TLR4 Endogenous Ligand S100A8/A9 Levels in Adult-Onset Still’s Disease and Their Association with Disease Activity and Clinical Manifestations
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of the 20 Adult-Onset Still’s Disease (AOSD) Patients, 20 Rheumatoid Arthritis (RA) Patietns, and 20 Healthy Controls (HCs)
2.2. Serum S100A8/A9 Levels in AOSD, RA Patients, and HCs
2.3. Serum Interleukin-1β (IL-1β), and Tumor Necrosis Factor-α (TNF-α) Levels in AOSD Patients and HCs
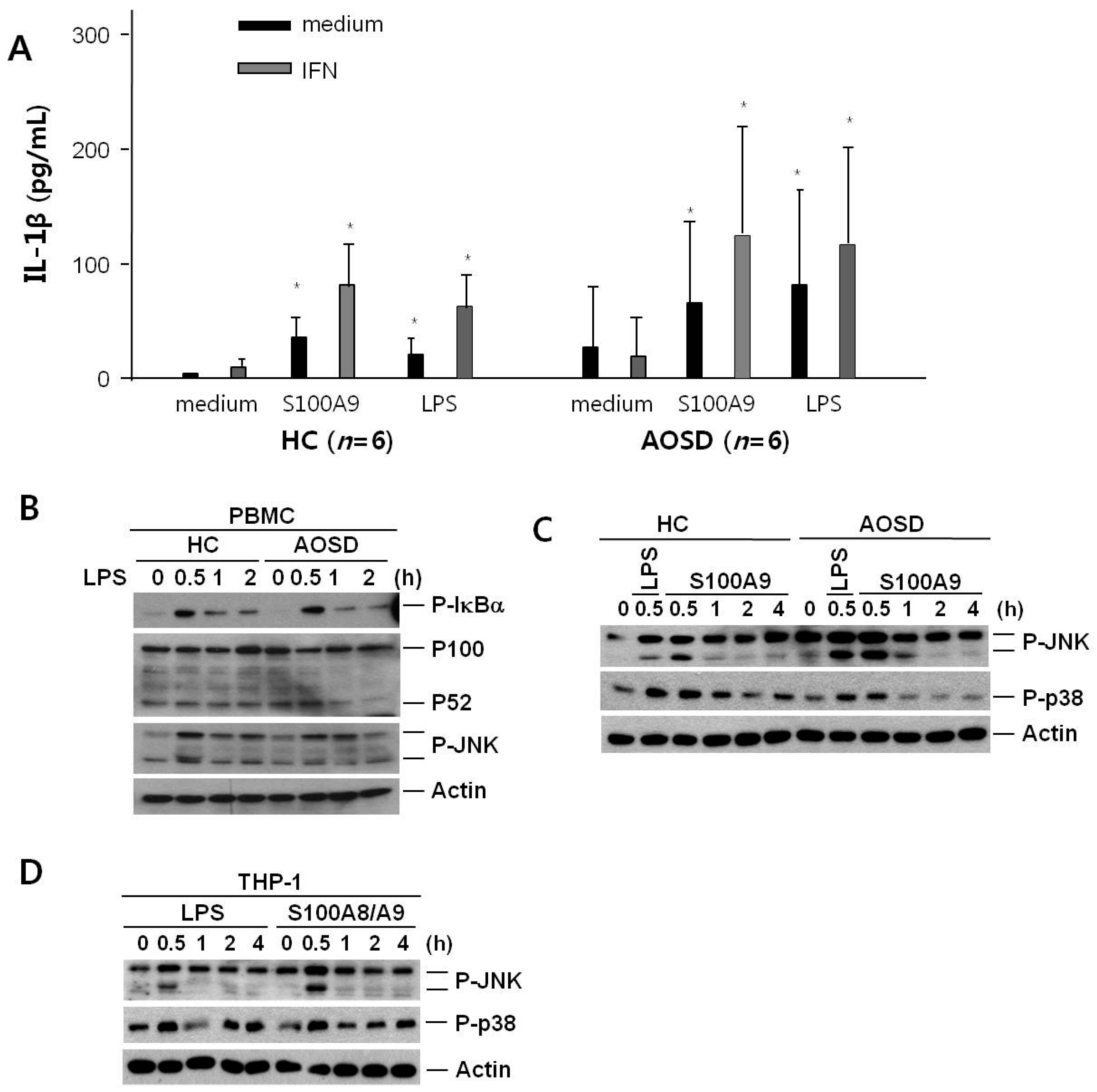
2.4. IL-1β Secretion after Treatment of S100A9 in PBMCs from Active AOSD Patients and HCs
2.5. Expression of c-JUN Amino-Terminal Kinase (JNK) and p38 after Treatment with S100A9 in PBMCs from Active AOSD Patients and HCs and in THP-1 Cells
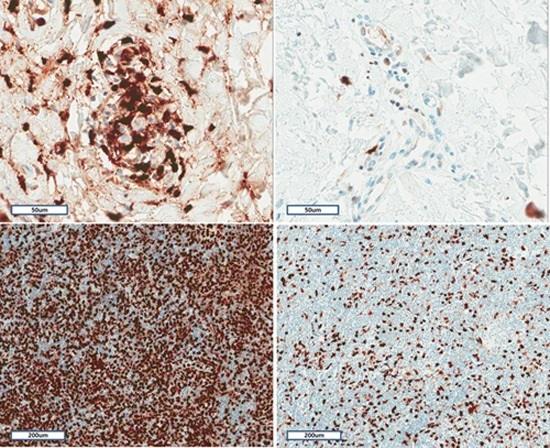
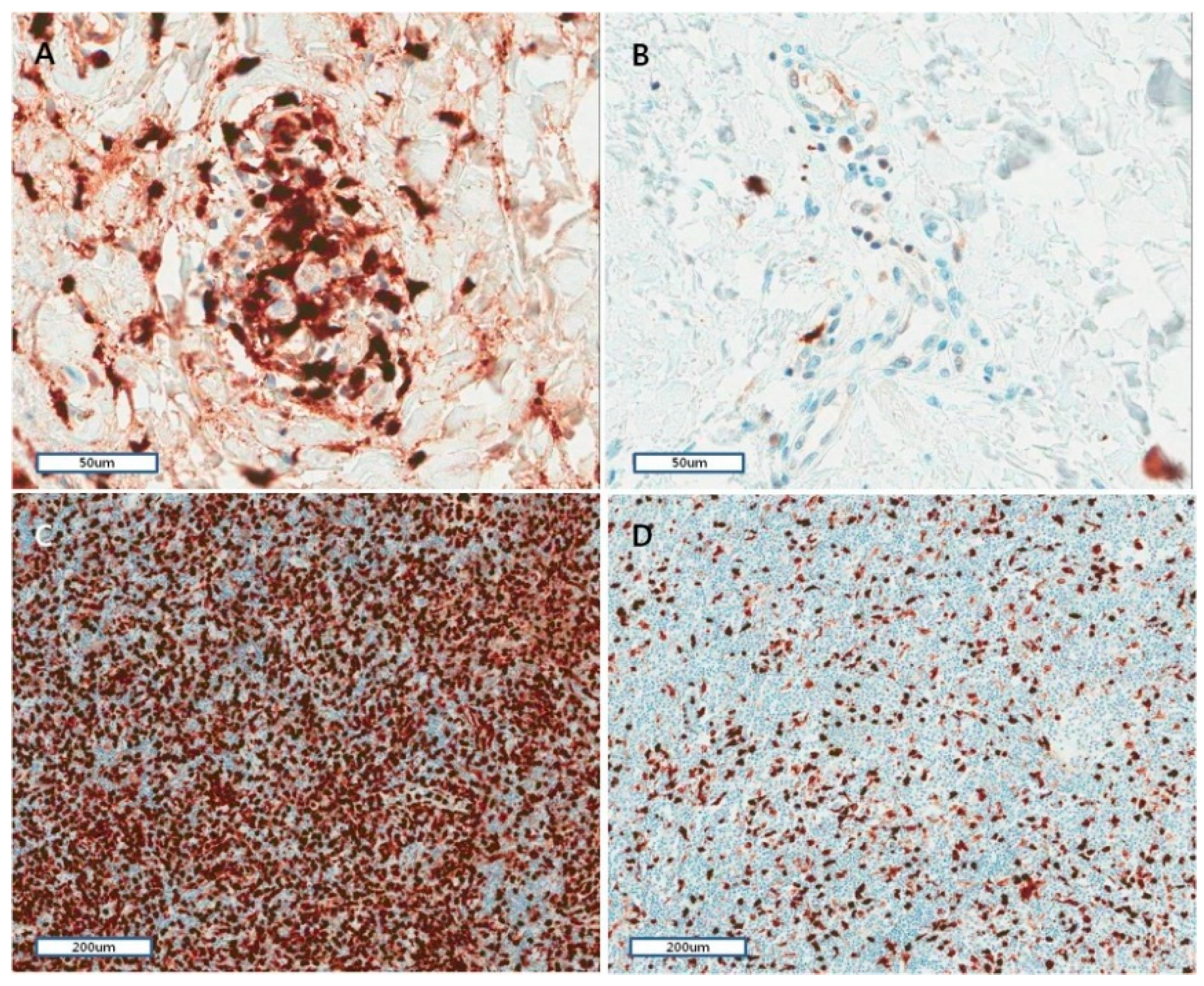
2.6. Histopathological and Immunohistochemical Characteristics of Skin and Lymph Nodes in AOSD
3. Discussion
4. Experimental Sections
4.1. Subjects
4.2. S100A8/A9, IL-1β, and TNF-α Assays
4.3. PBMC Preparation and Stimulation of Six Active AOSD Patients and Six HCs
4.4. Cell Culture
4.5. Immunobot
4.6. Histopathology of Skin Biopsy and Lymph Node
4.7. Immunohistochemistry and Evaluation
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fautrel, B. Adult-onset Still disease. Best Pract. Res. Clin. Rheumatol. 2008, 22, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Sung, J.M.; Suh, C.H. Therapeutic responses and prognosis in adult-onset Still’s disease. Rheumatol. Int. 2012, 32, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Efthimiou, P.; Moorthy, L.N.; Mavragani, C.P.; Skokos, D.; Fautrel, B. Adult onset Still’s disease and autoinflammation. Int. J. Inflamm. 2012, 2012, 964751. [Google Scholar] [CrossRef] [PubMed]
- Wouters, J.M.; van der Veen, J.; van de Putte, L.B.; de Rooij, D.J. Adult onset Still’s disease and viral infections. Ann. Rheum. Dis. 1988, 47, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Spyridakis, E.G.; Koutsilieris, M. Adult-onset Still’s disease: From Pathophysiology to targeted therapies. Int. J. Inflamm. 2012, 2012, 879020. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Vogl, T.; Sorg, C.; Sunderkotter, C. Phagocyte-specific S100 proteins: A novel group of proinflammatory molecules. Trends Immunol. 2003, 24, 155–158. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Edgeworth, J.; Gorman, M.; Bennett, R.; Freemont, P.; Hogg, N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 1991, 266, 7706–7713. [Google Scholar] [PubMed]
- Raquil, M.A.; Anceriz, N.; Rouleau, P.; Tessier, P.A. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J. Immunol. 2008, 180, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Van Zoelen, M.A.; Vogl, T.; Foell, D.; Van Veen, S.Q.; van Till, J.W.; Florquin, S.; Tanck, M.W.; Wittebole, X.; Laterre, P.F.; Boermeester, M.A.; et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am. J. Respir. Crit. Care Med. 2009, 180, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Mellins, E.D.; Macaubas, C.; Grom, A.A. Pathogenesis of systemic juvenile idiopathic arthritis: Some answers, more questions. Nat. Rev. Rheumatol. 2011, 7, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; Anceriz, N.; Plante, A.; Page, N.; Tardif, M.R.; Tessier, P.A. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PLoS ONE 2012, 7, e45478. [Google Scholar]
- Baillet, A.; Trocme, C.; Berthier, S.; Arlotto, M.; Grange, L.; Chenau, J.; Quétant, S.; Sève, M.; Berger, F.; Juvin, R.; et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology 2010, 49, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Nippe, N.; Vogl, T.; Marketon, K.; Mysore, V.; Weinhage, T.; Dalbeth, N.; Pool, B.; Merriman, T.; Baeten, D.; et al. Myeloid-related proteins 8 and 14 contribute to monosodium urate monohydrate crystal-induced inflammation in gout. Arthritis Rheumatol. 2014, 66, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vodovotz, Y.; Fan, L.; Li, Y.; Liu, Z.; Namas, R.; Barclay, D.; Zamora, R.; Billiar, T.R.; Wilson, M.A.; et al. Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in monocytes/macrophages. FASEB J. 2015, 29, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Frosch, M.; Kastrup, A.; Prince, F.H.; Otten, M.H.; van Suijlekom-Smit, L.W.; ten Cate, R.; Hoppenreijs, E.P.; Hansmann, S.; Moncrieffe, H.; et al. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 2012, 71, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Frosch, M.; Ahlmann, M.; Vogl, T.; Wittkowski, H.; Wulffraat, N.; Foell, D.; Roth, J. The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009, 60, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Frosch, M.; Vogl, T.; Seeliger, S.; Wulffraat, N.; Kuis, W.; Viemann, D.; Foell, D.; Sorg, C.; Sunderkötter, C.; Roth, J. Expression of myeloid-related proteins 8 and 14 in systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; An, J.M.; Nam, J.Y.; Jeon, J.Y.; Suh, C.H. Serum S100A8/A9, but not follistatin-like protein 1 and interleukin 18, may be a useful biomarker of disease activity in adult-onset Still’s disease. J. Rheumatol. 2012, 39, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.M.; Sunderkotter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.B.; Odegard, S.; Fagerhol, M.K.; Landewe, R.; van der Heijde, D.; Uhlig, T.; Mowinckel, P.; Kvien, T.K. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Frosch, M.; Strey, A.; Vogl, T.; Wulffraat, N.M.; Kuis, W.; Sunderkotter, C.; Harms, E.; Sorg, C.; Roth, J. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000, 43, 628–637. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Ren, Z.; Turton, J.; Pang, G.; Daebritz, J.; Ehrchen, J.; Heidemann, J.; Borody, T.; Roth, J.; et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J. Pathol. 2008, 216, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Tsan, M.F.; Gao, B. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J. Endotoxin Res. 2007, 13, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004, 50, 3762–3771. [Google Scholar] [CrossRef] [PubMed]
- Viemann, D.; Strey, A.; Janning, A.; Jurk, K.; Klimmek, K.; Vogl, T.; Hirono, K.; Ichida, F.; Foell, D.; Kehrel, B.; et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 2005, 105, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Roth, J.; Sorg, C.; Kolde, G. Epidermal expression of the calcium binding surface antigen 27E10 in inflammatory skin diseases. Arch. Dermatol. Res. 1992, 284, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, T.O.; Dale, I.; Brandtzaeg, P.; Hoel, P.S.; Fagerhol, M.K.; Larsen, T.E.; Thune, P.O. Epidermal and dermal distribution of a myelomonocytic antigen (L1) shared by epithelial cells in various inflammatory skin diseases. J. Am. Acad. Dermatol. 1986, 15, 173–179. [Google Scholar] [CrossRef]
- Nukui, T.; Ehama, R.; Sakaguchi, M.; Sonegawa, H.; Katagiri, C.; Hibino, T.; Huh, N.H. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J. Cell. Biochem. 2008, 104, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Benoit, S.; Toksoy, A.; Ahlmann, M.; Schmidt, M.; Sunderkotter, C.; Foell, D.; Pasparakis, M.; Roth, J.; Goebeler, M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br. J. Dermatol. 2006, 155, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Frosch, M.; Metze, D.; Foell, D.; Vogl, T.; Sorg, C.; Sunderkotter, C.; Roth, J. Early activation of cutaneous vessels and epithelial cells is characteristic of acute systemic onset juvenile idiopathic arthritis. Exp. Dermatol. 2005, 14, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ohta, A.; Tsunematsu, T.; Kasukawa, R.; Mizushima, Y.; Kashiwagi, H.; Kashiwazaki, S.; Tanimoto, K.; Matsumoto, Y.; Ota, T.; et al. Preliminary criteria for classification of adult Still’s disease. J. Rheumatol. 1992, 19, 424–430. [Google Scholar] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Pouchot, J.; Sampalis, J.S.; Beaudet, F.; Carette, S.; Decary, F.; Salusinsky-Sternbach, M.; Hill, R.O.; Gutkowski, A.; Harth, M.; Myhal, D.; et al. Adult Still’s disease: Manifestations, disease course, and outcome in 62 patients. Medicine (Baltimore) 1991, 70, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Kwon, J.E.; Yim, H.; Suh, C.H.; Jung, J.Y.; Han, J.H. The pathologic findings of skin, lymph node, liver, and bone marrow in patients with adult-onset still disease: A comprehensive analysis of 40 cases. Medicine (Baltimore) 2015, 94, e787. [Google Scholar] [CrossRef] [PubMed]


| Clinical Manifestations and Laboratory Findings | AOSD (n = 20) | RA (n = 20) | HC (n = 20) |
|---|---|---|---|
| Age (year) | 38 ± 13.7 | 41.8 ± 15.2 | 39.3 ± 8.3 |
| Gender (F/M) | 17/3 | 18/2 | 19/1 |
| Fever | 20 (100) | ||
| Sore throat | 11 (55) | ||
| Skin rash | 15 (75) | ||
| Lymphadenopathy | 8 (40) | ||
| Splenomegaly | 6 (30) | ||
| Hepatomegaly | 4 (20) | ||
| Pericarditis | 3 (15) | ||
| Pleuritis | 2 (10) | ||
| Arthritis | 19 (52.8) | 40 (100) | |
| Hemoglobin, g/dL | 11.1 ± 1.8 | ||
| Leukocyte, /µL | 15,554 ± 4553 | ||
| Platelet, ×103/µL | 324.7 ± 124.4 | ||
| Ferritin, ng/mL | 6100.4 ± 5158.3 | 72.7 ± 80.1 | 54.2 ± 58.4 |
| ESR, mm/h | 65.5 ± 22.9 | 30 ± 33.4 | |
| CRP, mg/dL | 10.6 ± 6.8 | 0.95 ± 1.7 | 0.09 ± 0.21 |
| AST, U/L | 96 ± 125.1 | ||
| ALT, U/L | 88.7 ± 105 | ||
| ANA positivity | 3 (15) | 4 (20) | |
| RF positivity | 5 (25) | 12 (60) | |
| Systemic score | 5.4 ± 1.43 | ||
| DAS-28 | 3.8 ± 1.22 | 4.05 ± 1.6 |
| Pathologic Finding and S100A8/A9 Staining Grade | Grade 1 | Grade 2 | Grade 3 | p-Value |
|---|---|---|---|---|
| Vacuole | ||||
| (+), n = 6 | 2 | 2 | 2 | 0.292 |
| (−), n = 20 | 4 | 4 | 12 | |
| Karyorrhexis | ||||
| (+), n = 14 | 1 | 3 | 10 | 0.028 |
| (−), n = 12 | 5 | 3 | 4 | |
| Mucin | ||||
| (+), n = 14 | 2 | 1 | 11 | 0.014 |
| (−), n = 12 | 4 | 5 | 3 | |
| Neutrophil infiltration | ||||
| (+), n = 9 | 0 | 1 | 8 | 0.006 |
| (−), n = 17 | 6 | 5 | 6 | |
| Necrosis | ||||
| (+), n = 6 | 2 | 0 | 4 | 0.794 |
| (−), n = 20 | 4 | 6 | 10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-A.; Han, J.H.; Kim, W.-J.; Noh, H.J.; An, J.-M.; Yim, H.; Jung, J.-Y.; Kim, Y.-S.; Suh, C.-H. TLR4 Endogenous Ligand S100A8/A9 Levels in Adult-Onset Still’s Disease and Their Association with Disease Activity and Clinical Manifestations. Int. J. Mol. Sci. 2016, 17, 1342. https://doi.org/10.3390/ijms17081342
Kim H-A, Han JH, Kim W-J, Noh HJ, An J-M, Yim H, Jung J-Y, Kim Y-S, Suh C-H. TLR4 Endogenous Ligand S100A8/A9 Levels in Adult-Onset Still’s Disease and Their Association with Disease Activity and Clinical Manifestations. International Journal of Molecular Sciences. 2016; 17(8):1342. https://doi.org/10.3390/ijms17081342
Chicago/Turabian StyleKim, Hyoun-Ah, Jae Ho Han, Woo-Jung Kim, Hyun Jin Noh, Jeong-Mi An, Hyunee Yim, Ju-Yang Jung, You-Sun Kim, and Chang-Hee Suh. 2016. "TLR4 Endogenous Ligand S100A8/A9 Levels in Adult-Onset Still’s Disease and Their Association with Disease Activity and Clinical Manifestations" International Journal of Molecular Sciences 17, no. 8: 1342. https://doi.org/10.3390/ijms17081342
APA StyleKim, H.-A., Han, J. H., Kim, W.-J., Noh, H. J., An, J.-M., Yim, H., Jung, J.-Y., Kim, Y.-S., & Suh, C.-H. (2016). TLR4 Endogenous Ligand S100A8/A9 Levels in Adult-Onset Still’s Disease and Their Association with Disease Activity and Clinical Manifestations. International Journal of Molecular Sciences, 17(8), 1342. https://doi.org/10.3390/ijms17081342