Parentage-Based Group Composition and Dispersal Pattern Studies of the Yangtze Finless Porpoise Population in Poyang Lake
Abstract
:1. Introduction
2. Results
2.1. Genetic Variation
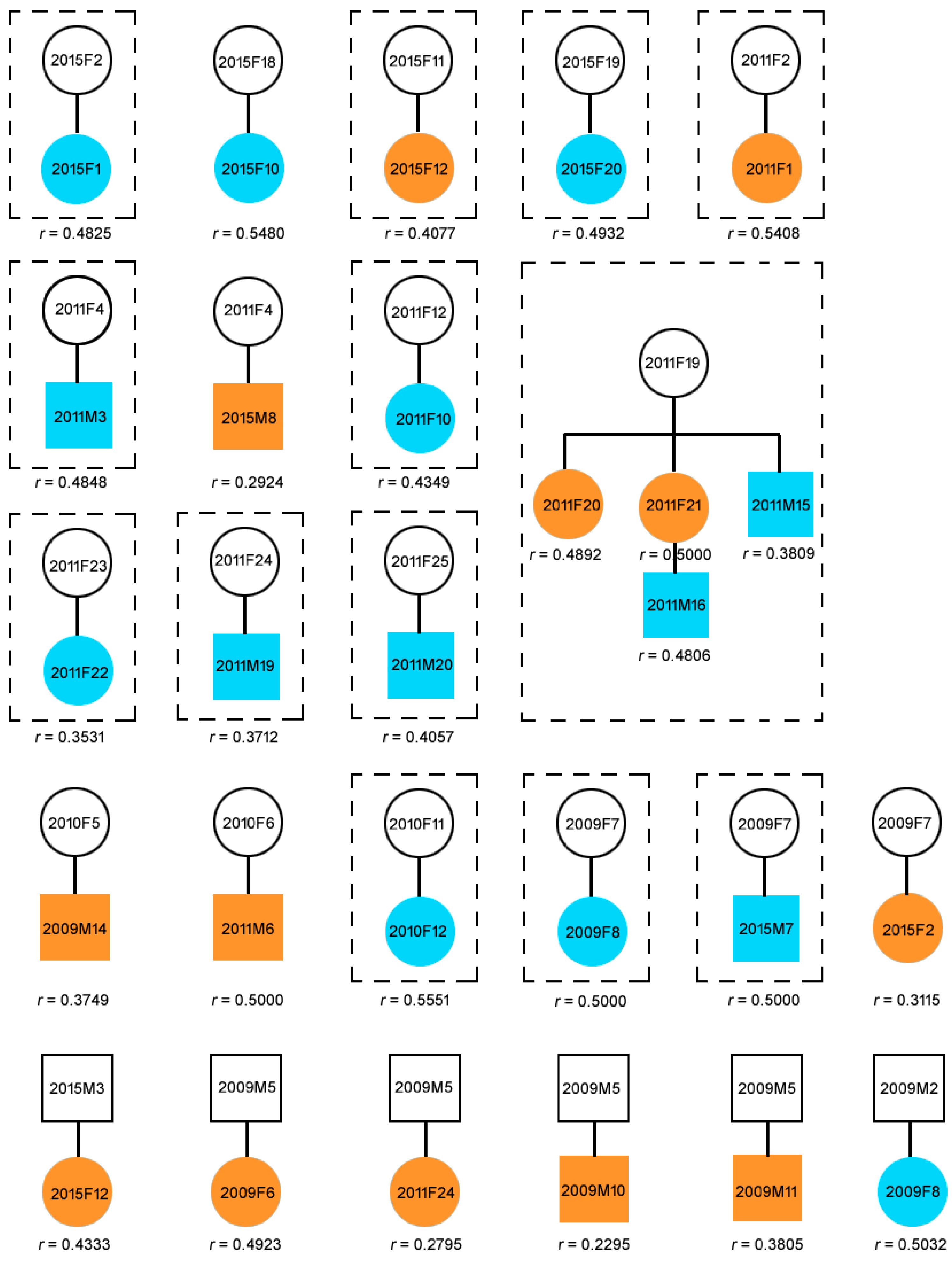
2.2. Parentage Assignment and Relatedness
2.3. Composition of Natural Groups
3. Discussion
3.1. Parentage and Relatedness
3.2. Group Composition
3.3. Dispersal Patterns
4. Materials and Methods
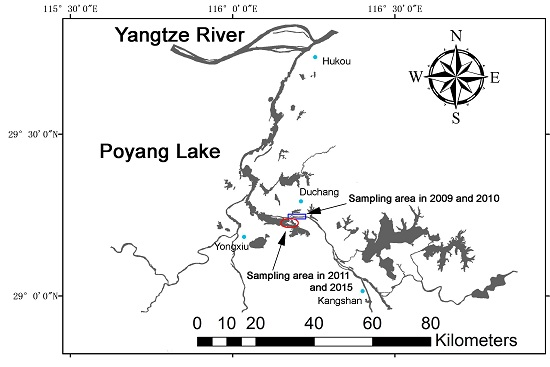
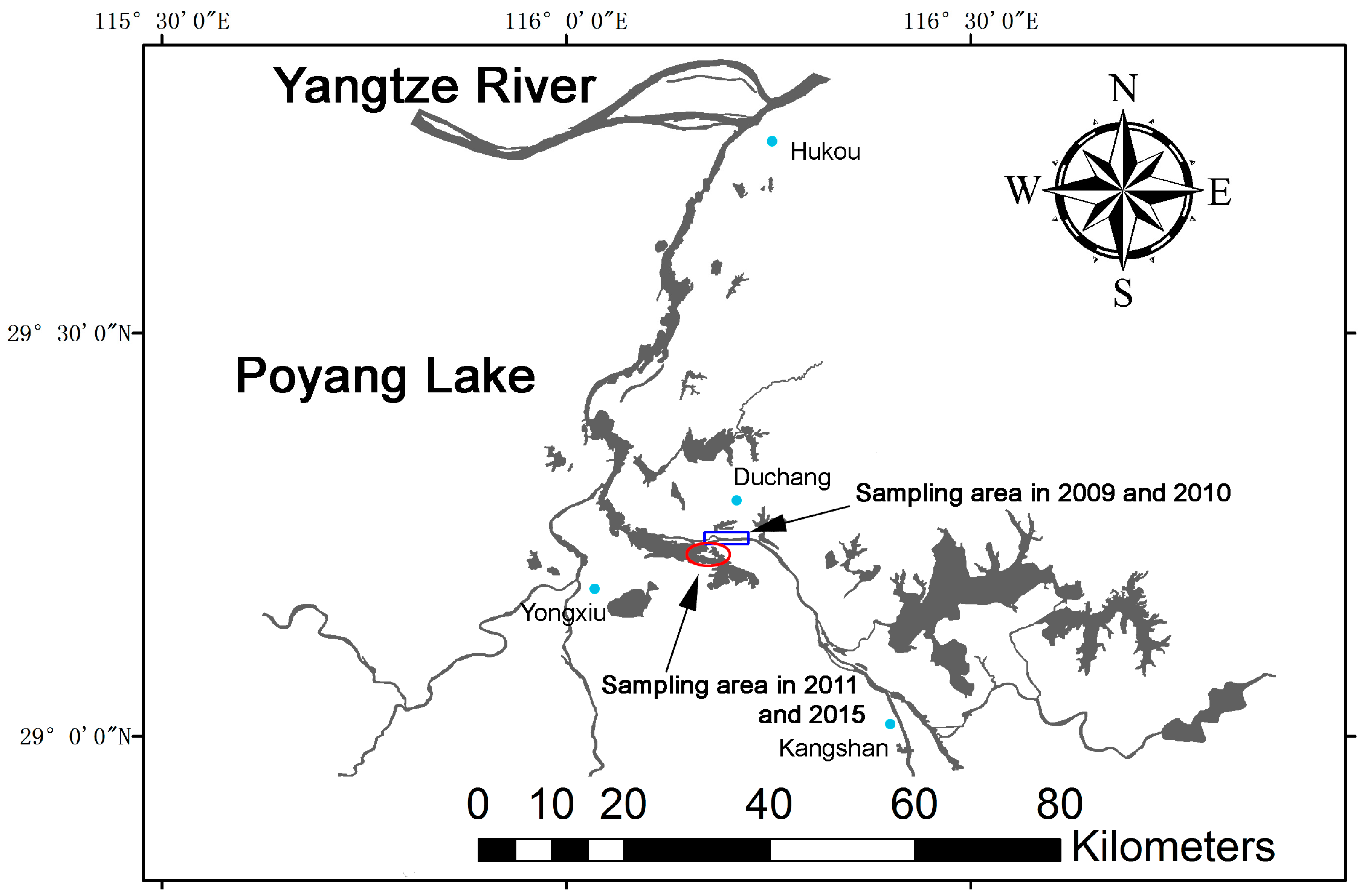
4.1. Study Location and Distribution of Porpoises
4.2. Sample Collection
4.3. DNA Extraction and PCR Amplification
4.4. Data Analysis
4.5. Detecting Potential Parents
4.6. Parentage Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mann, J. Cetacean Societies: Field Studies of Dolphins and Whales; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Michaud, R. Sociality and Ecology of the Odontocetes. In Sexual Segregation in Vertebrates: Ecology of the Two Sexes; Cambridge University Press: Cambridge, UK, 2005; pp. 303–326. [Google Scholar]
- Möller, L.M.; Beheregaray, L.B. Genetic evidence for sex-biased dispersal in resident bottlenose dolphins (Tursiops aduncus). Mol. Ecol. 2004, 13, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Karczmarski, L.; Würsig, B.; Gailey, G.; Larson, K.W.; Vanderlip, C. Spinner dolphins in a remote Hawaiian atoll: Social grouping and population structure. Behav. Ecol. 2005, 16, 675–685. [Google Scholar] [CrossRef]
- Baird, R.W.; Whitehead, H. Social organization of mammal-eating killer whales: Group stability and dispersal patterns. Can. J. Zool. 2000, 78, 2096–2105. [Google Scholar] [CrossRef]
- Ortega-Ortiz, J.G.; Engelhaupt, D.; Winsor, M.; Mate, B.R.; Rus Hoelzel, A. Kinship of long-term associates in the highly social sperm whale. Mol. Ecol. 2012, 21, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Mirimin, L.; Banguera-Hinestroza, E.; Dillane, E.; Hoelzel, A.R.; Cross, T.F.; Rogan, E. Insights into genetic diversity, parentage, and group composition of Atlantic white-sided dolphins (Lagenorhynchus acutus) off the west of Ireland based on nuclear and mitochondrial genetic markers. J. Hered. 2011, 102, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Reeves, R.R. River cetaceans and habitat change: Generalist resilience or specialist vulnerability? J. Mar. Biol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Gao, A.; Zhou, K. Geographical variation of external measurements and three subspecies of Neophocaena phocaenoides in Chinese waters. Acta Theriol. Sin. 1995, 15, 81–92. [Google Scholar]
- Mei, Z.; Huang, S.L.; Hao, Y.; Turvey, S.T.; Gong, W.; Wang, D. Accelerating population decline of Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis). Biol. Conserv. 2012, 153, 192–200. [Google Scholar] [CrossRef]
- Wang, D.; Turvey, S.T.; Zhao, X.; Mei, Z. Neophocaena asiaeorientalis ssp. asiaeorientalis. In IUCN Red List of Threatened Species Version 2013.1; Available online: http://www.iucnredlist.org/ (accessed on 2 September 2013).
- Taylor, A.; Horsup, A.; Johnson, C.; Sunnucks, P.; Sherwin, B. Relatedness structure detected by microsatellite analysis and attempted pedigree reconstruction in an endangered marsupial, the northern hairy-nosed wombat Lasiorhinus krefftii. Mol. Ecol. 1997, 6, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Onorato, D.P.; Hellgren, E.C.; Van Den Bussche, R.A.; Skiles, J.; Raymond, J. Paternity and relatedness of American black bears recolonizing a desert montane island. Can. J. Zool. 2004, 82, 1201–1210. [Google Scholar] [CrossRef]
- Itoh, T.; Sato, Y.; Kobayashi, K.; Mano, T.; Iwata, R. Effective dispersal of brown bears (Ursus arctos) in eastern Hokkaido, inferred from analyses of mitochondrial DNA and microsatellites. Mamm. Study 2012, 37, 29–41. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, R.; Zhao, Q.; Zhang, G.; Wei, Z.; Wang, X.; Yang, J. The population of finless porpoise in the middle and lower reaches of Yangtze River. Acta Theriol. Sin. 1993, 13, 260–270. [Google Scholar]
- Wei, Z.; Wang, D.; Zhang, X.; Zhao, Q.; Wang, K.; Kuang, X.A. Population size, behavior, movement pattern and protection of Yangtze finless porpoise at Balijiang section of the Yangtze River. Resour. Environ. Yangtze Basin 2002, 11, 427–432. [Google Scholar]
- Yang, J.; Chen, P. Movement and behavior of finless porpoise (Neophocaena phocaenoides) at Swan Oxbow, Hubei province. Acta Hydrobiol. Sin. 1996, 20, 32–40. [Google Scholar]
- Jiang, W. Observation on the group of the ChangJiang finless porpoise conserved in semi-nature conditions. J. Anhui Univ. (Nat. Sci.) 2000, 24, 106–111. [Google Scholar]
- Wei, Z.; Wang, D.; Zhang, X. Aggregation and spatio-temporal distribution of the Yangtze finless porpoise Neophocaena phocaenoides asiaeorientalis in Tian-E-Zhou National Baiji Reserve. Acta Hydrobiol. Sin. 2004, 28, 247–252. [Google Scholar]
- Mei, Z.; Zhang, X.; Huang, S.-L.; Turvey, S.T.; Gong, W.; Wang, D. The Yangtze finless porpoise: On an accelerating path to extinction? Biol. Conserv. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Csilléry, K.; Johnson, T.; Beraldi, D.; Clutton-Brock, T.; Coltman, D.; Hansson, B.; Spong, G.; Pemberton, J.M. Performance of marker-based relatedness estimators in natural populations of outbred vertebrates. Genetics 2006, 173, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Blouin, M.; Parsons, M.; Lacaille, V.; Lotz, S. Use of microsatellite loci to classify individuals by relatedness. Mol. Ecol. 1996, 5, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Isberg, S.; Chen, Y.; Barker, S.; Moran, C. Analysis of microsatellites and parentage testing in saltwater crocodiles. J. Hered. 2004, 95, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Haanes, H.; Rosef, O.; Veiberg, V.; Roed, K.H. Microsatellites with variation and heredity applicable to genetic studies of Norwegian red deer (Cervus elaphus atlanticus). Anim. Genet. 2005, 36, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Shideler, R.; Hechtel, J.; Strobeck, C.; Paetkau, D. Genetic relationships of grizzly bears (Ursus arctos) in the Prudhoe Bay region of Alaska: Inference from microsatellite DNA, mitochondrial DNA, and field observations. J. Hered. 1999, 90, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.A.; Shideler, R.; Waits, L.; Nelson, R.J. Genetic variation and relatedness in grizzly bears in the Prudhoe Bay region and adjacent areas in northern Alaska. Ursus 2005, 16, 70–84. [Google Scholar] [CrossRef]
- Mirimin, L.; Andrew, W.; Emer, R.; Patricia, R.; Andrew, R.; Jame, C.; Tom, C. Population structure of short-beaked common dolphins (Delphinus delphis) in the North Atlantic Ocean as revealed by mitochondrial and nuclear genetic markers. Mar. Biol. 2009, 156, 821–834. [Google Scholar] [CrossRef]
- Chen, L.; Bruford, M.W.; Xu, S.; Zhou, K.; Yang, G. Microsatellite variation and significant population genetic structure of endangered finless porpoises (Neophocaena phocaenoides) in Chinese coastal waters and the Yangtze River. Mar. Biol. 2010, 157, 1453–1462. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.; Gong, C.; Zhao, Q.; Wang, D. Inbreeding evaluation on the ex situ conserved Yangtze finless porpoise population in Tian’ezhou national natural reserve. Chin. J. Zool. 2014, 49, 305–316. [Google Scholar]
- Fullard, K.; Early, G.; Heide-Jørgensen, M.; Bloch, D.; Rosing-Asvid, A.; Amos, W. Population structure of long-finned pilot whales in the North Atlantic: A correlation with sea surface temperature? Mol. Ecol. 2000, 9, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Palsbøll, P.J.; Heide-Jørgensen, M.P.; Berubé, M. Analysis of Mitochondrial Control Region Nucleotide Sequences from Baffin Bay Belugas (Delphinapterus leucas): Detecting Pods or Sub-Populations? In Belugas in the North Atlantic and Russian Arctic Tromsø: North Atlantic Marine Mammal Commission; Heide-Jørgensen, M.P., Wiig, Ø., Eds.; NAMMCO Scientific Publications: Nuuk, Greenland, Denmark, 2002; pp. 39–50. [Google Scholar]
- Whitehead, H. Sperm Whales: Social Evolution in the Ocean; Chicago University Press: Chicago, IL, USA, 2003. [Google Scholar]
- Bigg, M.A.; Olesiuk, P.F.; Ellis, G.M.; Ford, J.K.B.; Balcomb, K.C. Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whal. Commun. 1990, 12, 383–405. [Google Scholar]
- Gowans, S.; Würsig, B.; Karczmarski, L. The social structure and strategies of delphinids: Predictions based on an ecological framework. Adv. Mar. Biol. 2008, 53, 195–294. [Google Scholar]
- Möller, L.M.; Beheregaray, L.B. Coastal bottlenose dolphins from southeastern Australia are Tursiops aduncus according to sequences of the mitochondrial DNA control region. Mar. Mamm. Sci. 2011, 17, 249–263. [Google Scholar] [CrossRef]
- Zheng, J.S.; Liao, X.L.; Tong, J.G.; Du, H.J.; Milinkovitch, M.C.; Wang, D. Development and characterization of polymorphic microsatellite loci in the endangered Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis). Conserv. Genet. 2008, 9, 1007–1009. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, J.; Zhou, Z.; Lin, G.; Wang, D.; Zheng, B.; Jiang, W. Paternity determination of captivity-bred Yangtze finless porpoises Neophocaena phocaenoides asiaeorientalis by microsatellite genotyping. Prog. Mod. Biomed. 2009, 9, 4015–4020. [Google Scholar]
- Zhou, Z.; Zheng, J.S.; Chen, M.M.; Zhao, Q.Z.; Wang, D. Genetic Evaluation and Development Prognosis on Ex Situ Conserved Yangtze Finless Porpoise Living in Tian-e-Zhou National Natural Reserve. Acta Hydrobiol. Sin. 2012, 36, 403–411. [Google Scholar]
- Rosel, P.; France, S.; Wang, J. Genetic structure of harbour porpoise Phocoena phocoena populations in the northwest Atlantic based on mitochondrial and nuclear markers. Mol. Ecol. 1999, 8, S41–S54. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bruford, M.; Yang, G. Isolation and characterization of microsatellite loci in the finless porpoise (Neophocaena phocaenoides). Mol. Ecol. Notes 2007, 7, 1129–1131. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. Development of tetranucleotide microsatellite loci for the finless porpoise (Neophocaena phocaenoides). Conserv. Genet. 2008, 9, 1033–1035. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. Micro-checker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Zheng, J.S.; Xia, J.H.; He, S.P.; Wang, D. Population genetic structure of the Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis): Implications for management and conservation. Biochem. Genet. 2005, 43, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zheng, J.; Wu, M.; Ruan, R.; Zhao, Q.; Wang, D. Genetic diversity and population structure of the critically endangered Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) as revealed by mitochondrial and microsatellite DNA. Int. J. Mol. Sci. 2014, 15, 11307–11323. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar] [PubMed]
- Hearne, C.M.; Ghosh, S.; Todd, J.A. Microsatellites for linkage analysis of genetic traits. Trends Genet. 1992, 8, 288–294. [Google Scholar] [CrossRef]
- Marshall, T.; Slate, J.; Kruuk, L.; Pemberton, J. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Taylor, C. Comparisons of three probability formulae for parentage exclusion. Anim. Genet. 1997, 28, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F. GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT 2.9.3.2, a Program to Estimate and Test Gene Diversities and Fixation Indices. Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 23 February 2002).
- Zhang, X. Studies on the age determination, growth and reproduction of finless porpoise Neophocaena phocaenoides. Acta Hydrobiol. Sin. 1992, 16, 289–298. [Google Scholar]
- Wu, H.P.; Hao, Y.J.; Li, X.; Zhao, Q.Z.; Chen, D.Q.; Kuang, X.A.; Kou, Z.B.; Feng, K.K.; Gong, W.M.; Wang, D.B. Mode ultrasonographic evaluation of the testis in relation to serum testosterone concentration in male Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis) during the breeding season. Theriogenology 2010, 73, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Queller, D.C.; Goodnight, K.F. Estimating relatedness using genetic markers. Evolution 1989, 43, 258–275. [Google Scholar] [CrossRef]
- Wang, J. Triadic IBD coefficients and applications to estimating pairwise relatedness. Genet. Res. 2007, 89, 135–153. [Google Scholar] [CrossRef] [PubMed]
| Locus | Na | Ho | He | PIC | Ne-1p | Ne-2p | Fis |
|---|---|---|---|---|---|---|---|
| SSR1 | 6 | 0.639 | 0.629 | 0.567 | 0.785 | 0.629 | −0.017 |
| SSR5 | 9 | 0.795 | 0.799 | 0.765 | 0.580 | 0.402 | 0.005 |
| SSR8 | 6 | 0.800 | 0.740 | 0.691 | 0.678 | 0.503 | −0.082 |
| SSR15 | 11 | 0.746 | 0.716 | 0.660 | 0.708 | 0.541 | −0.041 |
| SSR22 | 4 | 0.689 | 0.665 | 0.591 | 0.777 | 0.628 | −0.041 |
| SSR40 | 10 | 0.746 | 0.746 | 0.705 | 0.657 | 0.477 | 0.000 |
| SSR41 | 6 | 0.425 | 0.673 | 0.627 | 0.736 | 0.561 | 0.009 |
| SSR42 | 5 | 0.610 | 0.671 | 0.608 | 0.751 | 0.592 | 0.092 |
| SSR51 | 12 | 0.780 | 0.796 | 0.765 | 0.574 | 0.396 | 0.017 |
| SSR59 | 10 | 0.854 | 0.828 | 0.801 | 0.522 | 0.349 | −0.031 |
| SSR63 | 7 | 0.742 | 0.632 | 0.559 | 0.787 | 0.641 | −0.182 |
| SSR69 | 7 | 0.694 | 0.611 | 0.575 | 0.784 | 0.606 | −0.136 |
| SSR71 | 7 | 0.529 | 0.523 | 0.490 | 0.848 | 0.682 | −0.013 |
| SSR73 | 6 | 0.782 | 0.793 | 0.756 | 0.599 | 0.420 | 0.013 |
| SSR75 | 16 | 0.672 | 0.644 | 0.625 | 0.733 | 0.54 | −0.044 |
| NP391 | 12 | 0.415 | 0.475 | 0.441 | 0.877 | 0.723 | 0.128 |
| NP404 | 5 | 0.504 | 0.555 | 0.459 | 0.844 | 0.736 | 0.077 |
| NP409 | 9 | 0.664 | 0.748 | 0.706 | 0.652 | 0.476 | 0.113 |
| NP428 | 5 | 0.374 | 0.361 | 0.345 | 0.930 | 0.792 | −0.035 |
| NP464 | 9 | 0.628 | 0.764 | 0.724 | 0.634 | 0.456 | 0.178 |
| PPHO130 | 9 | 0.795 | 0.787 | 0.752 | 0.596 | 0.419 | −0.010 |
| Mean of 21 loci | 8.1 | 0.661 | 0.674 | 0.629 | Combined 7.20 × 10−4 | Combined 2.17 × 10−6 | 0.0002 (p > 0.05) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Zheng, Y.; Hao, Y.; Mei, Z.; Wang, K.; Zhao, Q.; Zheng, J.; Wang, D. Parentage-Based Group Composition and Dispersal Pattern Studies of the Yangtze Finless Porpoise Population in Poyang Lake. Int. J. Mol. Sci. 2016, 17, 1268. https://doi.org/10.3390/ijms17081268
Chen M, Zheng Y, Hao Y, Mei Z, Wang K, Zhao Q, Zheng J, Wang D. Parentage-Based Group Composition and Dispersal Pattern Studies of the Yangtze Finless Porpoise Population in Poyang Lake. International Journal of Molecular Sciences. 2016; 17(8):1268. https://doi.org/10.3390/ijms17081268
Chicago/Turabian StyleChen, Minmin, Yang Zheng, Yujiang Hao, Zhigang Mei, Kexiong Wang, Qingzhong Zhao, Jinsong Zheng, and Ding Wang. 2016. "Parentage-Based Group Composition and Dispersal Pattern Studies of the Yangtze Finless Porpoise Population in Poyang Lake" International Journal of Molecular Sciences 17, no. 8: 1268. https://doi.org/10.3390/ijms17081268