIE | clathrin-independent endocytosis |
| CME | clathrin-mediated endocytosis |
| COPII | coat protein complex II |
| CTxB | cholera toxin B |
| EGFR | epidermal growth factor receptor |
| ER | endoplasmic reticulum |
| ERES | ER exit site |
| ESCRT | endosomal sorting complex required for transport |
| GST | glutathione S-transferase |
| ILV | intraluminal vesicle |
| LBPA | lysobisphosphatidic acid |
| mTOR | mammalian target of rapamycin |
| MIT | microtubule-interacting and transport |
| MVB | multivesicular body |
| NE | nuclear envelope |
| NPC | nuclear pore complex |
| PEF | penta-EF-hand |
| PRR | Pro-rich region |
| TNFα | Tumor necrosis factor alpha |
| VSV-G | vesicular stomatitis virus envelope glycoprotein |
References
- Vito, P.; Lacanà, E.; D’Adamio, L. Interfering with apoptosis: Ca2+-binding protein ALG-2 and Alzheimer’s disease gene ALG-3. Science 1996, 271, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Vito, P.; Pellegrini, L.; Guiet, C.; D’Adamio, L. Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J. Biol. Chem. 1999, 274, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Missotten, M.; Nichols, A.; Rieger, K.; Sadoul, R. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 1999, 6, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Hu, R.; Lacanà, E.; D’Adamio, L.; Gu, H. Apoptosis-linked gene 2-deficient mice exhibit normal T-cell development and function. Mol. Cell. Biol. 2002, 22, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Tarabykina, S.; Mollerup, J.; Winding, P.; Berchtold, M.W. ALG-2, a multifunctional calcium binding protein? Front. Biosci. 2004, 9, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, R. Do Alix and ALG-2 really control endosomes for better or for worse? Biol. Cell 2006, 98, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Von Schwedler, U.K.; Stuchell, M.; Müller, B.; Ward, D.M.; Chung, H.Y.; Morita, E.; Wang, H.E.; Davis, T.; He, G.P.; Cimbora, D.M.; et al. The protein network of HIV budding. Cell 2003, 114, 701–713. [Google Scholar] [CrossRef]
- Strack, B.; Calistri, A.; Craig, S.; Popova, E.; Göttlinger, H.G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 2003, 114, 689–699. [Google Scholar] [CrossRef]
- Katoh, K.; Shibata, H.; Suzuki, H.; Nara, A.; Ishidoh, K.; Kominami, E.; Yoshimori, T.; Maki, M. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J. Biol. Chem. 2003, 278, 39104–39113. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Campsteijn, C.; Vietri, M.; Stenmark, H. Novel ESCRT functions in cell biology: Spiraling out of control? Curr. Opin. Cell Biol. 2016, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, J.G.; Stahmer, K.R.; Miller, E.A. Vesicle-mediated export from the ER: COPII coat function and regulation. Biochim. Biophys. Acta 2013, 1833, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Narayana, S.V.; Hitomi, K. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem. J. 1997, 328 Pt 2, 718–720. [Google Scholar] [PubMed]
- Maki, M.; Kitaura, Y.; Satoh, H.; Ohkouchi, S.; Shibata, H. Structures, functions and molecular evolution of the penta-EF-hand Ca2+-binding proteins. Biochim. Biophys. Acta 2002, 1600, 51–60. [Google Scholar] [CrossRef]
- Gifford, J.L.; Walsh, M.P.; Vogel, H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007, 405, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Denessiouk, K.; Permyakov, S.; Denesyuk, A.; Permyakov, E.; Johnson, M.S. Two structural motifs within canonical EF-hand calcium-binding domains identify five different classes of calcium buffers and sensors. PLoS ONE 2014, 9, e109287. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.; Zhang, Q.; Li, M.; Zhang, M. Apoptosis-linked gene product ALG-2 is a new member of the calpain small subunit subfamily of Ca2+-binding proteins. Biochemistry 1999, 38, 7498–7508. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, Y.; Matsumoto, S.; Satoh, H.; Hitomi, K.; Maki, M. Peflin and ALG-2, members of the penta-EF-hand protein family, form a heterodimer that dissociates in a Ca2+-dependent manner. J. Biol. Chem. 2001, 276, 14053–14058. [Google Scholar] [PubMed]
- Hamada, H.; Okochi, E.; Oh-hara, T.; Tsuruo, T. Purification of the Mr 22,000 calcium-binding protein (sorcin) associated with multidrug resistance and its detection with monoclonal antibodies. Cancer Res. 1988, 48, 3173–3178. [Google Scholar] [PubMed]
- Teahan, C.G.; Totty, N.F.; Segal, A.W. Isolation and characterization of grancalcin, a novel 28 kDa EF-hand calcium-binding protein from human neutrophils. Biochem. J. 1992, 286 Pt 2, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Tarabykina, S.; la Cour, J.M.; Lollike, K.; Berchtold, M.W. The PEF family proteins sorcin and grancalcin interact in vivo and in vitro. FEBS Lett. 2003, 545, 151–154. [Google Scholar] [CrossRef]
- Ono, Y.; Sorimachi, H. Calpains: An elaborate proteolytic system. Biochim. Biophys. Acta 2012, 1824, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Tarabykina, S.; Moller, A.L.; Durussel, I.; Cox, J.; Berchtold, M.W. Two forms of the apoptosis-linked protein ALG-2 with different Ca2+ affinities and target recognition. J. Biol. Chem. 2000, 275, 10514–10518. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Suzuki, H.; Shibata, H. Structure and function of ALG-2, a penta-EF-hand calcium-dependent adaptor protein. Sci. China Life Sci. 2011, 54, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Crabb, J.W.; Cox, J.; Durussel, I.; Walker, T.M.; van Ginkel, P.R.; Bhattacharya, S.; Dellaria, J.M.; Palczewski, K.; Polans, A.S. Ca2+ binding to EF hands 1 and 3 is essential for the interaction of apoptosis-linked gene-2 with Alix/AIP1 in ocular melanoma. Biochemistry 2004, 43, 11175–11186. [Google Scholar] [CrossRef] [PubMed]
- Henzl, M.T.; Frey, B.B.; Wolf, A.J. ALG-2 divalent-ion affinity: Calorimetric analysis of the des23 versions reveals high-affinity site for Mg2+. Biophys. Chem. 2016, 209, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Tarabykina, S.; Hansen, C.; Berchtold, M.; Cygler, M. Structure of apoptosis-linked protein ALG-2: Insights into Ca2+-induced changes in penta-EF-hand proteins. Structure 2001, 9, 267–275. [Google Scholar] [CrossRef]
- Suzuki, H.; Kawasaki, M.; Inuzuka, T.; Okumura, M.; Kakiuchi, T.; Shibata, H.; Wakatsuki, S.; Maki, M. Structural basis for Ca2+-dependent formation of ALG-2/Alix peptide complex: Ca2+/EF3-driven arginine switch mechanism. Structure 2008, 16, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Yamaguchi, K.; Kitaura, Y.; Satoh, H.; Hitomi, K. Calcium-induced exposure of a hydrophobic surface of mouse ALG-2, which is a member of the penta-EF-hand protein family. J. Biochem. 1998, 124, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Trioulier, Y.; Torch, S.; Blot, B.; Cristina, N.; Chatellard-Causse, C.; Verna, J.M.; Sadoul, R. Alix, a protein regulating endosomal trafficking, is involved in neuronal death. J. Biol. Chem. 2004, 279, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Yamada, K.; Mizuno, T.; Yorikawa, C.; Takahashi, H.; Satoh, H.; Kitaura, Y.; Maki, M. The penta-EF-hand protein ALG-2 interacts with a region containing PxY repeats in Alix/AIP1, which is required for the subcellular punctate distribution of the amino-terminal truncation form of Alix/AIP1. J. Biochem. 2004, 135, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, F.; Takaya, E.; Suzuki, H.; Kajigaya, S.; Buchman, V.L.; Shibata, H.; Maki, M. HD-PTP and Alix share some membrane-traffic related proteins that interact with their Bro1 domains or proline-rich regions. Arch. Biochem. Biophys. 2007, 457, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Suzuki, H.; Terasawa, Y.; Mizuno, T.; Yasuda, J.; Shibata, H.; Maki, M. The penta-EF-hand protein ALG-2 interacts directly with the ESCRT-I component TSG101, and Ca2+-dependently co-localizes to aberrant endosomes with dominant-negative AAA ATPase SKD1/VPS4B. Biochem. J. 2005, 391, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Ichioka, F.; Kobayashi, R.; Suzuki, H.; Yoshida, H.; Shibata, H.; Maki, M. Penta-EF-hand protein ALG-2 functions as a Ca2+-dependent adaptor that bridges Alix and TSG101. Biochem. Biophys. Res. Commun. 2009, 386, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Katsuyama, A.M.; Shibata, H.; Maki, M. Vps37 isoforms differentially modulate the ternary complex formation of ALIX, ALG-2, and ESCRT-I. Biosci. Biotechnol. Biochem. 2013, 77, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Takahashi, T.; Shibata, H.; Maki, M. Mammalian ESCRT-III-related protein IST1 has a distinctive met-pro repeat sequence that is essential for interaction with ALG-2 in the presence of Ca2+. Biosci. Biotechnol. Biochem. 2013, 77, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, A.; Tani, K.; Yamamoto, A.; Kitamura, N.; Komada, M. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol. Biol. Cell 2006, 17, 4876–4887. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Suzuki, H.; Yoshida, H.; Maki, M. ALG-2 directly binds Sec31A and localizes at endoplasmic reticulum exit sites in a Ca2+-dependent manner. Biochem. Biophys. Res. Commun. 2007, 353, 756–763. [Google Scholar] [CrossRef] [PubMed]
- La Cour, J.M.; Mollerup, J.; Berchtold, M.W. ALG-2 oscillates in subcellular localization, unitemporally with calcium oscillations. Biochem. Biophys. Res. Commun. 2007, 353, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Inuzuka, T.; Yoshida, H.; Sugiura, H.; Wada, I.; Maki, M. The ALG-2 binding site in Sec31A influences the retention kinetics of Sec31A at the endoplasmic reticulum exit sites as revealed by live-cell time-lapse imaging. Biosci. Biotechnol. Biochem. 2010, 74, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Suzuki, H.; Kakiuchi, T.; Inuzuka, T.; Yoshida, H.; Mizuno, T.; Maki, M. Identification of Alix-type and non-Alix-type ALG-2-binding sites in human phospholipid scramblase 3: Differential binding to an alternatively spliced isoform and amino acid-substituted mutants. J. Biol. Chem. 2008, 283, 9623–9632. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Shibata, H.; Nakano, Y.; Kitaura, Y.; Maki, M. ALG-2 interacts with the amino-terminal domain of annexin XI in a Ca2+-dependent manner. Biochem. Biophys. Res. Commun. 2002, 291, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Nakano, Y.; Shibata, H.; Maki, M. The penta-EF-hand domain of ALG-2 interacts with amino-terminal domains of both annexin VII and annexin XI in a Ca2+-dependent manner. Biochim. Biophys. Acta 2002, 1600, 61–67. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Snyder, S.L.; Creutz, C.E. Identification of targets for calcium signaling through the copine family of proteins. Characterization of a coiled-coil copine-binding motif. J. Biol. Chem. 2003, 278, 10048–10054. [Google Scholar] [CrossRef] [PubMed]
- Vergarajauregui, S.; Martina, J.A.; Puertollano, R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J. Biol. Chem. 2009, 284, 36357–36366. [Google Scholar] [CrossRef] [PubMed]
- Draeby, I.; Woods, Y.L.; la Cour, J.M.; Mollerup, J.; Bourdon, J.C.; Berchtold, M.W. The calcium binding protein ALG-2 binds and stabilizes Scotin, a p53-inducible gene product localized at the endoplasmic reticulum membrane. Arch. Biochem. Biophys. 2007, 467, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Kim, K.S.; Kim, K.D.; Lim, J.S.; Kim, J.W.; Kim, E. Apoptosis-linked gene 2 binds to the death domain of Fas and dissociates from Fas during Fas-mediated apoptosis in Jurkat cells. Biochem. Biophys. Res. Commun. 2001, 288, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Mahul-Mellier, A.L.; Strappazzon, F.; Petiot, A.; Chatellard-Causse, C.; Torch, S.; Blot, B.; Freeman, K.; Kuhn, L.; Garin, J.; Verna, J.M.; et al. Alix and ALG-2 are involved in tumor necrosis factor receptor 1-induced cell death. J. Biol. Chem. 2008, 283, 34954–34965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rho, S.B.; Song, Y.J.; Lim, M.C.; Lee, S.H.; Kim, B.R.; Park, S.Y. Programmed cell death 6 (PDCD6) inhibits angiogenesis through PI3K/mTOR/p70S6K pathway by interacting of VEGFR-2. Cell Signal. 2012, 24, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sytkowski, A.J. Apoptosis-linked gene-2 connects the Raf-1 and Ask1 signalings. Biochem. Biophys. Res. Commun. 2005, 333, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Rho, S.B.; Chun, T. Programmed cell death 6 (PDCD6) protein interacts with death-associated protein kinase 1 (DAPk1): Additive effect on apoptosis via caspase-3 dependent pathway. Biotechnol. Lett. 2005, 27, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Jung, Y.S.; Kim, E. Interaction of ALG-2 with Ask1 influences Ask1 localization and subsequent JNK activation. FEBS Lett. 2002, 529, 183–187. [Google Scholar] [CrossRef]
- Montaville, P.; Dai, Y.; Cheung, C.Y.; Giller, K.; Becker, S.; Michalak, M.; Webb, S.E.; Miller, A.L.; Krebs, J. Nuclear translocation of the calcium-binding protein ALG-2 induced by the RNA-binding protein RBM22. Biochim. Biophys. Acta 2006, 1763, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Osugi, K.; Suzuki, H.; Nomura, T.; Ariumi, Y.; Shibata, H.; Maki, M. Identification of the P-body component PATL1 as a novel ALG-2-interacting protein by in silico and far-western screening of proline-rich proteins. J. Biochem. 2012, 151, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Osugi, K.; Imoto, C.; Takahara, T.; Shibata, H.; Maki, M. Nuclear ALG-2 protein interacts with Ca2+ homeostasis endoplasmic reticulum protein (CHERP) Ca2+-dependently and participates in regulation of alternative splicing of inositol trisphosphate receptor type 1 (IP3R1) pre-mRNA. J. Biol. Chem. 2013, 288, 33361–33375. [Google Scholar] [CrossRef] [PubMed]
- Ilari, A.; Fiorillo, A.; Poser, E.; Lalioti, V.S.; Sundell, G.N.; Ivarsson, Y.; Genovese, I.; Colotti, G. Structural basis of Sorcin-mediated calcium-dependent signal transduction. Sci. Rep. 2015, 5, 16828. [Google Scholar] [CrossRef] [PubMed]
- Mollerup, J.; Krogh, T.N.; Nielsen, P.F.; Berchtold, M.W. Properties of the co-chaperone protein p23 erroneously attributed to ALG-2 (apoptosis-linked gene 2). FEBS Lett. 2003, 555, 478–482. [Google Scholar] [CrossRef]
- Takahashi, T.; Kojima, K.; Zhang, W.; Sasaki, K.; Ito, M.; Suzuki, H.; Kawasaki, M.; Wakatsuki, S.; Takahara, T.; Shibata, H.; et al. Structural analysis of the complex between penta-EF-hand ALG-2 protein and Sec31A peptide reveals a novel target recognition mechanism of ALG-2. Int. J. Mol. Sci. 2015, 16, 3677–3699. [Google Scholar] [CrossRef] [PubMed]
- Crivici, A.; Ikura, M. Molecular and structural basis of target recognition by calmodulin. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 85–116. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, T.; Suzuki, H.; Kawasaki, M.; Shibata, H.; Wakatsuki, S.; Maki, M. Molecular basis for defect in Alix-binding by alternatively spliced isoform of ALG-2 (ALG-2ΔGF122) and structural roles of F122 in target recognition. BMC Struct. Biol. 2010, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Votteler, J.; Sundquist, W.I. Virus budding and the ESCRT pathway. Cell Host Microbe 2013, 14, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Dores, M.R.; Chen, B.; Lin, H.; Soh, U.J.; Paing, M.M.; Montagne, W.A.; Meerloo, T.; Trejo, J. ALIX binds a YPX3L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J. Cell Biol. 2012, 197, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Dores, M.R.; Grimsey, N.J.; Mendez, F.; Trejo, J. ALIX regulates the ubiquitin-independent lysosomal sorting of the P2Y1 purinergic receptor via a YPX3L motif. PLoS ONE 2016, 11, e0157587. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, D.P.; Sandrin, V.; Vivona, S.; Shaler, T.A.; Kaiser, S.E.; Melandri, F.; Sundquist, W.I.; Kopito, R.R. ALIX is a Lys63-specific polyubiquitin binding protein that functions in retrovirus budding. Dev. Cell 2012, 23, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Si, J.; Corvera, J.; Gallick, G.E.; Kuang, J. Decoding the intrinsic mechanism that prohibits ALIX interaction with ESCRT and viral proteins. Biochem. J. 2010, 432, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhou, X.; Corvera, J.; Gallick, G.E.; Lin, S.H.; Kuang, J. ALG-2 activates the MVB sorting function of ALIX through relieving its intramolecular interaction. Cell Discov. 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sun, L.; Zhou, X.; Wu, C.; Wang, R.; Lin, S.H.; Kuang, J. Phosphorylation-dependent activation of the ESCRT function of ALIX in cytokinetic abscission and retroviral budding. Dev. Cell 2016, 36, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhou, X.; Zhang, W.; Gallick, G.E.; Kuang, J. Unravelling the pivotal role of Alix in MVB sorting and silencing of the activated EGFR. Biochem. J. 2015, 466, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, A.; Bache, K.G.; Brech, A.; Stenmark, H. Alix regulates cortical actin and the spatial distribution of endosomes. J. Cell Sci. 2005, 118, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Doyotte, A.; Mironov, A.; McKenzie, E.; Woodman, P. The Bro1-related protein HD-PTP/PTPN23 is required for endosomal cargo sorting and multivesicular body morphogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 6308–6313. [Google Scholar] [CrossRef] [PubMed]
- Mercier, V.; Laporte, M.H.; Destaing, O.; Blot, B.; Blouin, C.M.; Pernet-Gallay, K.; Chatellard, C.; Saoudi, Y.; Albiges-Rizo, C.; Lamaze, C.; et al. ALG-2 interacting protein-X (ALIX) is essential for clathrin-independent endocytosis and signaling. Sci. Rep. 2016, 6, 26986. [Google Scholar] [CrossRef] [PubMed]
- Chatellard-Causse, C.; Blot, B.; Cristina, N.; Torch, S.; Missotten, M.; Sadoul, R. Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization. J. Biol. Chem. 2002, 277, 29108–29115. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Hurley, J.H. Proline-rich regions and motifs in trafficking: From ESCRT interaction to viral exploitation. Traffic 2011, 12, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zhang, L.; Taylor, S.; Mironov, A.; Urbe, S.; Woodman, P. Recruitment of UBPY and ESCRT exchange drive HD-PTP-dependent sorting of EGFR to the MVB. Curr. Biol. 2013, 23, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Stefani, F.; Zhang, L.; Taylor, S.; Donovan, J.; Rollinson, S.; Doyotte, A.; Brownhill, K.; Bennion, J.; Pickering-Brown, S.; Woodman, P. UBAP1 is a component of an endosome-specific ESCRT-I complex that is essential for MVB sorting. Curr. Biol. 2011, 21, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Wunderley, L.; Brownhill, K.; Stefani, F.; Tabernero, L.; Woodman, P. The molecular basis for selective assembly of the UBAP1-containing endosome-specific ESCRT-I complex. J. Cell Sci. 2014, 127, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M.C.; Zhang, Y.L.; Kharitidi, D.; Barr, A.J.; Knapp, S.; Tremblay, M.L.; Pause, A. HD-PTP is a catalytically inactive tyrosine phosphatase due to a conserved divergence in its phosphatase domain. PLoS ONE 2009, 4, e5105. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Chevallier, J.; Mayran, N.; Le Blanc, I.; Ferguson, C.; Faure, J.; Blanc, N.S.; Matile, S.; Dubochet, J.; Sadoul, R.; et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 2004, 303, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Bissig, C.; Lenoir, M.; Velluz, M.C.; Kufareva, I.; Abagyan, R.; Overduin, M.; Gruenberg, J. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev. Cell 2013, 25, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Bissig, C.; Gruenberg, J. ALIX and the multivesicular endosome: ALIX in wonderland. Trends Cell Biol. 2014, 24, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.J.; Perez, F. Physico-chemical and biological considerations for membrane wound evolution and repair in animal cells. Semin. Cell Dev. Biol. 2015, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.J.; Maiuri, P.; Lafaurie-Janvore, J.; Divoux, S.; Piel, M.; Perez, F. ESCRT machinery is required for plasma membrane repair. Science 2014, 343, 1247136. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, L.L.; Sreetama, S.C.; Sharma, N.; Medikayala, S.; Brown, K.J.; Defour, A.; Jaiswal, J.K. Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nat. Commun. 2014, 5, 5646. [Google Scholar] [CrossRef] [PubMed]
- Rescher, U.; Gerke, V. Annexins—Unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Draeger, A.; Monastyrskaya, K.; Babiychuk, E.B. Plasma membrane repair and cellular damage control: The annexin survival kit. Biochem. Pharmacol. 2011, 81, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Elia, N.; Ghirlando, R.; Lippincott-Schwartz, J.; Hurley, J.H. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science 2008, 322, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Budnik, A.; Stephens, D.J. ER exit sites—Localization and control of COPII vesicle formation. FEBS Lett. 2009, 583, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Venditti, R.; Wilson, C.; de Matteis, M.A. Exiting the ER: What we know and what we don’t. Trends Cell Biol. 2014, 24, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.; Nycz, D.C.; Joglekar, A.; Fertschai, I.; Malli, R.; Graier, W.F.; Hay, J.C. Vesicular calcium regulates coat retention, fusogenicity, and size of pre-Golgi intermediates. Mol. Biol. Cell 2010, 21, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- La Cour, J.M.; Schindler, A.J.; Berchtold, M.W.; Schekman, R. ALG-2 attenuates COPII budding in vitro and stabilizes the Sec23/Sec31A complex. PLoS ONE 2013, 8, e75309. [Google Scholar] [CrossRef] [PubMed]
- Helm, J.R.; Bentley, M.; Thorsen, K.D.; Wang, T.; Foltz, L.; Oorschot, V.; Klumperman, J.; Hay, J.C. Apoptosis-linked gene-2 (ALG-2)/Sec31 interactions regulate endoplasmic reticulum (ER)-to-Golgi transport: A potential effector pathway for luminal calcium. J. Biol. Chem. 2014, 289, 23609–23628. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kanadome, T.; Sugiura, H.; Yokoyama, T.; Yamamuro, M.; Moss, S.E.; Maki, M. A new role for annexin A11 in the early secretory pathway via stabilizing Sec31A protein at the endoplasmic reticulum exit sites (ERES). J. Biol. Chem. 2015, 290, 4981–4993. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, J.C.; Renzing, J.; Robertson, P.L.; Fernandes, K.N.; Lane, D.P. Scotin, a novel p53-inducible proapoptotic protein located in the ER and the nuclear membrane. J. Cell Biol. 2002, 158, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Dashzeveg, N.; Lu, Z.G.; Taira, N.; Miki, Y.; Yoshida, K. Programmed cell death 6, a novel p53-responsive gene, targets to the nucleus in the apoptotic response to DNA damage. Cancer Sci. 2012, 103, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Grishin, N.V. Unexpected diversity in SHISA-like proteins suggests the importance of their roles as transmembrane adaptors. Cell Signal. 2012, 24, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Wong, C.O.; Zhu, M.X. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 2015, 58, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Gustafson, A.M.; Sidransky, E.; Goldin, E. Mucolipidosis type IV: An update. Mol. Genet. Metab. 2011, 104, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Onyenwoke, R.U.; Sexton, J.Z.; Yan, F.; Diaz, M.C.; Forsberg, L.J.; Major, M.B.; Brenman, J.E. The mucolipidosis IV Ca2+ channel TRPML1 (MCOLN1) is regulated by the TOR kinase. Biochem. J. 2015, 470, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A tumor suppressor complex with gap activity for the rag gtpases that signal amino acid sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Finn, R.D.; Sims, P.J.; Wiedmer, T.; Biegert, A.; Soding, J. Phospholipid scramblases and tubby-like proteins belong to a new superfamily of membrane tethered transcription factors. Bioinformatics 2009, 25, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Bevers, E.M.; Williamson, P.L. Phospholipid scramblase: An update. FEBS Lett. 2010, 584, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dai, Q.; Chen, J.; Durrant, D.; Freeman, A.; Liu, T.; Grossman, D.; Lee, R.M. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol. Cancer Res. 2003, 1, 892–902. [Google Scholar] [PubMed]
- Van, Q.; Liu, J.; Lu, B.; Feingold, K.R.; Shi, Y.; Lee, R.M.; Hatch, G.M. Phospholipid scramblase-3 regulates cardiolipin de novo biosynthesis and its resynthesis in growing HeLa cells. Biochem. J. 2007, 401, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.T.; Ji, J.; Dagda, R.K.; Jiang, J.F.; Tyurina, Y.Y.; Kapralov, A.A.; Tyurin, V.A.; Yanamala, N.; Shrivastava, I.H.; Mohammadyani, D.; et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 2013, 15, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, T.; Zhao, J.; Li, L.; Zhou, Q.; Hevener, A.; Olefsky, J.M.; Curtiss, L.K.; Sims, P.J. Adiposity, dyslipidemia, and insulin resistance in mice with targeted deletion of phospholipid scramblase 3 (PLSCR3). Proc. Natl. Acad. Sci. USA 2004, 101, 13296–13301. [Google Scholar] [CrossRef] [PubMed]
- Inokawa, A.; Inuzuka, T.; Takahara, T.; Shibata, H.; Maki, M. Tubby-like protein superfamily member PLSCR3 functions as a negative regulator of adipogenesis in mouse 3T3-L1 preadipocytes by suppressing induction of late differentiation stage transcription factors. Biosci. Rep. 2016, 36, e00287. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, T.; Inokawa, A.; Chen, C.; Kizu, K.; Narita, H.; Shibata, H.; Maki, M. ALG-2-interacting Tubby-like protein superfamily member PLSCR3 is secreted by an exosomal pathway and taken up by recipient cultured cells. Biosci. Rep. 2013, 33, e00026. [Google Scholar] [CrossRef] [PubMed]
- Rayl, M.; Truitt, M.; Held, A.; Sargeant, J.; Thorsen, K.; Hay, J.C. Penta-EF-hand protein peflin is a negative regulator of ER-to-Golgi transport. PLoS ONE 2016, 11, e0157227. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Maemoto, Y.; Osako, Y.; Shibata, H. Evolutionary and physical linkage between calpains and penta-EF-hand Ca2+-binding proteins. FEBS J. 2012, 279, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Noordeen, N.A.; Meur, G.; Rutter, G.A.; Leclerc, I. Glucose-induced nuclear shuttling of ChREBP is mediated by sorcin and Ca2+-ions in pancreatic β-cells. Diabetes 2012, 61, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Lalioti, V.S.; Ilari, A.; O’Connell, D.J.; Poser, E.; Sandoval, I.V.; Colotti, G. Sorcin links calcium signaling to vesicle trafficking, regulates Polo-like kinase 1 and is necessary for mitosis. PLoS ONE 2014, 9, e85438. [Google Scholar]
- Brownawell, A.M.; Creutz, C.E. Calcium-dependent binding of sorcin to the N-terminal domain of synexin (annexin VII). J. Biol. Chem. 1997, 272, 22182–22190. [Google Scholar] [CrossRef] [PubMed]
- Tomas, A.; Futter, C.; Moss, S.E. Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J. Cell Biol. 2004, 165, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Høj, B.R.; la Cour, J.M.; Mollerup, J.; Berchtold, M.W. ALG-2 knockdown in HeLa cells results in G2/M cell cycle phase accumulation and cell death. Biochem. Biophys. Res. Commun. 2009, 378, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, R.L.; Miyake, K.; Kramerova, I.; Spencer, M.J.; Bourg, N.; Bartoli, M.; Richard, I.; Greer, P.A.; McNeil, P.L. Calcium-dependent plasma membrane repair requires m- or μ-calpain, but not calpain-3, the proteasome, or caspases. Biochim. Biophys. Acta 2009, 1793, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T. The signaling mechanism of ambient pH sensing and adaptation in yeast and fungi. FEBS J. 2012, 279, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Osako, Y.; Maemoto, Y.; Tanaka, R.; Suzuki, H.; Shibata, H.; Maki, M. Autolytic activity of human calpain 7 is enhanced by ESCRT-III-related protein ist1 through MIT-MIM interaction. FEBS J. 2010, 277, 4412–4426. [Google Scholar] [CrossRef] [PubMed]
- Maemoto, Y.; Kiso, S.; Shibata, H.; Maki, M. Analysis of limited proteolytic activity of calpain-7 using non-physiological substrates in mammalian cells. FEBS J. 2013, 280, 2594–2607. [Google Scholar] [CrossRef] [PubMed]
- Maemoto, Y.; Ono, Y.; Kiso, S.; Shibata, H.; Takahara, T.; Sorimachi, H.; Maki, M. Involvement of calpain-7 in epidermal growth factor receptor degradation via the endosomal sorting pathway. FEBS J. 2014, 281, 3642–3655. [Google Scholar] [CrossRef] [PubMed]
- La Cour, J.M.; Høj, B.R.; Mollerup, J.; Simon, R.; Sauter, G.; Berchtold, M.W. The apoptosis linked gene ALG-2 is dysregulated in tumors of various origin and contributes to cancer cell viability. Mol. Oncol. 2008, 1, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Aviel-Ronen, S.; Coe, B.P.; Lau, S.K.; da Cunha Santos, G.; Zhu, C.Q.; Strumpf, D.; Jurisica, I.; Lam, W.L.; Tsao, M.S. Genomic markers for malignant progression in pulmonary adenocarcinoma with bronchioloalveolar features. Proc. Natl. Acad. Sci. USA 2008, 105, 10155–10160. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Arao, T.; Gotoda, T.; Taniguchi, H.; Oda, I.; Shirao, K.; Shimada, Y.; Hamaguchi, T.; Kato, K.; Hamano, T.; et al. Identification of prognostic biomarkers in gastric cancer using endoscopic biopsy samples. Cancer Sci. 2008, 99, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Briffa, R.; Um, I.; Faratian, D.; Zhou, Y.; Turnbull, A.K.; Langdon, S.P.; Harrison, D.J. Multi-scale genomic, transcriptomic and proteomic analysis of colorectal cancer cell lines to identify novel biomarkers. PLoS ONE 2015, 10, e0144708. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, J.H.; Lee, G.B.; Byun, H.J.; Kim, B.R.; Park, C.Y.; Kim, H.B.; Rho, S.B. PDCD6 additively cooperates with anti-cancer drugs through activation of NF-κB pathways. Cell Signal. 2012, 24, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Janowicz, A.; Michalak, M.; Krebs, J. Stress induced subcellular distribution of ALG-2, RBM22 and hSlu7. Biochim. Biophys. Acta 2011, 1813, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Marnef, A.; Weil, D.; Standart, N. RNA-related nuclear functions of human Pat1b, the P-body mRNA decay factor. Mol. Biol. Cell 2012, 23, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, O.; Gerasimenko, J. New aspects of nuclear calcium signalling. J. Cell Sci. 2004, 117, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Raab, M.; Gentili, M.; de Belly, H.; Thiam, H.R.; Vargas, P.; Jimenez, A.J.; Lautenschlaeger, F.; Voituriez, R.; Lennon-Dumenil, A.M.; Manel, N.; et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 2016, 352, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Denais, C.M.; Gilbert, R.M.; Isermann, P.; McGregor, A.L.; te Lindert, M.; Weigelin, B.; Davidson, P.M.; Friedl, P.; Wolf, K.; Lammerding, J. Nuclear envelope rupture and repair during cancer cell migration. Science 2016, 352, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Tomas, A.; Moss, S.E. Calcium- and cell cycle-dependent association of annexin 11 with the nuclear envelope. J. Biol. Chem. 2003, 278, 20210–20216. [Google Scholar] [CrossRef] [PubMed]
- Yoshibori, M.; Yorimitsu, T.; Sato, K. Involvement of the penta-EF-hand protein Pef1p in the Ca2+-dependent regulation of COPII subunit assembly in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e40765. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, A.R.; Friedberg, F. Sequence motifs for calmodulin recognition. FASEB J. 1997, 11, 331–340. [Google Scholar] [PubMed]
- Yamniuk, A.P.; Vogel, H.J. Calmodulin’s flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol. Biotechnol. 2004, 27, 33–57. [Google Scholar] [CrossRef]
- Batters, C.; Brack, D.; Ellrich, H.; Averbeck, B.; Veigel, C. Calcium can mobilize and activate myosin-VI. Proc. Natl. Acad. Sci. USA 2016, 113, E1162–E1169. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.A.; Sorensen, B.R.; Kilpatrick, A.M.; Shea, M.A. Calcium-dependent energetics of calmodulin domain interactions with regulatory regions of the ryanodine receptor type 1 (RyR1). Biophys. Chem. 2014, 193, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Suzuki, H.; Inuzuka, T.; Shibata, H.; Maki, M. Prediction of a new ligand-binding site for type 2 motif based on the crystal structure of ALG-2 by dry and wet approaches. Int. J. Mol. Sci. 2012, 13, 7532–7549. [Google Scholar] [CrossRef] [PubMed]



| Protein Name | Alternative Name | UniProt ID (Human) | Binding Domain/Region | Binding Motif | Binding to ΔGF122 | Method | Function System | Reference |
|---|---|---|---|---|---|---|---|---|
| ALIX | AIP1, PDCD6IP | Q8WUM4 | PRR | 1, 3-like | No | Y2H, IP, PD, FW, SPR | ESCRT accessory | [2,3,25,28,30,31] |
| HD-PTP | PTPN23 | Q9H3S7 | PRR | 1-like | nd | Y2H, PD, FW | ESCRT accessory | [32,54] |
| TSG101 | - | Q99816 | PRR | 1-like | No | Y2H, IP, PD, FW | ESCRT-I | [33,34,54] |
| VPS37B | - | Q9H9H4 | PRR | 1-like | No | IP, PD, FW | ESCRT-I | [35] |
| VPS37C | - | A5D8V6 | PRR | 1-like | nd | IP, PD, FW | ESCRT-I | [35,54] |
| IST1 | - | P53990 | PRR | 3 | No | PD, FW | ESCRT-III | [36] |
| Sec31A | - | O94979 | PRR | 2 | Yes | IP, PD, FW | COPII outer shell | [37,38,39,40,54] |
| PLSCR3 | PLS3, Scr3 | Q9NRY6 | PRR | 1, 2 | Yes | IP, PD, FW, SPR | cardiolipin translocation | [41,54] |
| annexin A11 | AnxA11 | P50995 | PRR | 1-like | No | Y2H, PD, FW, SPR | phospholipid binding | [41,42,43,54] |
| annexin A7 | AnxA7, synexin | P20073 | PRR | 1-like | No | PD, FW, SPR | phospholipid binding | [41,43,54] |
| copine-4 | CPNE4 | Q96A23 | VWFA | nd | nd | Y2H, PD | phospholipid binding | [44] |
| Mucolipin-1 | MCOLN1, TRPML1 | Q9GZU1 | N-tail | ABH | Yes/No | PD | ion channel | [45] |
| Scotin | SHISA5 | Q8N114 | PRR | 1-like | No | PD, IP, FW | apoptosis | [46,54] |
| Fas *1 | APO-1, CD95 | P25445 | nd | nd | nd | Y2H, IP, PD | apoptosis | [47] |
| pro-caspase 8 | - | Q14790 | nd | nd | nd | IP | apoptosis | [48] |
| VEGFR2 | FLK1, KDR | P35968 | 801–1180 | nd | nd | Y2H, IP | RTK, angiogenesis | [49] |
| Raf-1 | RAF1 | P04049 | nd | nd | nd | Y2H, IP | Ser/Thr kinase | [50] |
| DAPK1 | - | P53355 | nd | nd | nd | Y2H, IP | Ser/Thr kinase | [51] |
| ASK1 *2 | MAP3K5 | Q99683 | 941–1375 | nd | No | PD, IP | Ser/Thr kinase | [52] |
| RBM22 | ZC3H16 | Q9NW64 | PRR | 2-like | n/a *3 | Y2H, FW | pre-mRNA splicing | [53,54] |
| PATL1 | Pat1b | Q86TB9 | PRR | 2 | Yes | PD, IP, FW | RNA processing | [54] |
| CHERP | SCAF6 | Q8IWX8 | PRR | 1, 2-like | nd | IP, FW | pre-mRNA splicing | [54,55] |
| ALG-2 | PDCD6 | O75340 | EF5 | nd | nd | Y2H, IP | PEF family | [3,17] |
| peflin | PEF1 | Q9UBV8 | EF5 | nd | nd | Y2H, IP | PEF family | [18] |
| sorcin *4 | - | P30626 | PEF | nd | nd | SPR | PEF family | [56] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maki, M.; Takahara, T.; Shibata, H. Multifaceted Roles of ALG-2 in Ca2+-Regulated Membrane Trafficking. Int. J. Mol. Sci. 2016, 17, 1401. https://doi.org/10.3390/ijms17091401
Maki M, Takahara T, Shibata H. Multifaceted Roles of ALG-2 in Ca2+-Regulated Membrane Trafficking. International Journal of Molecular Sciences. 2016; 17(9):1401. https://doi.org/10.3390/ijms17091401
Chicago/Turabian StyleMaki, Masatoshi, Terunao Takahara, and Hideki Shibata. 2016. "Multifaceted Roles of ALG-2 in Ca2+-Regulated Membrane Trafficking" International Journal of Molecular Sciences 17, no. 9: 1401. https://doi.org/10.3390/ijms17091401