Molecular Insights into the Potential Toxicological Interaction of 2-Mercaptothiazoline with the Antioxidant Enzyme—Catalase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Binding Interaction between 2-MT and CAT by Fluorescence Analysis
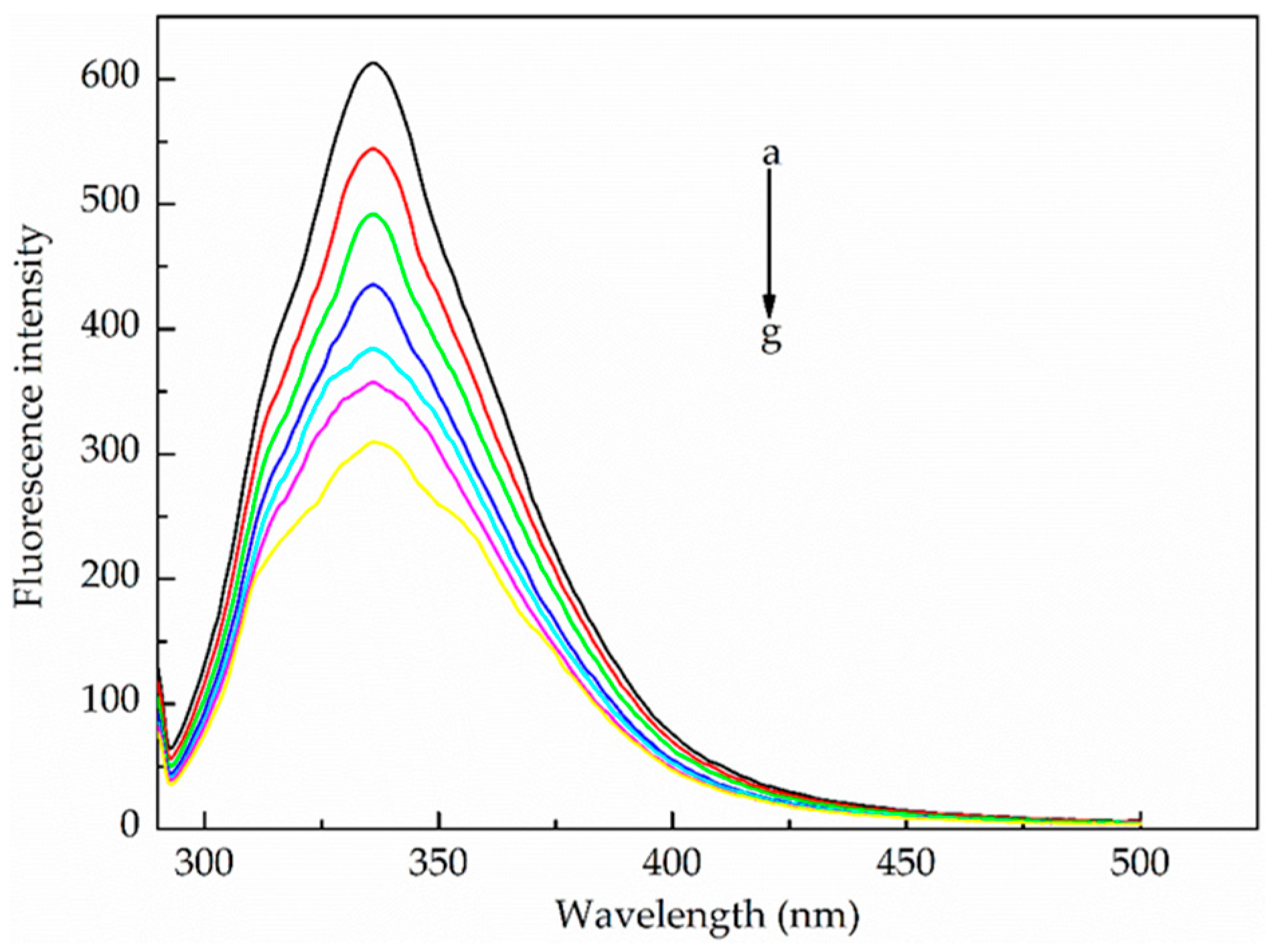
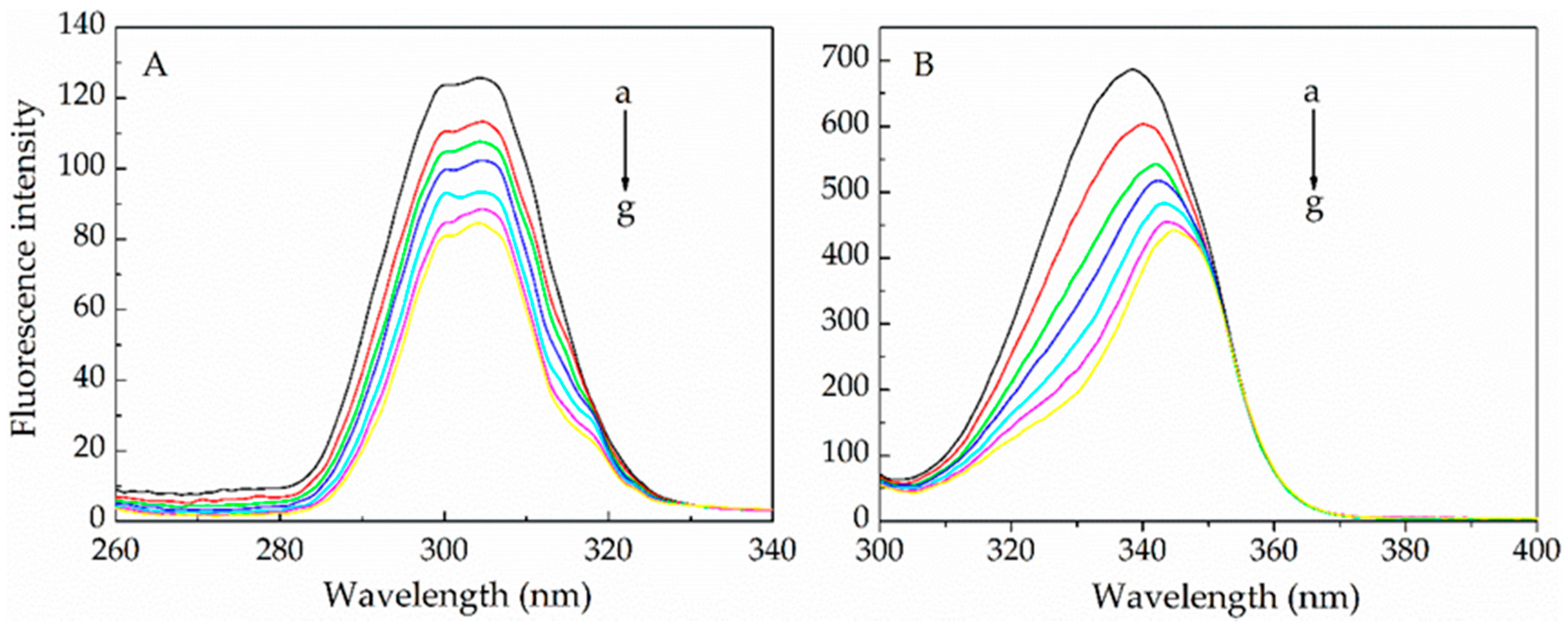
2.1.1. Fluorescence Quenching Mechanism
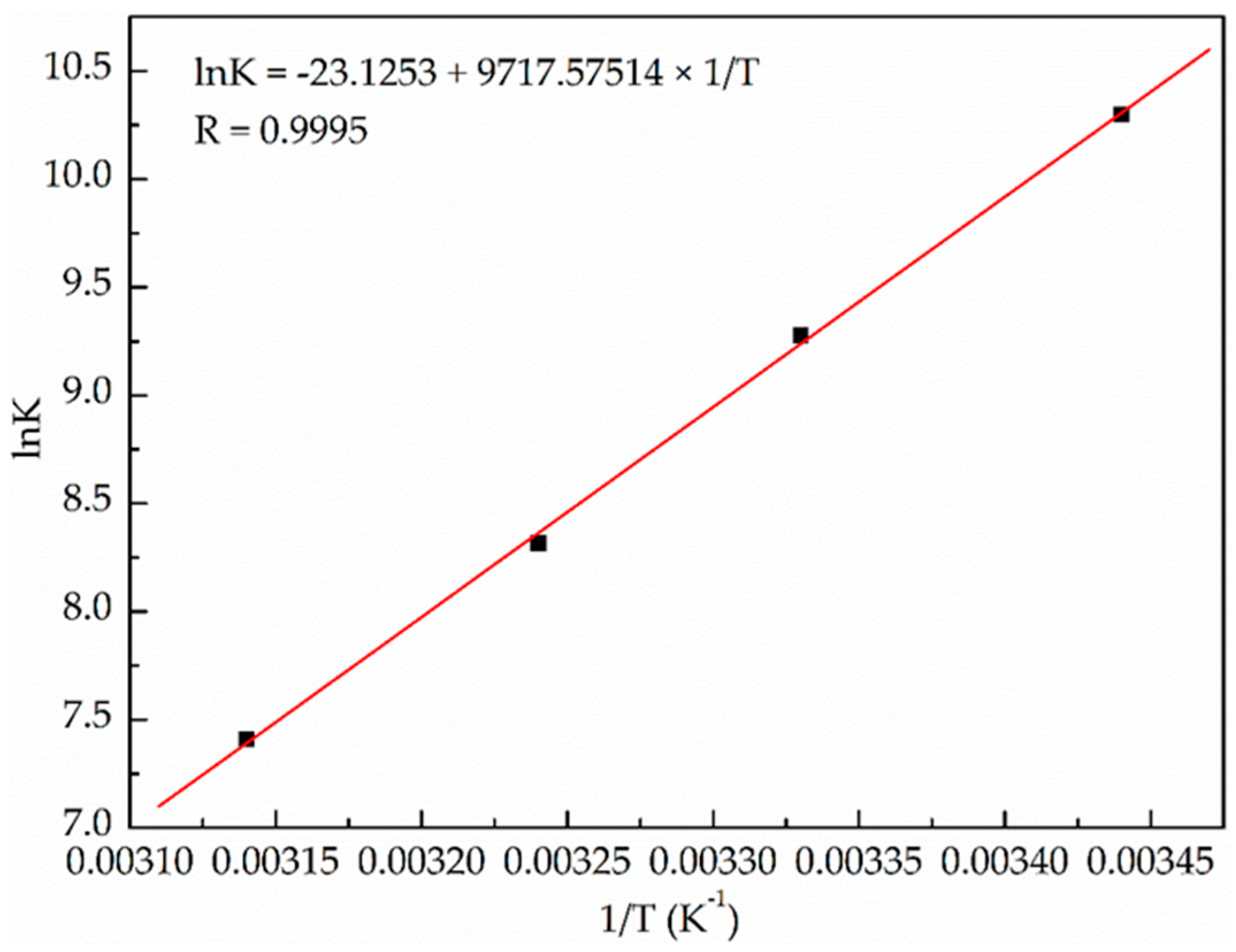
2.1.2. Binding Parameters Determination
2.1.3. Identification of Binding Forces
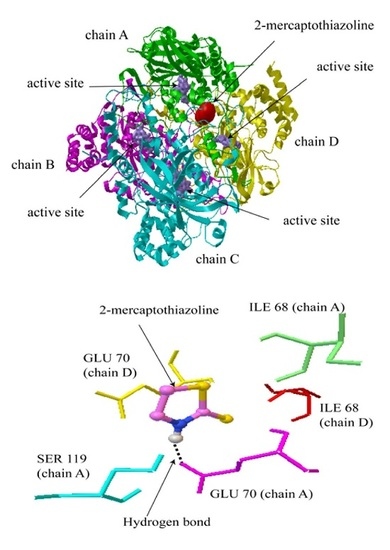
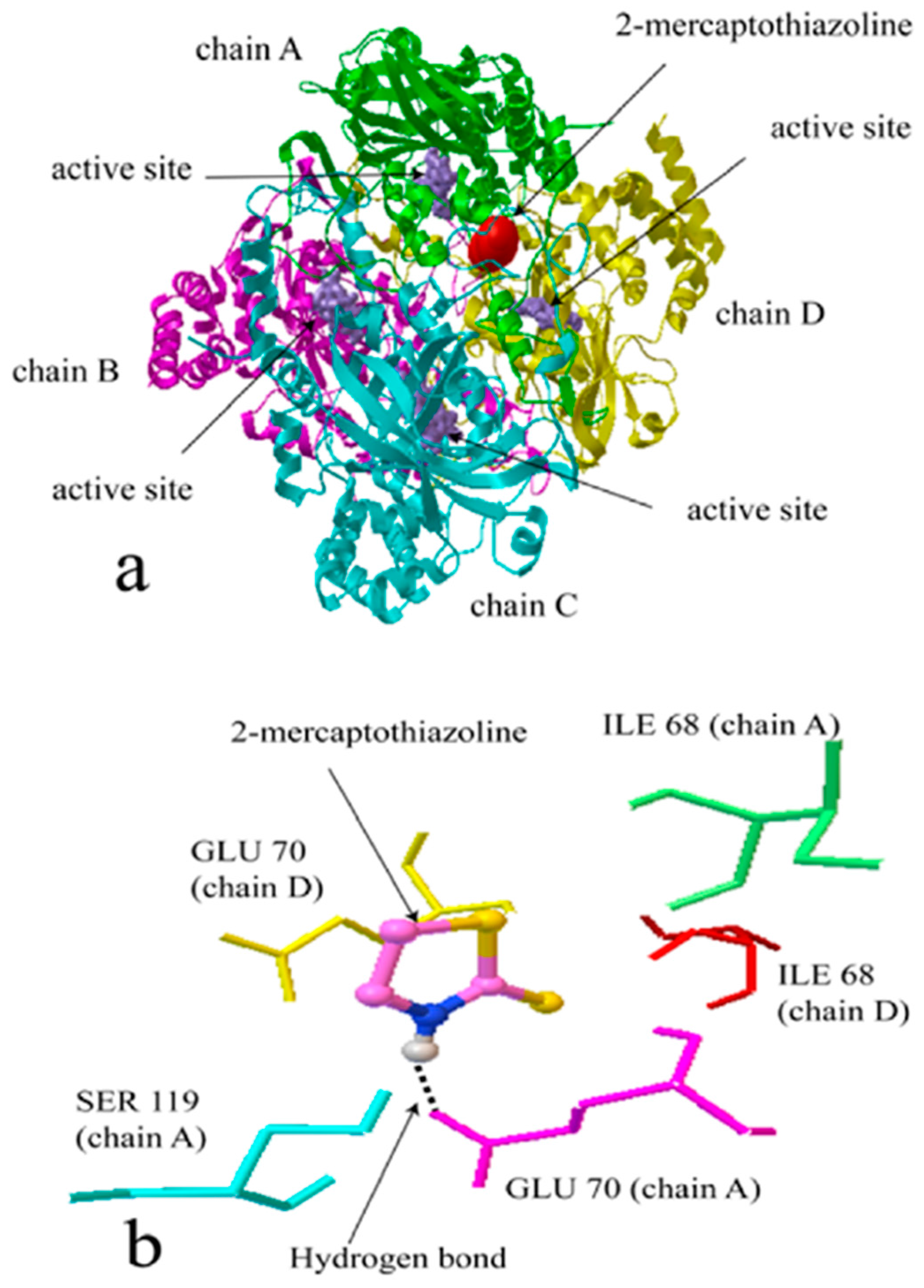
2.2. Molecular Docking Simulation
2.3. CAT Conformation Dynamic Characterization
2.3.1. UV–Vis Absorption Spectroscopy
2.3.2. Synchronous Fluorescence Spectroscopy
2.3.3. Circular Dichroism
2.4. Evaluation of 2-MT Inhibitory Effect on CAT Activity
3. Materials and Methods
3.1. Reagents
3.2. Apparatus and Measurements
3.2.1. Fluorescence Measurements
3.3.2. Molecular Docking Investigation
3.3.3. UV–Visible Absorption Measurements
3.3.4. Synchronous Fluorescence Measurements
3.3.5. Circular Dichroism (CD) Measurements
3.3.6. CAT Activity Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaetani, G.F.; Ferraris, A.M.; Rolfo, M.; Mangerini, R.; Arena, S.; Kirkman, H.N. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood 1996, 87, 1595–1599. [Google Scholar] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [PubMed]
- Giustarini, D.; Dalledonne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar] [PubMed]
- Gonenc, A.; Hacisevki, A.; Aslan, S.; Torun, M.; Simsek, B. Increased oxidative DNA damage and impaired antioxidant defense system in patients with gastrointestinal cancer. Eur. J. Intern. Med. 2012, 23, 350–354. [Google Scholar] [CrossRef]
- Noguer, M.A.; Cerezo, A.B.; Navarro, E.D.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012, 65, 609–614. [Google Scholar] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H. Study on the interaction between salicylic acid and catalase by spectroscopic methods. J. Pharm. Biomed. 2007, 44, 796–801. [Google Scholar]
- Chi, Z.X.; Liu, R.T.; Zhang, H. Potential enzyme toxicity of oxytetracycline to catalase. Sci. Total Environ. 2010, 408, 5399–5404. [Google Scholar] [PubMed]
- Cao, Z.Z.; Liu, R.T.; Yang, B.J. Potential toxicity of sarafloxacin to catalase: Spectroscopic, ITC and molecular docking descriptions. Spectrochim. Acta A 2013, 115, 457–463. [Google Scholar] [CrossRef] [PubMed]
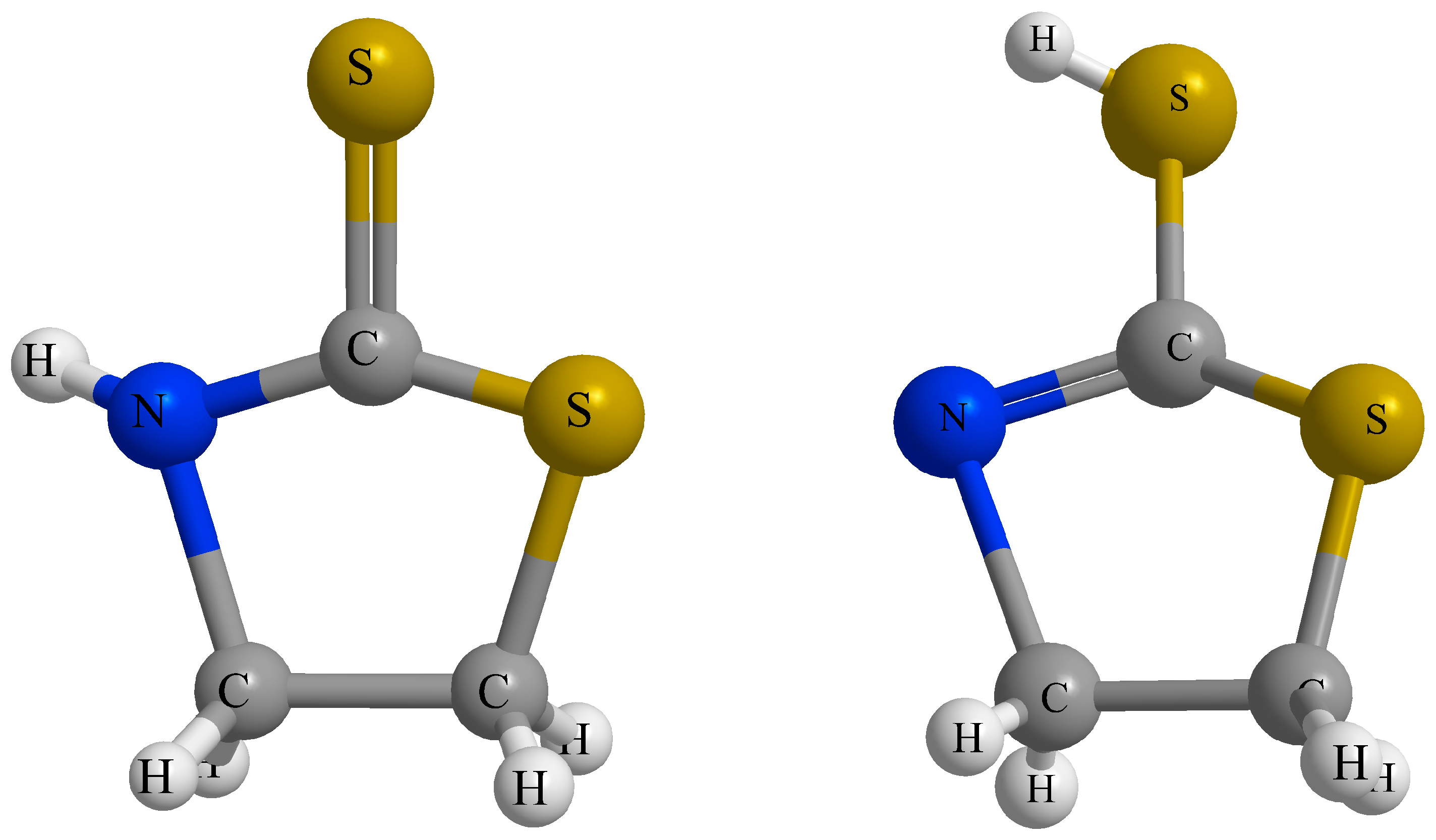
- Park, Y.; Yang, S.; Lee, M.; Lim, H.; Kim, Y.; Kim, S.; Lee, H. Confirmation of the coexistence of two tautomers of 2-mercaptothiazoline on the Ge(100) surface. Phys. Chem. Chem. Phys. 2013, 15, 16594–16598. [Google Scholar] [CrossRef] [PubMed]
- Rabie, U.M.; Abou-El-Wafa, M.H.M.; Nassar, H. Multiple and sequential charge transfer interactions occurring in situ: A redox reaction of thiazolidine-2-thione with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Spectrochim. Acta A 2011, 79, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Abbehausen, C.; Paiva, R.E.F.D.; Formiga, A.L.B.; Corbi, P.P. Studies of the tautomeric equilibrium of 1,3-thiazolidine-2-thione: Theoretical and experimental approaches. Chem. Phys. 2012, 408, 62–68. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, C.Y.; Chen, C.C.; Chiu, C.Y.; Yu, Y.H.; Chiang, P.C.; Chang, C.F.; Shie, J.L. Kinetics of ozonation of 2-Mercaptothiazoline in an electroplating solution. Ind. Eng. Chem. Res. 2004, 43, 6935–6942. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardas, G.; Culha, M.; Yazici, B.; Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Hierro, I.D.; Fajardo, M.; Sierra, I. 2-Mercaptothiazoline modified mesoporous silica for mercury removal from aqueous media. J. Hazard. Mater. 2006, 134, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Rabie, U.M.; Abou-El-Wafa, M.H.M.; Nassar, H. In vitro simulation of the chemical scenario of the action of an anti-thyroid drug: Charge transfer interaction of thiazolidine-2-thione with iodine. Spectrochim. Acta A 2011, 78, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Chang, C.Y.; Holl, W. Investigating the adsorption of 2-mercaptothiazoline on activated carbon from aqueous systems. J. Colloid Interface Sci. 2004, 272, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chang, C.Y.; Chen, C.C.; Chiu, C.Y. Kinetics of ozonation of 2-Mercaptothiazoline in an electroplating solution combined with UV radiation. Ind. Eng. Chem. Res. 2006, 45, 4936–4943. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, C.Y.; Chen, C.C.; Chiu, C.Y.; Yu, Y.H.; Chiang, P.C.; Ku, Y.; Chen, J.N.; Chang, C.F. Decomposition of 2-mercaptothiazoline in aqueous solution by ozonation. Chemosphere 2004, 56, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Wang, X.; Zou, L.; Huang, M.; Du, X. Experimental and theoretical study on the binding of 2-mercaptothiazoline to bovine serum albumin. J. Lumin. 2015, 161, 14–19. [Google Scholar] [CrossRef]
- Thomes, J.C.; Comby, F.; Lagorce, J.F.; Buxeraud, J.; Raby, J. Sites of action of 2-thiazoline-2-thiol on biogenesis of thyroid hormones. Jpn. J. Pharmacol. 1992, 58, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Vercillo, N.C.; Herald, K.J.; Fox, J.M.; Der, B.S.; Dattebaum, J.D. Analysis of ligand binding to a ribose biosensor using site-directed mutagenesis and fluorescence spectroscopy. Protein Sci. 2007, 16, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, O.A.; Feroz, S.R.; Shilpi, J.A.; Anouar, E.H.; Mukarram, A.K.; Mohamad, S.B.; Tayyab, S.; Awang, K. Spectrofluorometric and molecular docking studies on the binding of curcumenol and curcumenone to human serum albumin. Int. J. Mol. Sci. 2015, 16, 5180–5193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Zhou, B.; Zhang, X.P.; Huang, P.; Li, C.H.; Liu, Y. Interaction of malachite green with bovine serum albumin: Determination of the binding mechanism and binding site by spectroscopic methods. J. Hazard. Mater. 2009, 163, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.L.; Fan, J.; Li, J.P.; Hu, Z.D. Interactions between 1-benzoyl-4-p-chlorophenyl thiosemicarbazide and serum albumin: Investigation by fluorescence spectroscopy. Bioorgan. Med. Chem. 2004, 12, 151–157. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry 1973, 12, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.X.; Liu, R.T.; Yang, B.J.; Zhang, H. Toxic interaction mechanism between oxytetracycline and bovine hemoglobin. J. Hazard. Mater. 2010, 180, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Xi, P.X.; Chen, F.J.; Xu, Z.H.; Zeng, Z.Z. Spectroscopic studies on binding of 1-phenyl-3-(coumarin-6-yl)sulfonylurea to bovine serum albumin. J. Photochnol. Photobiol. B 2008, 92, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Paramaguru, G.; Kathiravan, A.; Selvaraj, S.; Venuvanalingam, P.; Renganathan, R. Interaction of anthraquinone dyes with lysozyme: Evidences from spectroscopic and docking studies. J. Hazard. Mater. 2010, 175, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Islam, B.; Yennamalli, R.; Sultan, A.; Subbarao, N.; Khan, A.U. Interaction of mitoxantrone with human serum albumin: Spectroscopic and molecular modeling studies. Eur. J. Pharm. Sci. 2008, 35, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yao, N.; Xu, H.; Wang, T.; Li, G.; Li, Z. Characterization of the interaction between eupatorin and bovine serum albumin by spectroscopic and molecular modeling methods. Int. J. Mol. Sci. 2013, 14, 14185–14203. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Liang, J.G.; Han, H.Y. Probing the Interaction of Magnetic Iron Oxide Nanoparticles with Bovine Serum Albumin by Spectroscopic Techniques. J. Phys. Chem. B 2009, 113, 10454–10458. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ji, F.Y.; Li, C.; Yu, Z.H.; Liu, R.T. Interaction mechanism between 4-aminoantipyrine and the enzyme lysozyme. J. Lumin. 2011, 131, 2661–2667. [Google Scholar] [CrossRef]
- Guo, M.; Lu, W.J.; Li, M.H.; Wang, W. Study on the binding interaction between carnitine optical isomer and bovine serum albumin. Eur. J. Med. Chem. 2008, 43, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.F.; Zhang, X.; Li, D.J.; Jin, J. Probing the binding of flavonoids to catalase by molecular spectroscopy. J. Mol. Struct. 2007, 843, 38–44. [Google Scholar] [CrossRef]
- Lu, J.Q.; Jin, F.; Sun, T.Q.; Zhou, X.W. Multi-spectroscopic study on interaction of bovine serum albumin with lomefloxacin-copper(II) complex. Int. J. Biol. Macromol. 2007, 40, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hideyuki, K.; Kazuaki, Y.; Hidetoshi, M.; Isao, Y. Characterization of catalase from psychrotolerant psychrobacter piscatorii T-3 exhibiting high catalase activity. Int. J. Mol. Sci. 2012, 13, 1733–1746. [Google Scholar]
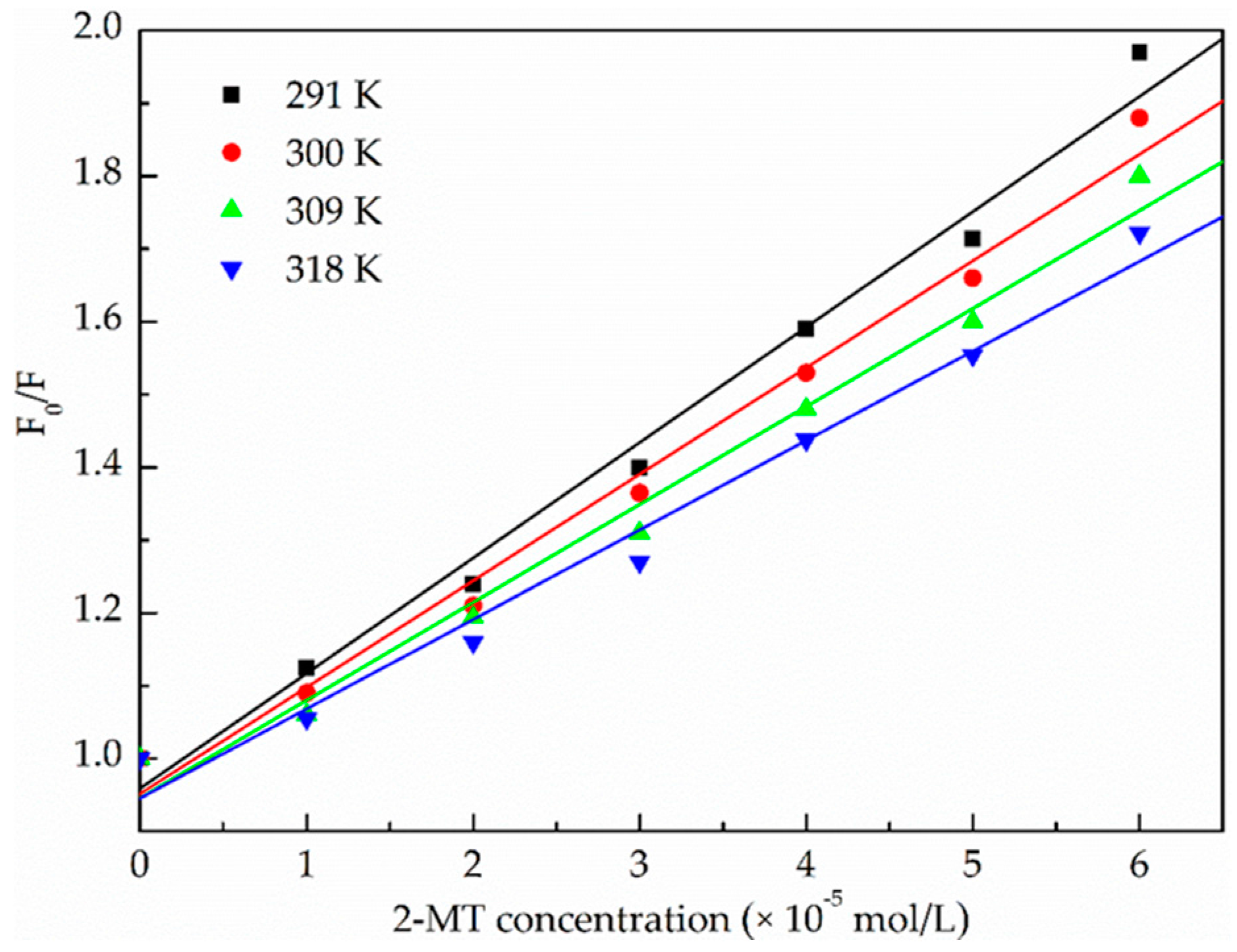



| pH | T (K) | KSV (×104 L·mol−1·s−1) | kq (×1012 L·mol−1·s−1) | R 1 | S.D. 2 |
|---|---|---|---|---|---|
| 7.4 | 291 | 1.59 | 1.59 | 0.9935 | 0.0429 |
| 7.4 | 300 | 1.46 | 1.46 | 0.9939 | 0.0384 |
| 7.4 | 309 | 1.35 | 1.35 | 0.9922 | 0.0398 |
| 7.4 | 318 | 1.23 | 1.23 | 0.9910 | 0.0391 |
| T (K) | Ka (× 104 mol·L−1) | n | R 1 | ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔG° (kJ·mol−1) |
|---|---|---|---|---|---|---|
| 291 | 2.97 | 1.14 | 0.9987 | −80.79 | −192.26 | −24.84 |
| 300 | 1.07 | 1.07 | 0.9973 | −23.11 | ||
| 309 | 0.41 | 0.96 | 0.9955 | −21.38 | ||
| 318 | 0.17 | 0.83 | 0.9956 | −19.65 |
| Molar Ratio of CAT to 2-MT | Secondary Structural Elements in CAT | |||
|---|---|---|---|---|
| α-Helix (%) | β-Sheet (%) | Turns (%) | Unordered (%) | |
| 1:0 | 19.7 | 31.1 | 23.3 | 25.8 |
| 1:10 | 19.0 | 31.5 | 23.6 | 25.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Huang, M.; Mi, C.; Wang, T.; Chen, D.; Teng, Y. Molecular Insights into the Potential Toxicological Interaction of 2-Mercaptothiazoline with the Antioxidant Enzyme—Catalase. Int. J. Mol. Sci. 2016, 17, 1330. https://doi.org/10.3390/ijms17081330
Huang Z, Huang M, Mi C, Wang T, Chen D, Teng Y. Molecular Insights into the Potential Toxicological Interaction of 2-Mercaptothiazoline with the Antioxidant Enzyme—Catalase. International Journal of Molecular Sciences. 2016; 17(8):1330. https://doi.org/10.3390/ijms17081330
Chicago/Turabian StyleHuang, Zhenxing, Ming Huang, Chenyu Mi, Tao Wang, Dong Chen, and Yue Teng. 2016. "Molecular Insights into the Potential Toxicological Interaction of 2-Mercaptothiazoline with the Antioxidant Enzyme—Catalase" International Journal of Molecular Sciences 17, no. 8: 1330. https://doi.org/10.3390/ijms17081330