A Δ-9 Fatty Acid Desaturase Gene in the Microalga Myrmecia incisa Reisigl: Cloning and Functional Analysis
Abstract
:1. Introduction
2. Results and Discussion
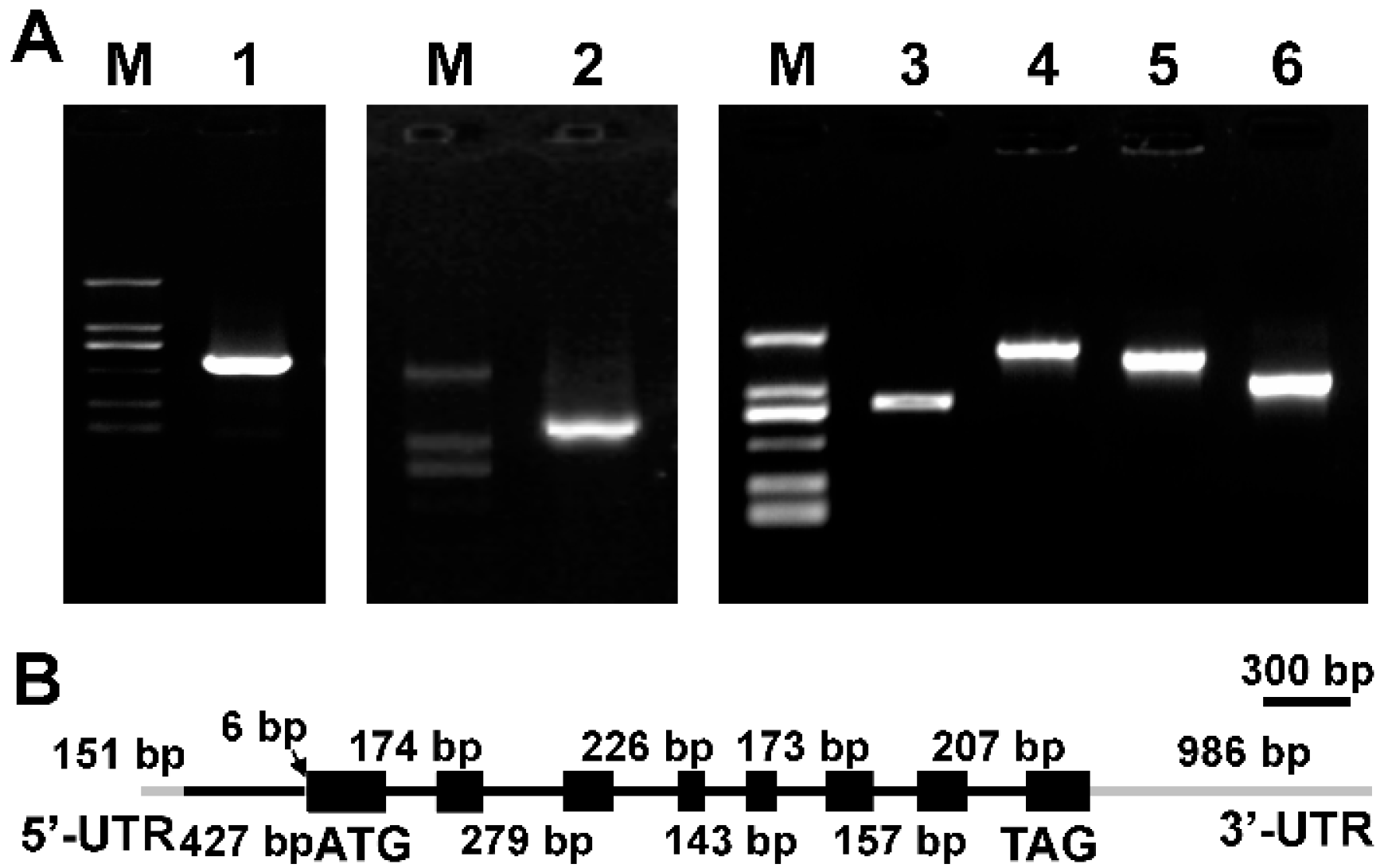
2.1. Cloning of Δ9 FAD Gene from M. incisa
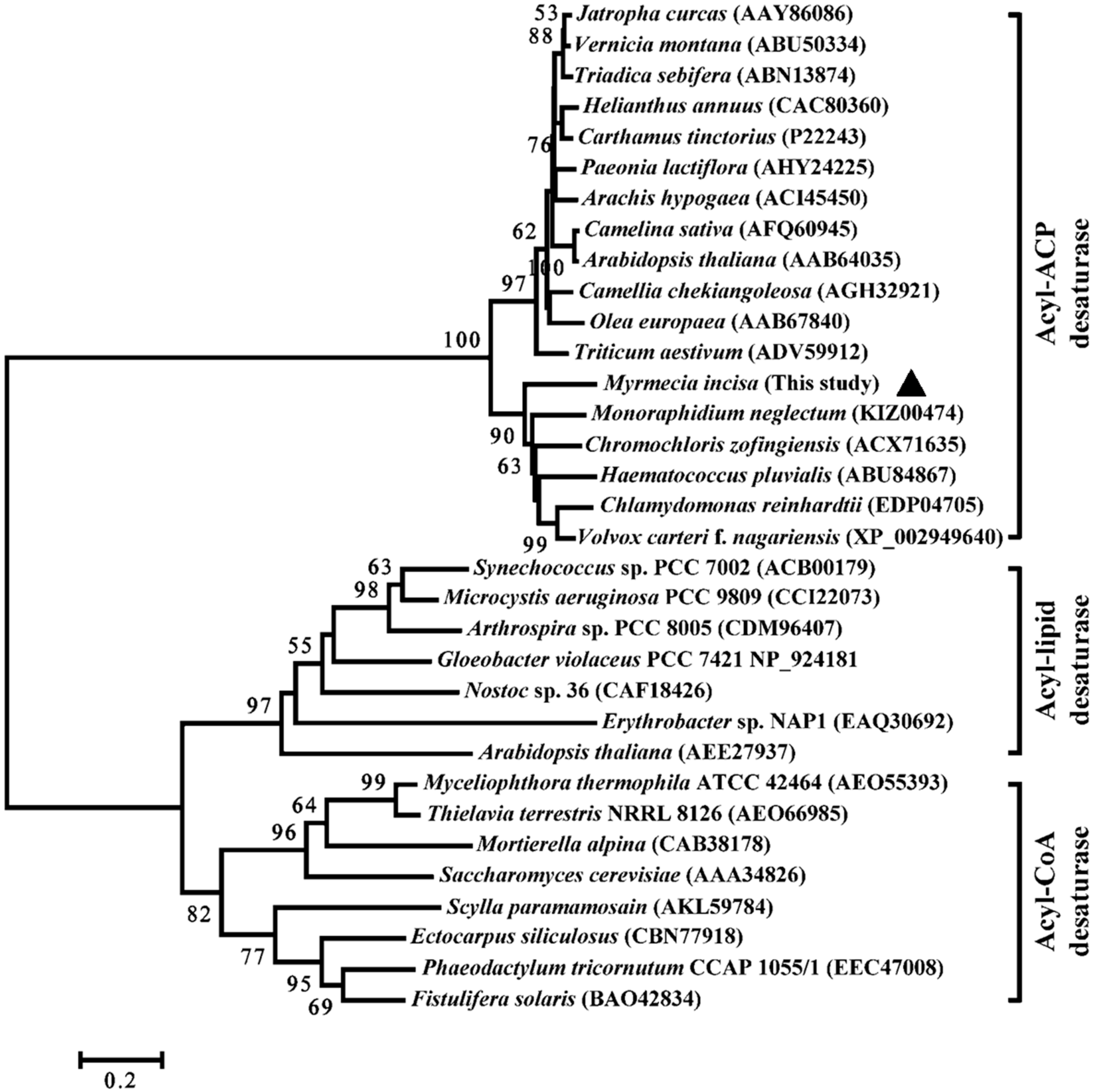
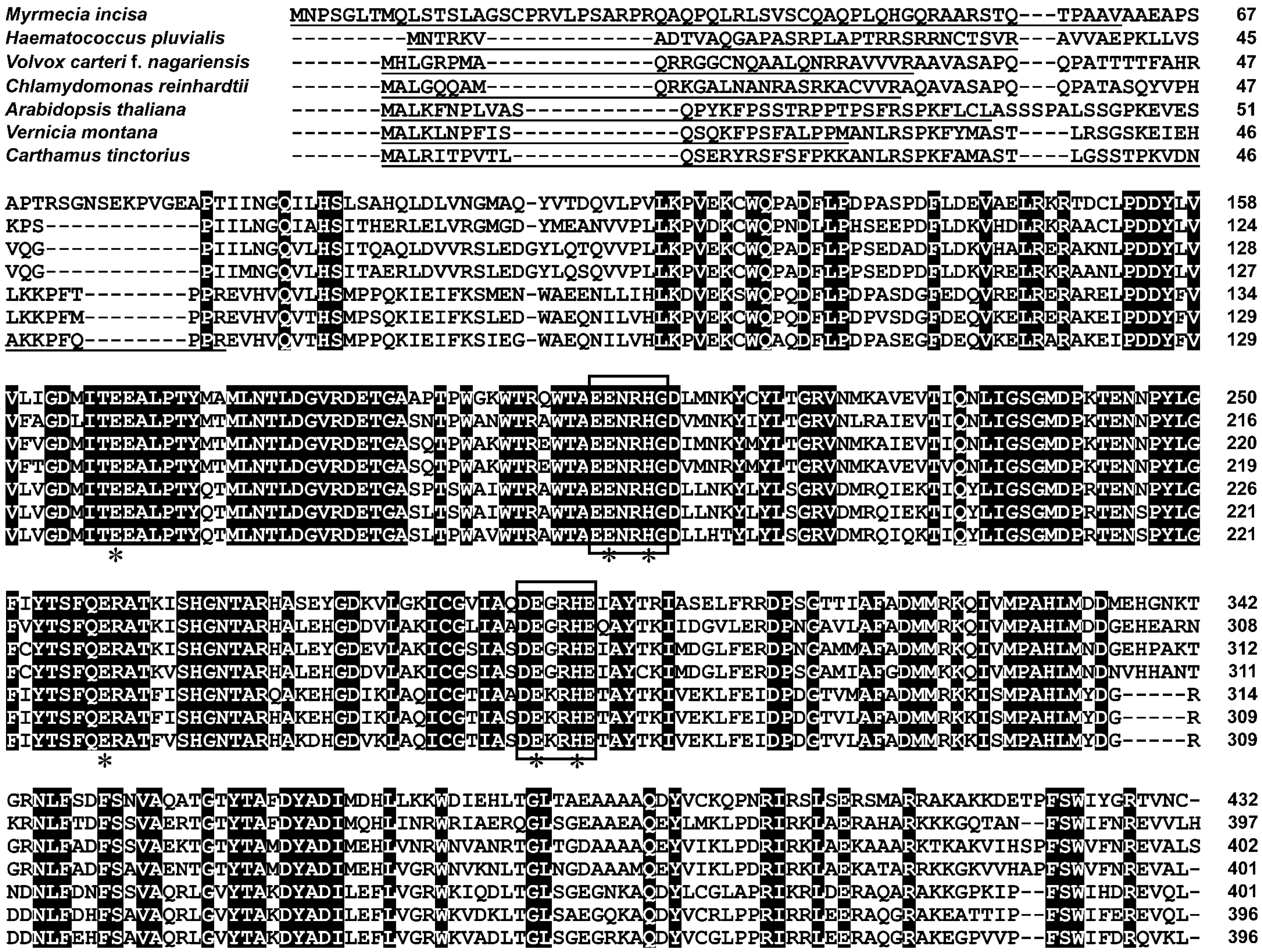
2.2. Characterization of MiΔ9FAD
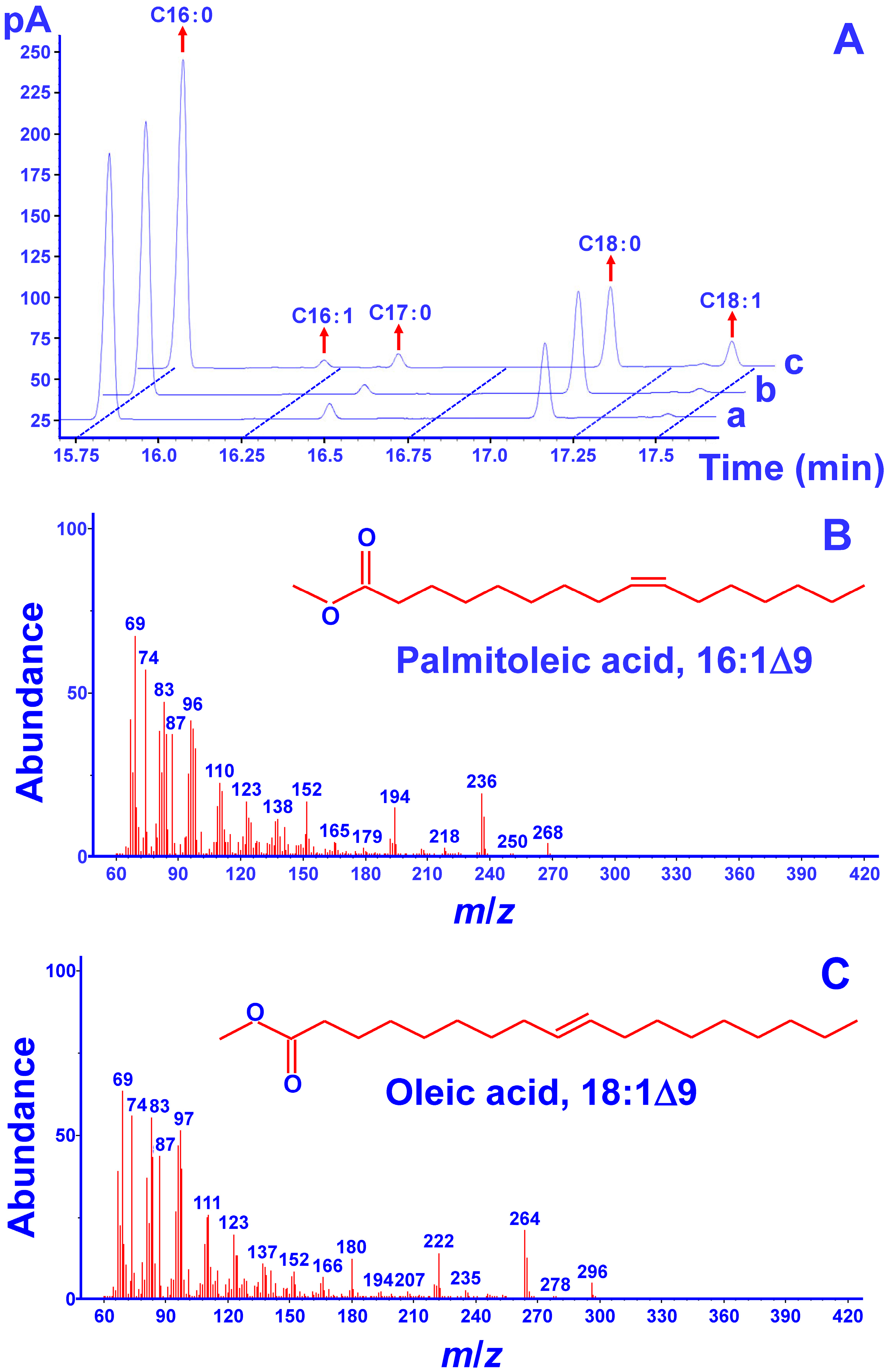
2.3. Functional Complementation Assays in S. cerevisiae
3. Materials and Methods
3.1. Algal Strain and Culture Conditions
3.2. Yeast Strain and Culture Conditions
3.3. Genomic DNA and RNA Isolation
3.4. Cloning of Δ9 FAD cDNA and Its Corresponding Gene
3.5. Bioinformatics Analysis
3.6. Vector Construction and Heterologous Expression in S. cerevisiae
3.7. Preparation of Fatty Acid Methyl Esters and GC-MS Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hwang, D.H.; Chow, C.K. Dietary fatty acids and eicosanoids. In Fatty Acids in Foods and Their Health Implications; Chow, C.K., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2000; pp. 585–595. [Google Scholar]
- FAO/WHO Joint Consultation. Dietary fatty acids and eicosanoids. Nutr. Rev. 1995, 53, 202–205. [Google Scholar]
- Nichols, B.W.; Appleby, R.S. The distribution and biosynthesis of arachidonic acid in algae. Phytochemistry 1969, 8, 1907–1915. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Belarbi, E.H.; Rebolloso-Fuentes, M.M. Eicosapentaenoic and arachidonic acids purification from the red microalga Porphyridium cruentum. Bioseparation 2000, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bigogno, C.; Khozin-Goldberg, I.; Adlerstein, D.; Cohen, Z. Biosynthesis of arachidonic acid in the oleaginous microalga Parietochloris incisa (Chlorophyceae): Radiolabeling studies. Lipids 2002, 37, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.L.; Li, H.; Liu, F.; Tong, M.; Yu, S.Y.; Zhou, Z.G. Accumulation of arachidonic acid in a green microalga, Myrmecia incisa H4301, enhanced by nitrogen starvation and its molecular mechanisms. In Arachidonic Acid: Dietary Sources and General Functions; Dumancas, G.G., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 1–20. [Google Scholar]
- Reisigl, H. Zur Systematik und Ökologie alpiner Bodenalgen. Plant Syst. Evol. 1964, 111, 402–499. [Google Scholar] [CrossRef]
- Tong, M.; Yu, S.Y.; Ouyang, L.L.; Zhou, Z.G. Comparison of increased arachidonic acid content in Myrmecia incisa cultured during the course of nitrogen or phosphorus starvation. J. Fish. China 2011, 35, 763–773. [Google Scholar]
- Shiran, D.; Khozin, I.; Heimer, Y.M.; Cohen, Z. Biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum. I: The use of externally supplied fatty acids. Lipids 1996, 31, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Khozin, I.; Adlerstein, D.; Bigongo, C.; Heimer, Y.M.; Cohen, Z. Elucidation of the biosynthesis of eicosapentaenoic acid in the Microalga Porphyridium cruentum (II Studies with Radiolabeled Precursors). Plant Physiol. 1997, 114, 223–230. [Google Scholar] [PubMed]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Sakuradani, E.; Kobayashi, M.; Shimizu, S. Δ9-fatty acid desaturase from arachidonic acid-producing fungus. Unique gene sequence and its heterologous expression in a fungus, Aspergillus. Eur. J. Biochem. 1999, 260, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Zhou, Z.G. The transcriptome pyrosequencing and gene function annotation of the green microalga Myrmecia incisa. J. Shanghai Ocean Univ. 2012, 21, 662–670. [Google Scholar]
- Ouyang, L.L.; Chen, S.H.; Li, Y.; Zhou, Z.G. Transcriptome analysis reveals unique C4-like photosynthesis and oil body formation in an arachidonic acid-rich microalga Myrmecia incisa Reisigl H4301. BMC Genom. 2012, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Macartney, A.; Maresca, B.; Cossins, A.R. Temperature Adaptation of Biological Membranes; Cossins, A.R., Ed.; Portland Press: London, UK, 1994. [Google Scholar]
- Holloway, P.W. Fatty acid desaturation. In The Enzymes; Boyer, P.D., Ed.; Academic Press: New York, NY, USA, 1983; pp. 63–83. [Google Scholar]
- Murata, N.; Wada, H.; Gombos, Z. Modes of fatty-acid desaturation in cyanobacteria. Plant Cell Physiol. 1992, 33, 933–941. [Google Scholar]
- Murata, N.; Wada, H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem. J. 1995, 308, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, P.; Lyle, K.S.; Kaul, S.P.; Turco, M.M.; Arabshahi, I.; Marwah, A.; Fox, B.G. Identification of the binding region of the [2Fe-2S] ferredoxin in stearoyl-acyl carrier protein desaturase: Insight into the catalytic complex and mechanism of action. Biochemistry 2006, 45, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yang, Q.; Pan, L.; Chen, M.; He, Y.; Yang, Z.; Yu, S. Isolation and characterization of fatty acid desaturase genes from peanut (Arachis hypogaea L.). Plant Cell Rep. 2011, 30, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, K.; Ahn, J.W.; Lim, J.M.; Park, Y.I.; Liu, J.R.; Jeong, W.J. Overexpression of stearoyl-ACP desaturase enhances accumulations of oleic acid in the green alga Chlamydomonas reinhardtii. Plant Biotechnol. Rep. 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Fox, B.G.; Shanklin, J.; Somerville, C.; Münck, E. Stearoyl-acyl carrier protein Δ9 desaturase from Ricinus communis is a diiron-oxo protein. Proc. Natl. Acad. Sci. USA 1993, 90, 2486–2490. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, Y.; Huang, W.J.; Schneider, G.J. Crystal structure of Δ9 stearoyl-acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins. EMBO J. 1996, 15, 4081–4092. [Google Scholar] [PubMed]
- Stukey, J.E.; Mcdonough, V.M.; Martin, C.E. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J. Biol. Chem. 1989, 264, 16537–16544. [Google Scholar] [PubMed]
- Stukey, J.E.; Mcdonough, V.M.; Martin, C.E. The OLE1 gene of Saccharomyces cerevisiae encodes the Δ9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 1990, 265, 20144–20149. [Google Scholar] [PubMed]
- Harood, J.L. Fatty acid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 101–138. [Google Scholar] [CrossRef]
- Lightner, J.; Wu, J.; Browse, J. A mutant of arabidopsis with increased levels of stearic acid. Plant Physiol. 1994, 106, 1443–1451. [Google Scholar] [PubMed]
- Li, Y.; Dietrich, M.; Schmid, R.D.; He, B.; Ouyang, P.; Urlacher, V.B. Identification and functional expression of a Δ9-fatty acid desaturase from Psychrobacter urativorans in Escherichia coli. Lipids 2008, 43, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.C.; Sun, R.H.; Liang, Y.X.; Zhang, M.D.; Zheng, Y.S.; Li, D.D. Cloning and functional expression of a cDNA encoding stearoyl-ACP Δ9-desaturase from the endosperm of coconut (Cocos nucifera L.). Gene 2014, 549, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [PubMed]
- Ouyang, L.L.; Du, D.H.; Yu, S.Y.; Li, C.Y.; Zhang, C.W.; Gao, H.J.; Zhou, Z.G. Expressed sequence tags analysis revealing the taxonomic position and fatty acid biosynthesis in an oleaginous green microalga, Myrmecia incisa Reisigl (Trebouxiophyceae, Chlorophyta). Chin. Sci. Bull. 2012, 57, 3342–3352. (In Chinese) [Google Scholar] [CrossRef]
- Suga, M.; Isobe, M.; Hatakeyama, T. Cryopreservation of competent intact yeast cells for efficient electroporation. Yeast 2000, 16, 889–896. [Google Scholar] [CrossRef]
- Yu, S.Y.; Liu, S.C.; Li, C.Y.; Zhou, Z.G. Submesoscale characteristics and transcription of a fatty acid elongase gene from a freshwater green microalgae, Myrmecia incisa Reisigl. Chin. J. Oceanol. Limnol. 2011, 29, 87–95. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Li, H.; Tong, M.; Ouyang, L.L.; Zhou, Z.G. Identification of a Δ6 fatty acid elongase gene for arachidonic acid biosynthesis localized to the endoplasmic reticulum in the green microalga Myrmecia incisa Reisigl. Gene 2012, 493, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.X.; Fraser, T.; Mugford, S.; Dobson, G.; Sayanova, O.; Butler, J.; Napier, J.A.; Stobart, A.K.; Lazarus, C.M. Production of very long chain polyunsaturated ω-3 and ω-6 fatty acids in plants. Nat. Biotechnol. 2004, 22, 739–745. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence 5′-3′ | Annealing Temperature/°C | Size of Amplificant/bp |
|---|---|---|---|
| RACE for cDNA cloning | |||
| acp-1 Forward | CTGTCGGCCCACCAGTTAGA | – | – |
| acp-1 Reverse | GCCGTAGATCCAGGAGAAGG | 60.9 | 1002 |
| acp-5-gsp1 | TCCTGGAAGGAGGTGTAGATGAAGC | 66 | – |
| acp-5-gsp2 | GGCAGCACCTGGTCCGTAACATA | 68 | 501 |
| acp-3-gsp1 | CCGCAACCTGTTTTCAGACTTCTCC | 65 | 1230 |
| DNA cloning | |||
| D1-Forward | ACGCGGGGAGTGACAACACCAGCTGT | 69.6 | 812 |
| D1-Reverse | CTCTGAGTTGCCGCTCCTTGTGGGGG | – | – |
| D2-Forward | ATGAACCCGAGTGGGCTCACGATG | 66.3 | 1542 |
| D2-Reverse | GTCCATGCCAGAGCCAATCAGGTT | – | – |
| D3-Forward | ACAGCCGAGGAGAACCGTCATGGT | 68.1 | 1376 |
| D3-Reverse | CTAGCAGTTGACGGTGCGGCCGTAGAT | – | – |
| D4-Forward | GGACGAGACGCCCTTCTCCTGGAT | 64.9 | 1005 |
| D4-Reverse | GCCCTGCAGCGTTTTACAGCGC | – | – |
| Heterologous expression in yeast | |||
| pY-Forward | cgGAATTCATGGCCGCAGAGGCCCCAT 1,2 | 68.5 | 1138 |
| pY-Reverse | gcTCTAGACATCTAGCAGTTGACGGTGC 1,2 | – | – |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, W.-B.; Liu, F.; Sun, Z.; Zhou, Z.-G. A Δ-9 Fatty Acid Desaturase Gene in the Microalga Myrmecia incisa Reisigl: Cloning and Functional Analysis. Int. J. Mol. Sci. 2016, 17, 1143. https://doi.org/10.3390/ijms17071143
Xue W-B, Liu F, Sun Z, Zhou Z-G. A Δ-9 Fatty Acid Desaturase Gene in the Microalga Myrmecia incisa Reisigl: Cloning and Functional Analysis. International Journal of Molecular Sciences. 2016; 17(7):1143. https://doi.org/10.3390/ijms17071143
Chicago/Turabian StyleXue, Wen-Bin, Fan Liu, Zheng Sun, and Zhi-Gang Zhou. 2016. "A Δ-9 Fatty Acid Desaturase Gene in the Microalga Myrmecia incisa Reisigl: Cloning and Functional Analysis" International Journal of Molecular Sciences 17, no. 7: 1143. https://doi.org/10.3390/ijms17071143







