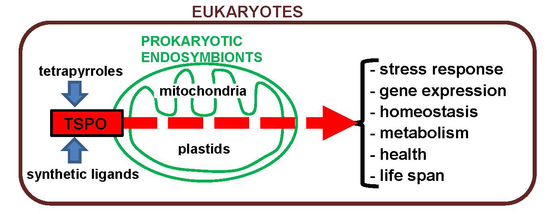
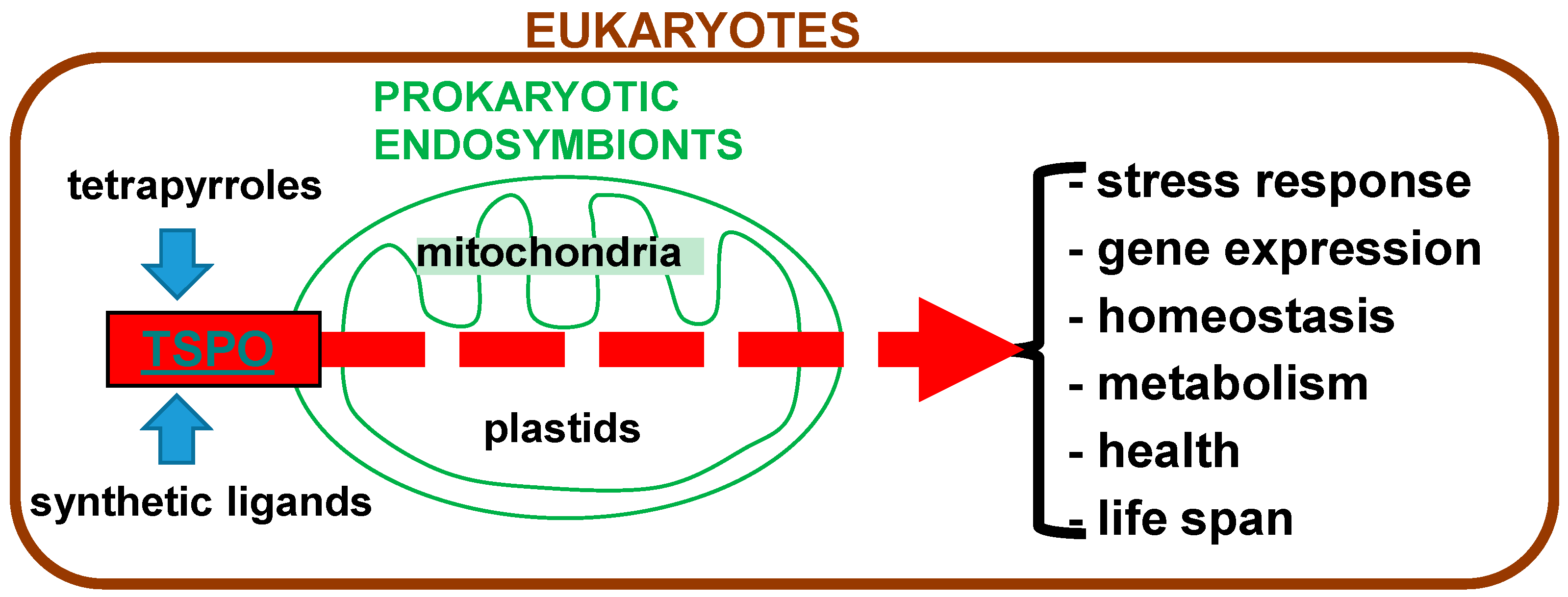
Tetrapyrroles as Endogenous TSPO Ligands in Eukaryotes and Prokaryotes: Comparisons with Synthetic Ligands
Abstract
:1. Concise Introduction to the Theme
2. The 18 kDa Translocator Protein (TSPO)
2.1. General TSPO Characteristics and Functions
2.2. TSPO Ligands, Endogenous and Synthetic
3. Tetrapyrroles, including Protoporphyrins such as PPIX, as Ligands for TSPO
3.1. Tetrapyrroles Binding to TSPO
3.2. Implications of Tetrapyrrole-TSPO Interactions
3.3. Implications of Tetrapyrrole-TSPO Interactions for Brain Disease
3.4. Tetrapyrrole-TSPO Interactions in Insects
4. Functional Effects of PPIX and Synthetic TSPO Ligands in Mammals, including Human
4.1. Differences between PPIX and Synthetic Ligands in Interactions with TSPO
4.2. Effects of Synthetic TSPO Ligands and the Endogenous TSPO Ligand PPIX in Blood Cells
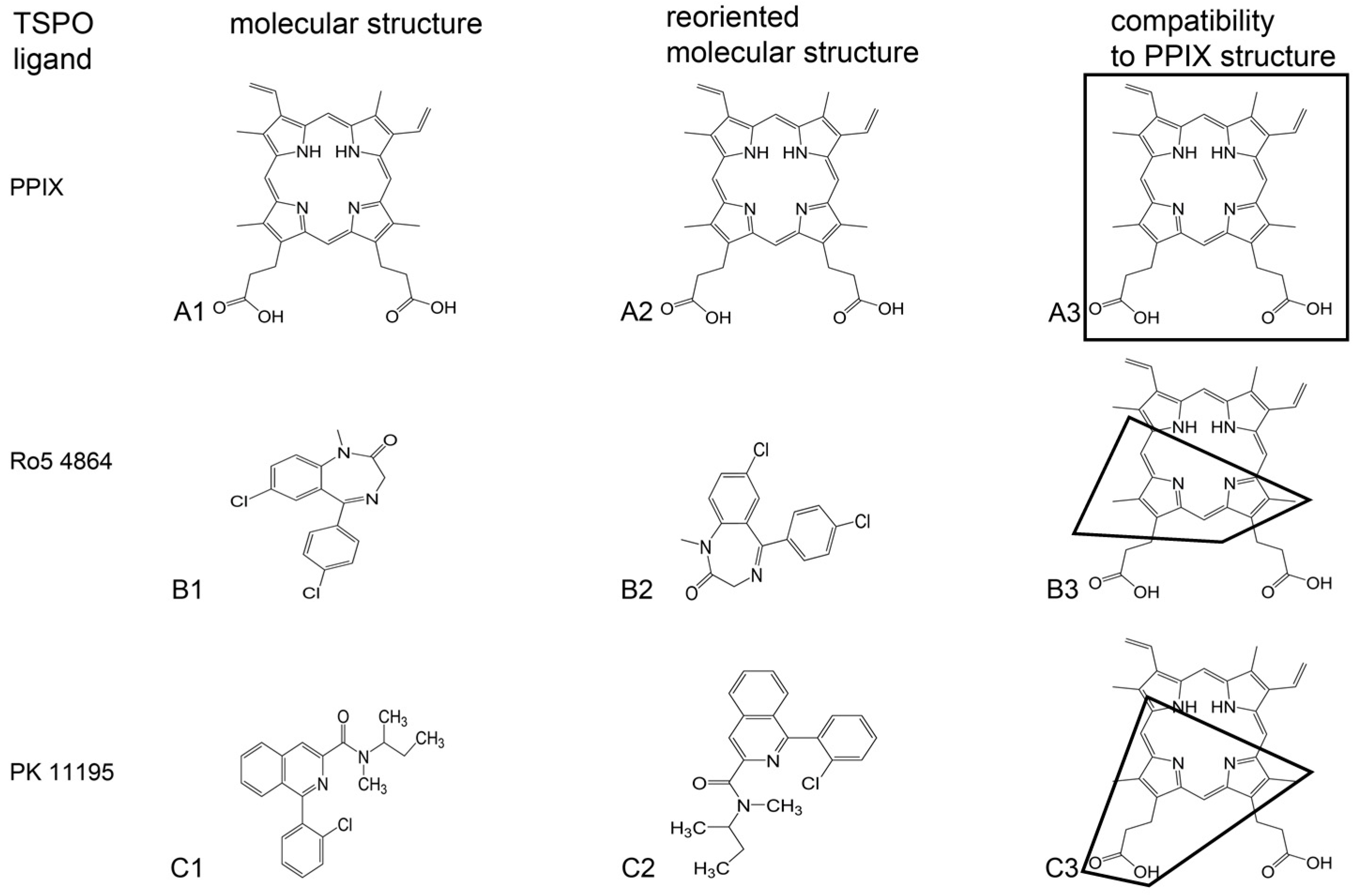
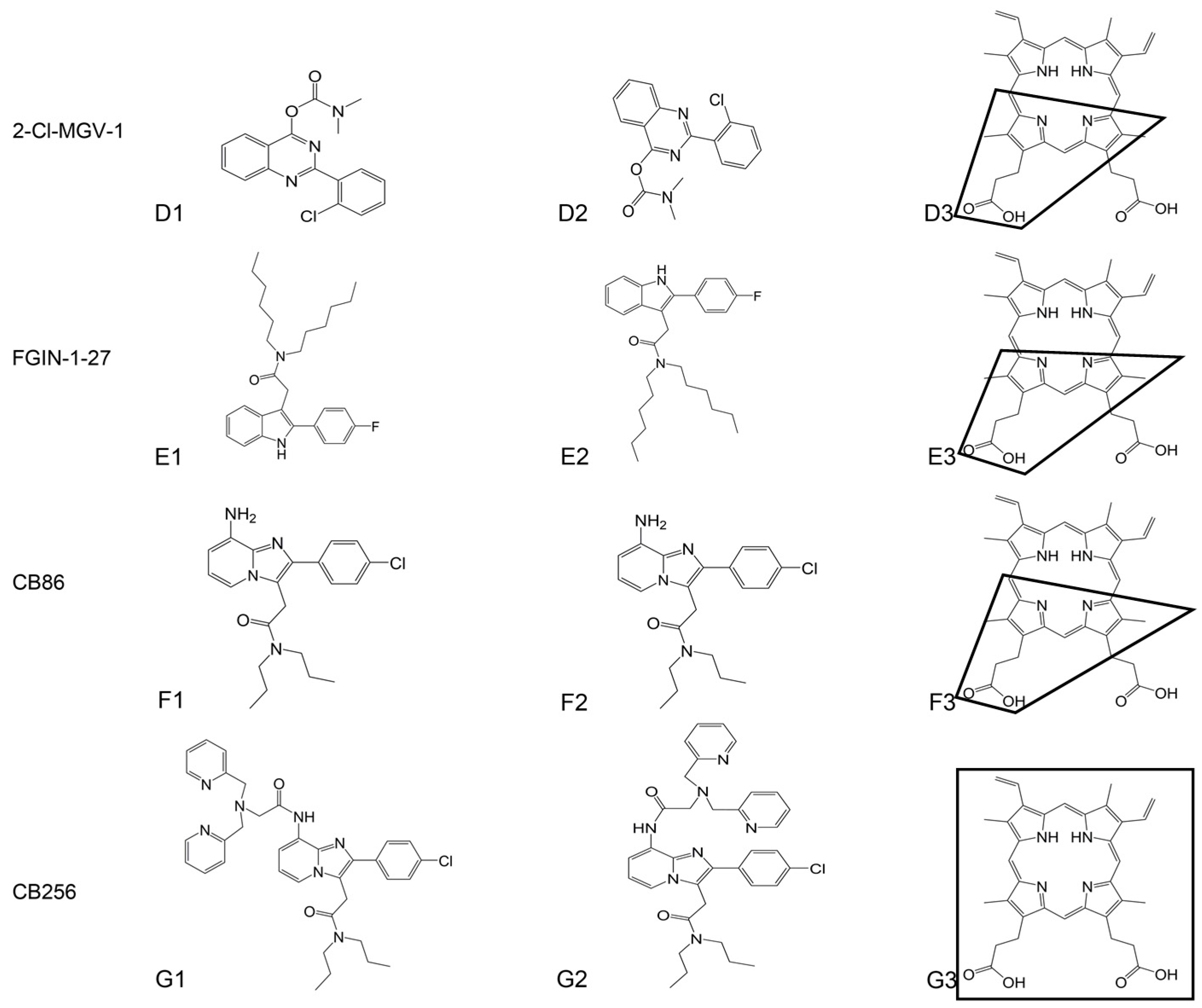
5. Structural Relationships between Synthetic and Endogenous TSPO Ligands and Their Binding Sites
5.1. Differences between PPIX and Synthetic Ligands in Interactions with TSPO
5.2. Accommodation of Various Types of TSPO Ligands by Binding Sites on the TSPO
6. Bacteria, TSPO, and Tetrapyrroles
7. Plants, TSPO, and Tetrapyrroles
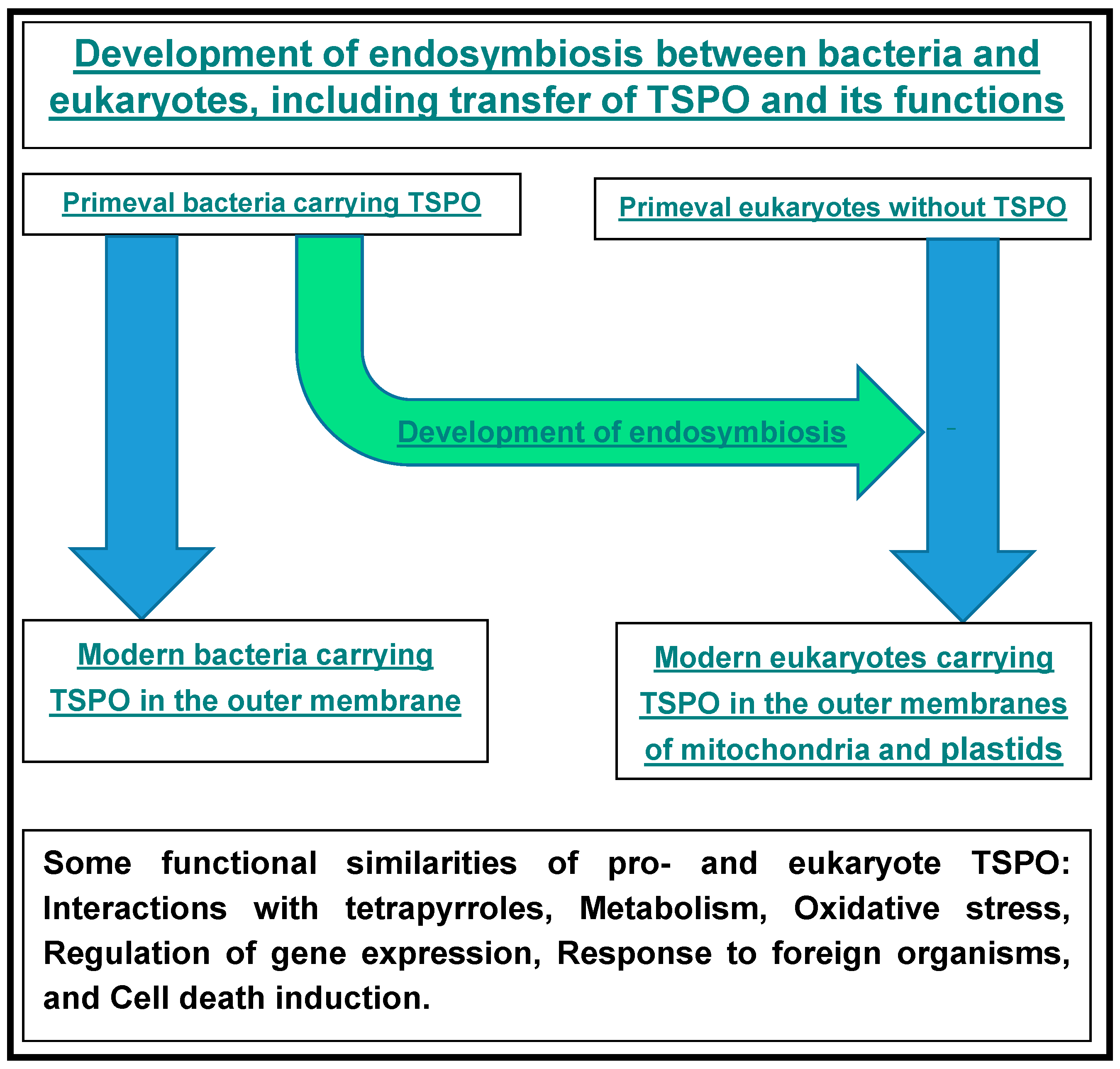
8. TSPO-Tetrapyrrole Interactions from an Evolutionary Perspective
9. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gavish, M.; Bachman, I.; Shoukrun, R.; Katz, Y.; Veenman, L.; Weisinger, G.; Weizman, A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 1999, 51, 629–650. [Google Scholar] [PubMed]
- Fan, J.; Lindemann, P.; Feuilloley, M.G.; Papadopoulos, V. Structural and functional evolution of the translocator protein (18 kDa). Curr. Mol. Med. 2012, 12, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Braestrup, C.; Squires, R.F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc. Natl. Acad. Sci. USA 1977, 74, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Anholt, R.R.; Pedersen, P.L.; De Souza, E.B.; Snyder, S.H. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J. Biol. Chem. 1986, 261, 576–583. [Google Scholar] [PubMed]
- McEnery, M.W.; Snowman, A.M.; Trifiletti, R.R.; Snyder, S.H. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA 1992, 89, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Gavish, M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol. Ther. 2006, 110, 503–524. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Nye, J.S.; Snyder, S.H. Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc. Natl. Acad. Sci. USA 1987, 84, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, J.; Zheng, Y.; Garavito, R.M.; Ferguson-Miller, S. Protein structure. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science 2015, 347, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Papadopoulos, V.; Gavish, M. Channel-like functions of the 18-kDa translocator protein (TSPO): Regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr. Pharm. Des. 2007, 13, 2385–2405. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Gavish, M.; Kugler, W. Apoptosis induction by erucylphosphohomocholine via the 18 kDa mitochondrial translocator protein: Implications for cancer treatment. Anticancer Agents Med. Chem. 2014, 14, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Lecanu, L. Translocator protein (18 kDa) TSPO: An emerging therapeutic target in neurotrauma. Exp. Neurol. 2009, 219, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zeno, S.; Veenman, L.; Katz, Y.; Bode, J.; Gavish, M.; Zaaroor, M. The 18 kDa mitochondrial translocator protein (TSPO) prevents accumulation of protoporphyrin IX. Involvement of reactive oxygen species (ROS). Curr. Mol. Med. 2012, 12, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Zeno, S.; Zaaroor, M.; Leschiner, S.; Veenman, L.; Gavish, M. CoCl2 induces apoptosis via the 18 kDa translocator protein in U118MG human glioblastoma cells. Biochemistry 2009, 48, 4652–4661. [Google Scholar] [CrossRef] [PubMed]
- Fares, F.; Bar-Ami, S.; Brandes, J.M.; Gavish, M. Gonadotropin- and estrogen-induced increase of peripheral-type benzodiazepine binding sites in the hypophyseal-genital axis of rats. Eur. J. Pharmacol. 1987, 133, 97–102. [Google Scholar] [CrossRef]
- Gavish, M.; Laor, N.; Bidder, M.; Fisher, D.; Fonia, O.; Muller, U.; Reiss, A.; Wolmer, L.; Karp, L.; Weizman, R. Altered platelet peripheral-type benzodiazepine receptor in posttraumatic stress disorder. Neuropsychopharmacology 1996, 14, 181–186. [Google Scholar] [CrossRef]
- Veenman, L.; Gavish, M. Peripheral-type benzodiazepine receptors: Their implication in brain disease. Drug Dev. Res. 2000, 50, 355–370. [Google Scholar] [CrossRef]
- Veenman, L.; Gavish, M. The role of 18 kDa mitochondrial translocator protein (TSPO) in programmed cell death, and effects of steroids on TSPO expression. Curr. Mol. Med. 2012, 12, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Angelin, A.; da Settimo, F.; Martini, C.; Taliani, S.; Zhu, S.; Wallace, D.C. Genetic analysis of dTSPO, an outer mitochondrial membrane protein, reveals its functions in apoptosis, longevity, and Ab42-induced neurodegeneration. Aging Cell 2014, 13, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, V.M.; Rupprecht, R.; Wetzel, C.H. The Translocator protein 18 kDa (TSPO) and its role in mitochondrial biology and psychiatric disorders. Mini Rev. Med. Chem. 2015, 15, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Vainshtein, A.; Gavish, M. TSPO as a target for treatments of diseases, including neuropathological disorders. Cell Death Dis. 2015, 6, e1911. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Veenman, L.; Shterenberg, A.; Singh, S.; Masarwa, A.; Dutta, B.; Island, B.; Tsoglin, E.; Levin, E.; Leschiner, S.; et al. Quinazoline-based tricyclic compounds that regulate programmed cell death, induce neuronal differentiation, and are curative in animal models for excitotoxicity and hereditary brain disease. Cell Death Discov. 2015, 1, 15027. [Google Scholar] [CrossRef]
- Veenman, L.; Levin, E.; Weisinger, G.; Leschiner, S.; Spanier, I.; Snyder, S.H.; Weizman, A.; Gavish, M. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol. 2004, 68, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Shandalov, Y.; Gavish, M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J. Bioenerg. Biomembr. 2008, 40, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.; Premkumar, A.; Veenman, L.; Kugler, W.; Leschiner, S.; Spanier, I.; Weisinger, G.; Lakomek, M.; Weizman, A.; Snyder, S.H.; et al. The peripheral-type benzodiazepine receptor and tumorigenicity: Isoquinoline binding protein (IBP) antisense knockdown in the C6 glioma cell line. Biochemistry 2005, 44, 9924–9935. [Google Scholar] [CrossRef] [PubMed]
- Kugler, W.; Veenman, L.; Shandalov, Y.; Leschiner, S.; Spanier, I.; Lakomek, M.; Gavish, M. Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomocholine. Cell Oncol. 2008, 30, 435–450. [Google Scholar] [PubMed]
- Caballero, B.; Veenman, L.; Gavish, M. Role of mitochondrial translocator protein (18 kDa) on mitochondrial-related cell death processes. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Veenman, L.; Bode, J.; Leschiner, S.; Gavish, M. Concentration-dependent bimodal effect of specific 18 kDa translocator protein (TSPO) ligands on cell death processes induced by ammonium chloride: Potential implications for neuropathological effects due to hyperammonemia. CNS Neurol. Disord. Drug Targets 2014, 13, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, A.G.; Papadopoulos, V.; Costa, E.; Krueger, K.E. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc. Natl. Acad. Sci. USA 1989, 86, 9813–9816. [Google Scholar] [CrossRef] [PubMed]
- Nothdurfter, C.; Baghai, T.C.; Schüle, C.; Rupprecht, R. Translocator protein (18 kDa) (TSPO) as a therapeutic target for anxiety and neurologic disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Aghazadeh, Y.; Fan, J.; Campioli, E.; Zirkin, B.; Midzak, A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 2015, 408, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Markó, K.; Hádinger, N.; Jelitai, M.; Demeter, K.; Tihanyi, K.; Vas, A.; Madarász, E. Translocator protein (TSPO 18 kDa) is expressed by neural stem and neuronal precursor cells. Neurosci. Lett. 2009, 462, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Wang, Y.; Thuillier, R.; Rhodes, C.; Culty, M. Developmental expression of the translocator protein 18 kDa (TSPO) in testicular germ cells. Curr. Mol. Med. 2012, 12, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Bode, J.; Gaitner, M.; Caballero, B.; Pe’er, Y.; Zeno, S.; Kietz, S.; Kugler, W.; Lakomek, M.; Gavish, M. Effects of 18-kDa translocator protein knockdown on gene expression of glutamate receptors, transporters, and metabolism, and on cell viability affected by glutamate. Pharmacogenet. Genom. 2012, 22, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.; Veenman, L.; Gavish, M. Regulation of nuclear gene expression by PK 11195, a ligand specific for the 18 kDa mitochondrial translocator protein (TSPO). In Proceedings of the Annual Meeting of the Israel Society for Neuroscience, Eilat, Israel, 20–22 December 2015. Abstract #98.
- Yasin, N.; Veenman, L.; Gavish, M. NCBI. Available online: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77998 (accessed on 2 June 2016).
- Verma, A.; Snyder, S.H. Characterization of porphyrin interactions with peripheral type benzodiazepine receptors. Mol. Pharmacol. 1988, 34, 800–805. [Google Scholar] [PubMed]
- Mantione, C.R.; Goldman, M.E.; Martin, B.; Bolger, G.T.; Lueddens, H.W.; Paul, S.M.; Skolnick, P. Purification and characterization of an endogenous protein modulator of radioligand binding to “peripheral-type” benzodiazepine receptors and dihydropyridine Ca2+-channel antagonist binding sites. Biochem. Pharmacol. 1988, 37, 339–347. [Google Scholar] [CrossRef]
- Alho, H.; Fremeau, R.T., Jr.; Tiedge, H.; Wilcox, J.; Bovolin, P.; Brosius, J.; Roberts, J.L.; Costa, E. Diazepam binding inhibitor gene expression: Location in brain and peripheral tissues of rat. Proc. Natl. Acad. Sci. USA 1988, 85, 7018–7022. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.A.; Ghatei, M.A.; Sekiya, K.; Krausz, T.; Bloom, S.R. Diazepam binding inhibitor-like immunoreactivity (51–70): Distribution in human brain, spinal cord and peripheral tissues. Brain Res. 1989, 479, 300–305. [Google Scholar] [CrossRef]
- Slobodyansky, E.; Guidotti, A.; Wambebe, C.; Berkovich, A.; Costa, E. Isolation and characterization of a rat brain triakontatetraneuropeptide, a posttranslational product of diazepam binding inhibitor: Specific action at the Ro 5-4864 recognition site. J. Neurochem. 1989, 53, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Bovolin, P.; Schlichting, J.; Miyata, M.; Ferrarese, C.; Guidotti, A.; Alho, H. Distribution and characterization of diazepam binding inhibitor (DBI) in peripheral tissues of rat. Regul. Pept. 1990, 29, 267–281. [Google Scholar] [CrossRef]
- Perrone, M.; Moon, B.S.; Park, H.S.; Laquintana, V.; Jung, J.H.; Cutrignelli, A.; Lopedota, A.; Franco, M.; Kim, S.E.; Lee, B.C.; et al. A novel PET imaging probe for the detection and monitoring of translocator protein 18 kDa expression in pathological disorders. Sci. Rep. 2016, 6, 20422. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, G.; Vaucher, N.; Perrier, M.L.; Flamier, A.; Benavides, J.; Renault, C.; Dubroeucq, M.C.; Guérémy, C.; Uzan, A. Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies. Life Sci. 1983, 33, 449–457. [Google Scholar] [CrossRef]
- Romeo, E.; Cavallaro, S.; Korneyev, A.; Kozikowski, A.P.; Ma, D.; Polo, A.; Costa, E.; Guidotti, A. Stimulation of brain steroidogenesis by 2-aryl-indole-3-acetamidederivatives acting at the mitochondrial diazepam-binding inhibitor receptor complex. J. Pharmacol. Exp. Ther. 1993, 267, 462–471. [Google Scholar] [PubMed]
- Denora, N.; Margiotta, N.; Laquintana, V.; Lopedota, A.; Cutrignelli, A.; Losacco, M.; Franco, M.; Natile, G. Synthesis, characterization, and in vitro evaluation of a new TSPO-selective bifunctional chelate ligand. ACS Med. Chem. Lett. 2014, 5, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Schlicke, H.; Richter, A.; Rothbart, M.; Brzezowski, P.; Hedtke, B.; Grimm, B. Function of tetrapyrroles, regulation of tetrapyrrole metabolism and methods for analyses of tetrapyrroles. Procedia Chem. 2015, 14, 171–175. [Google Scholar] [CrossRef]
- Fujiwara, T.; Harigae, H. Biology of heme in mammalian erythroid cells and related disorders. BioMed Res. Int. 2015, 2015, 278536. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, O.; Grimm, B. Post-translational control of tetrapyrrole biosynthesis in plants, algae, and cyanobacteria. J. Exp. Bot. 2012, 63, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.S.; Klein, D.C.; Skolnick, P. Characterization of benzodiazepine receptors in the bovine pineal gland: Evidence for the presence of an atypical binding site. Brain Res. 1986, 387, 127–135. [Google Scholar] [CrossRef]
- Awad, M.; Gavish, M. Binding of [3H]Ro 5-4864 and [3H]PK 11195 to cerebral cortex and peripheral tissues of various species: Species differences and heterogeneity in peripheral benzodiazepine binding sites. J. Neurochem. 1987, 49, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Weizman, A.; Gavish, M. Ligands specific to peripheral benzodiazepine receptors for treatment of porphyrias. Lancet 1989, 1, 932–933. [Google Scholar] [CrossRef]
- Lourenço, C.; Lee, C.; Anderson, K.E. Disorders of haem biosynthesis. In Inborn Metabolic Diseases: Diagnosis and Treatment, 5th ed.; Saudubray, J.M., van den Berghe, G., Walter, J.H., Eds.; Springer: New York, NY, USA, 2012; pp. 521–532. [Google Scholar]
- Cantoni, L.; Rizzardini, M.; Skorupska, M.; Cagnotto, A.; Codegoni, A.; Pecora, N.; Frigo, L.; Ferrarese, C.; Mennini, T. Hepatic protoporphyria is associated with a decrease in ligand binding for the mitochondrial benzodiazepine receptors in the liver. Biochem. Pharmacol. 1992, 44, 1159–1164. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Simbula, G.; Gilfor, E.; Hoek, J.B.; Farber, J.L. Protoporphyrin IX, an endogenous ligand of the peripheral benzodiazepine receptor, potentiates induction of the mitochondrial permeability transition and the killing of cultured hepatocytes by rotenone. J. Biol. Chem. 1994, 269, 31041–31046. [Google Scholar] [PubMed]
- Gemelli, C.; Dongmo, B.M.; Ferrarini, F.; Grande, A.; Corsi, L. Cytotoxic effect of hemin in colonic epithelial cell line: Involvement of 18 kDa translocator protein (TSPO). Life Sci. 2014, 107, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerrish, K.E.; Putnam, C.W.; Laird, H.E., 2nd. Prolactin-stimulated mitogenesis in the Nb2 rat lymphoma cell: Lack of protoporphyrin IX effects. Life Sci. 1990, 47, 1647–1653. [Google Scholar] [CrossRef]
- Fonia, O.; Weizman, R.; Coleman, R.; Kaganovskaya, E.; Gavish, M. PK 11195 aggravates 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced hepatic porphyria in rats. Hepatology 1996, 24, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, V.; Magistrelli, A.; Cantoni, L.; Tacconi, M.T. Peripheral benzodiazepine receptor ligands in rat liver mitochondria: Effect on cholesterol translocation. Eur. J. Pharmacol. 1995, 294, 601–607. [Google Scholar] [CrossRef]
- Awad, M.; Gavish, M. Species differences and heterogeneity of solubilized peripheral-type benzodiazepine binding sites. Biochem. Pharmacol. 1989, 38, 3843–3849. [Google Scholar] [CrossRef]
- Tsankova, V.; Visentin, M.; Cantoni, L.; Carelli, M.; Tacconi, M.T. Peripheral benzodiazepine receptor ligands in rat liver mitochondria: Effect on 27-hydroxylation of cholesterol. Eur. J. Pharmacol. 1996, 299, 197–203. [Google Scholar] [CrossRef]
- Kanegawa, N.; Collste, K.; Forsberg, A.; Schain, M.; Arakawa, R.; Jucaite, A.; Lekander, M.; Olgart Höglund, C.; Kosek, E.; Lampa, J.; et al. In vivo evidence of a functional association between immune cells in blood and brain in healthy human subjects. Brain Behav. Immun. 2016, 54, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Gunn, R.N.; Rabiner, E.A.; Bennacef, I.; Fujita, M.; Kreisl, W.C.; Innis, R.B.; Pike, V.W.; Reynolds, R.; Matthews, P.M.; et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J. Nucl. Med. 2011, 52, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Lewis, A.J.; Reynolds, R.; Rupprecht, R.; Eser, D.; Wilkins, M.R.; Bennacef, I.; Nutt, D.J.; Parker, C.A. Variation in binding affinity of the novel anxiolytic XBD173 for the 18 kDa translocator protein in human brain. Synapse 2011, 65, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Yeo, A.J.; Gunn, R.N.; Song, K.; Wadsworth, G.; Lewis, A.; Rhodes, C.; Pulford, D.J.; Bennacef, I.; Parker, C.A.; et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 2012, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, M.; Jaremko, Ł.; Giller, K.; Becker, S.; Zweckstetter, M. Structural integrity of the A147T polymorph of mammalian TSPO. ChemBioChem 2015, 16, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, M.; Jaremko, Ł.; Giller, K.; Becker, S.; Zweckstetter, M. Backbone and side-chain resonance assignment of the A147T polymorph of mouse TSPO in complex with a high-affinity radioligand. Biomol. NMR Assign. 2016, 10, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, S.L.; Matthews, E.K. Modification of the photodynamic action of δ aminolaevulinic acid (ALA) on rat pancreatoma cells by mitochondrial benzodiazepine receptor ligands. Br. J. Cancer 1995, 71, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kalathur, R.C.; Liu, Q.; Kloss, B.; Bruni, R.; Ginter, C.; Kloppmann, E.; Rost, B.; Hendrickson, W.A. Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science 2015, 347, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.H.; Tu, L.N.; Mukai, C.; Sirivelu, M.P.; Pillai, V.V.; Morohaku, K.; Cohen, R.; Selvaraj, V. Mitochondrial translocator protein (TSPO) function is not essential for heme biosynthesis. J. Biol. Chem. 2016, 291, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Wendler, G.; Lindemann, P.; Lacapère, J.J.; Papadopoulos, V. Protoporphyrin IX binding and transport by recombinant mouse PBR. Biochem. Biophys. Res. Commun. 2003, 11, 847–852. [Google Scholar] [CrossRef]
- Bisland, S.K.; Goebel, E.A.; Hassanali, N.S.; Johnson, C.; Wilson, B.C. Increased expression of mitochondrial benzodiazepine receptors following low-level light treatment facilitates enhanced protoporphyrin IX production in glioma-derived cells in vitro. Lasers Surg. Med. 2007, 39, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Zoghbi, S.S.; Hong, J.; Verma, A.; Pike, V.W.; Innis, R.B.; Fujita, M. In vivo binding of protoporphyrin IX to rat translocator protein imaged with positron emission tomography. Synapse 2010, 64, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.S.; Norenberg, M.D. Effect of benzodiazepines and neurosteroids on ammonia-induced swelling in cultured astrocytes. J. Neurosci. Res. 1998, 54, 673–680. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Panickar, K.S.; Norenberg, M.D. Effects on free radical generation by ligands of the peripheral benzodiazepine receptor in cultured neural cells. J. Neurochem. 2002, 83, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Repalli, J. Translocator protein (TSPO) role in aging and Alzheimer’s disease. Curr. Aging Sci. 2014, 7, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Zweckstetter, M.; Banati, R.B. Lost in translocation: The functions of the 18-kD translocator protein. Trends Endocrinol. Metab. 2015, 26, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Leschiner, S.; Spanier, I.; Weisinger, G.; Weizman, A.; Gavish, M. PK 11195 attenuates kainic acid-induced seizures and alterations in peripheral-type benzodiazepine receptor (PBR) protein components in the rat brain. J. Neurochem. 2002, 80, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, E.; Luz, M.; Vidal, L.M.; Cruces, M.P.; Janczur, M.K. Action of protoporphyrin-IX (PP-IX) in the lifespan of Drosophila melanogaster deficient in endogenous antioxidants, Sod and Cat. Open J. Anim. Sci. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Vidal, L.M.E.; Pimentel, E.P.; Cruces, M.P.; Sánchez, J.C.M. Genetic damage induced by CrO3 can be reduced by low doses of Protoporphyrin-IX in somatic cells of Drosophila melanogaster. Toxicol. Rep. 2014, 1, 894–899. [Google Scholar] [CrossRef]
- Curtis, C.; Landis, G.N.; Folk, D.; Wehr, N.B.; Hoe, N.; Waskar, M.; Abdueva, D.; Skvortsov, D.; Ford, D.; Luu, A.; et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007, 8, R262. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.; Reichenbecher, V. The effects of superoxide and the peripheral benzodiazepine receptor ligands on the mitochondrial processing of manganese-dependent superoxide dismutase. Exp. Cell Res. 1999, 246, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Park, J.H.; Chung, C.G.; Shim, H.J.; Jeon, K.H.; Yu, S.W.; Lee, S.B. Parkin-mediated responses against infection and wound involve TSPO-VDAC complex in Drosophila. Biochem. Biophys. Res. Commun. 2015, 463, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Bicker, G. Nitric oxide and cyclic nucleotides are regulators of neuronal migration in an insect embryo. Development 2003, 130, 3977–3987. [Google Scholar] [CrossRef] [PubMed]
- Caiaffa, C.D.; Stiebler, R.; Oliveira, M.F.; Lara, F.A.; Paiva-Silva, G.O.; Oliveira, P.L. Sn-protoporphyrin inhibits both heme degradation and hemozoin formation in Rhodnius prolixus midgut. Insect Biochem. Mol. Biol. 2010, 40, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Rosenberg, O.; Weizman, A.; Leschiner, S.; Sakoury, Y.; Fares, F.; Soudry, M.; Weisinger, G.; Veenman, L.; Gavish, M. In vitro mitochondrial effects of PK 11195, a synthetic translocator protein 18 kDa (TSPO) ligand, in human osteoblast-like cells. J. Bioenerg. Biomembr. 2011, 43, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Rosenberg, O.; Weizman, A.; Veenman, L.; Gavish, M. In vitro catabolic effect of protoporphyrin IX in human osteoblast-like cells: Possible role of the 18 kDa mitochondrial translocator protein. J. Bioenerg. Biomembr. 2013, 45, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Rosenberg, O.; Weizman, A.; Veenman, L.; Gavish, M. In vitro effect of FGIN-1–27, a ligand to 18 kDa mitochondrial translocator protein, in human osteoblast-like cells. J. Bioenerg. Biomembr. 2014, 46, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Rosenberg, O.; Weizman, A.; Veenman, L.; Gavish, M. In vitro effects of the specific mitochondrial TSPO ligand Ro5 4864 in cultured human osteoblasts. J. Bioenerg. Biomembr. 2016. submitted. [Google Scholar]
- Gavish, M.; Veenman, J.A.; Shterenberg, A.; Marek, I. Heterocyclic Derivatives, Pharmaceutical Compositions and Methods of Use Thereof. U.S. Patent 8,541,428, 24 September 2013. [Google Scholar]
- Gavish, M.; Marek, I.; Avital, A.; Shterenberg, A.; Vainshtein, A.; Veenman, L. Quinazoline Derivatives, Pharmaceutical Compositions and Methods of Use Thereof. WO 2015162615 A1, 29 October 2015. [Google Scholar]
- Ferrarese, C.; Appollonio, I.; Frigo, M.; Perego, M.; Pierpaoli, C.; Trabucchi, M.; Frattola, L. Characterization of peripheral benzodiazepine receptors in human blood mononuclear cells. Neuropharmacology 1990, 29, 375–378. [Google Scholar] [CrossRef]
- Berkovich, A.; Ferrarese, C.; Cavaletti, G.; Alho, H.; Marzorati, C.; Bianchi, G.; Guidotti, A.; Costa, E. Topology of two DBI receptors in human lymphocytes. Life Sci. 1993, 52, 1275–1277. [Google Scholar] [CrossRef]
- Kinnally, K.W.; Zorov, D.B.; Antonenko, Y.N.; Snyder, S.H.; McEnery, M.W.; Tedeschi, H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc. Natl. Acad. Sci. USA 1993, 90, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Kinnally, K.W.; Muro, C.; Campo, M.L. MCC and PSC, the putative protein import channels of mitochondria. J. Bioenerg. Biomembr. 2000, 32, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Taketani, S.; Kohno, H.; Furukawa, T.; Tokunaga, R. Involvement of peripheral-type benzodiazepine receptors in the intracellular transport of heme and porphyrins. J. Biochem. 1995, 117, 875–880. [Google Scholar] [PubMed]
- Nakajima, O.; Hashimoto, Y.; Iwasaki, S. Possible involvement of peripheral-type benzodiazepine receptors in erythroid differentiation of human leukemia cell line, K562. Biol. Pharm. Bull. 1995, 18, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Odber, J.; Cutler, M.; Dover, S.; Moore, M.R. Haem precursor effects on [3H]-PK 11195 binding to platelets. Neuroreport 1994, 5, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xia, Y.; Meiler, J.; Ferguson-Miller, S. Characterization and modeling of the oligomeric state and ligand binding behavior of purified translocator protein 18 kDa from Rhodobacter sphaeroides. Biochemistry 2013, 52, 5884–5889. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, L.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 2014, 343, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Hinsen, K.; Vaitinadapoule, A.; Ostuni, M.A.; Etchebest, C.; Lacapere, J.J. Construction and validation of an atomic model for bacterial TSPO from electron microscopy density, evolutionary constraints, and biochemical and biophysical data. Biochim. Biophys. Acta 2015, 1848, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Comment on “Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism”. Science 2015, 350, 519. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Sumlin, A.B.; Greco, W.R.; Weishaupt, K.R.; Vaughan, L.A.; Pandey, R.K. The role of the peripheral benzodiazepine receptor in photodynamic activity of certain pyropheophorbide ether photosensitizers: Albumin site II as a surrogate marker for activity. Photochem. Photobiol. 2002, 76, 91–97. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Dobhal, MP.; Gryshuk, A.; Morgan, J.; Dougherty, TJ.; Oseroff, A.; Pandey, R.K. Methyl pyropheophorbide-a analogues: Potential fluorescent probes for the peripheral-type benzodiazepine receptor. Effect of central metal in photosensitizing efficacy. J. Med. Chem. 2005, 48, 3692–3695. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Guo, H.L.; Shi, T.Y.; Yang, L.; Wang, M.; Zhang, K.; Guo, Y.Y.; Wu, Y.M.; Liu, S.B.; Zhao, M.G. Neuroprotective effects of a novel translocator protein (18 kDa) ligand, ZBD-2, against focal cerebral ischemia and NMDA-induced neurotoxicity. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Tian, Z.; Guo, Y.Y.; Guo, H.L.; Kang, W.B.; Li, S.; Den, Y.T.; Li, X.B.; Feng, B.; Feng, D.; et al. Anxiolytic-like effects of translocator protein (TSPO) ligand ZBD-2 in an animal model of chronic pain. Mol. Pain 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, Ł.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Conformational flexibility in the transmembrane protein TSPO. Chemistry 2015, 21, 16555–16563. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.A.; Alberti, M.; Leach, F.; Hearst, J.E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter. capsulatus. Mol. Gen. Genet. 1989, 216, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Bui, E.T.; Bradley, P.J.; Johnson, P.J. A common evolutionary origin for mitochondria and hydrogenosomes. Proc. Natl. Acad. Sci. USA 1996, 93, 9651–9656. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E.; Fanestil, D.D. Mammalian peripheral-type benzodiazepine receptor is homologous to CrtK protein of Rhodobacter capsulatus, a photosynthetic bacterium. Cell 1991, 65, 721–722. [Google Scholar] [CrossRef]
- Yeliseev, A.A.; Kaplan, S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 1995, 270, 21167–21175. [Google Scholar] [CrossRef] [PubMed]
- Chapalain, A.; Chevalier, S.; Orange, N.; Murillo, L.; Papadopoulos, V.; Feuilloley, M.G.J. Bacterial ortholog of mammalian translocator protein (TSPO) with virulence regulating activity. PLoS ONE 2009, 4, e6096. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Krueger, K.E.; Kaplan, S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc. Natl. Acad. Sci. USA 1997, 94, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, A.F.; Daldal, F. Cytochrome c biogenesis System I: An intricate process catalyzed by a maturase supercomplex? Biochim. Biophys. Acta 2014, 1837, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Kaplan, S. A novel mechanism for the regulation of photosynthesis gene expression by the TspO outer membrane protein of Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 1999, 274, 21234–21243. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Kaplan, S. TspO as a modulator of the repressor/antirepressor (PpsR/AppA) regulatory system in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 2001, 183, 6355–6364. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Kaplan, S. TspO of Rhodobacter sphaeroides. A structural and functional model for the mammalian peripheral benzodiazepine receptor. J. Biol. Chem. 2000, 275, 5657–5667. [Google Scholar] [CrossRef] [PubMed]
- Scarf, A.M.; Auman, K.M.; Kassiou, M. Is there any correlation between binding and functional effects at the translocator protein (TSPO) (18 kDa)? Curr. Mol. Med. 2012, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Z.; Degenhardt, B.; Teper, G.; Papadopoulos, V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 1277–1272. [Google Scholar] [CrossRef]
- Lindemann, P.; Koch, A.; Degenhardt, B.; Hause, G.; Grimm, B.; Papadopoulos, V. A novel Arabidopsis thaliana protein is a functional peripheral-type benzodiazepine receptor. Plant Cell Physiol. 2004, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Guillaumot, D.; Guillon, S.; Morsomme, P.; Batoko, H. ABA, porphyrins and plant TSPO-related protein. Plant Signal. Behav. 2009, 4, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Avallone, R.; Geminiani, E.; Cosenza, F.; Venturini, I.; Baraldi, M. Peripheral benzodiazepine receptors in potatoes (Solanum tuberosum). Biochem. Biophys. Res. Commun. 2004, 313, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Guillaumot, D.; Guillon, S.; Déplanque, T.; Vanhee, C.; Gumy, C.; Masquelier, D.; Morsomme, P.; Batoko, H. The Arabidopsis TSPO-related protein is a stress and abscisic acid-regulated, endoplasmic reticulum-Golgi-localized membrane protein. Plant J. 2009, 60, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Balsemão-Pires, E.; Jaillais, Y.; Olson, B.J.; Andrade, L.R.; Umen, J.G.; Chory, J.; Sachetto-Martins, G. The Arabidopsis translocator protein (AtTSPO) is regulated at multiple levels in response to salt stress and perturbations in tetrapyrrole metabolism. BMC Plant Biol. 2011, 11, 108. [Google Scholar] [CrossRef]
- Vanhee, C.; Zapotoczny, G.; Masquelier, D.; Ghislain, M.; Batoko, H. The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell 2011, 23, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, C.; Batoko, H. Arabidopsis TSPO and porphyrins metabolism: A transient signaling connection? Plant Signal. Behav. 2011, 6, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, C.; Batoko, H. Autophagy involvement in responses to abscisic acid by plant cells. Autophagy 2011, 7, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Frank, W.; Baar, K.M.; Qudeimat, E.; Woriedh, M.; Alawady, A.; Ratnadewi, D.; Gremillon, L.; Grimm, B.; Reski, R. A mitochondrial protein homologous to the mammalian peripheral-type benzodiazepine receptor is essential for stress adaptation in plants. Plant J. 2007, 51, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, M.T.; Akita, M.; Frank, W.; Reski, R.; Valkonen, J.P. Involvement of a class III peroxidase and the mitochondrial protein TSPO in oxidative burst upon treatment of moss plants with a fungal elicitor. Mol. Plant Microbe Interact. 2012, 25, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Sherameti, I.; Oelmuller, R. Siroheme: An essential component for life on earth. Plant Signal. Behav. 2010, 5, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Garg, S.; Zimorski, V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140330. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Towards understanding the evolution and functional diversification of DNA containing plant organelles. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- French, K.L.; Hallmann, C.; Hope, J.M.; Schoon, P.L.; Zumberge, J.A.; Hoshino, Y.; Peters, C.A.; George, S.C.; Love, G.D.; Brocks, J.J.; et al. Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc. Natl. Acad. Sci. USA 2015, 112, 5915–5920. [Google Scholar] [CrossRef] [PubMed]
- Weisinger, G.; Kelly-Hershkovitz, E.; Veenman, L.; Spanier, I.; Leschiner, S.; Gavish, M. Peripheral benzodiazepine receptor antisense knockout increases tumorigenicity of MA-10 Leydig cells in vivo and in vitro. Biochemistry 2004, 43, 12315–12321. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ando, H.; Hanaoka, M.; Tanaka, K. Abscisic acid participates in the control of cell cycle initiation through heme homeostasis in the unicellular red alga Cyanidioschyzon merolae. Plant Cell Physiol. 2016, 57, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.W.; Montgomery, B. The tryptophan-rich sensory protein (TSPO) is involved in stress-related and light-dependent processes in the cyanobacterium Fremyella diplosiphon. Front. Microbiol. 2015, 6, 1393. [Google Scholar] [CrossRef] [PubMed]
- Stowe-Evans, E.L.; Ford, J.; Kehoe, D.M. Genomic DNA microarray analysis: Identification of new genes regulated by light color in the cyanobacterium Fremyella diplosiphon. J. Bacteriol. 2004, 186, 4338–4349. [Google Scholar] [CrossRef] [PubMed]
- Pecoits, E.; Smith, M.L.; Catling, D.C.; Philippot, P.; Kappler, A.; Konhauser, K.O. Atmospheric hydrogen peroxide and Eoarchean iron formations. Geobiology 2015, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Leneveu-Jenvrin, C.; Connil, N.; Bouffartigues, E.; Papadopoulos, V.; Feuilloley, M.G.; Chevalier, S. Structure-to-function relationships of bacterial translocator protein (TSPO): A focus on Pseudomonas. Front. Microbiol. 2014, 5, 631. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Masuda, T.; Tajima, N.; Wada, H.; Sato, N. Molecular phylogeny and intricate evolutionary history of the three isofunctional enzymes involved in the oxidation of protoporphyrinogen IX. Genome Biol. Evol. 2014, 6, 2141–2155. [Google Scholar] [CrossRef] [PubMed]
- Boldareva-Nuianzina, E.N.; Bláhová, Z.; Sobotka, R.; Koblízek, M. Distribution and origin of oxygen-dependent and oxygen-independent forms of Mg-protoporphyrin monomethylester cyclase among phototrophic proteobacteria. Appl. Environ. Microbiol. 2013, 79, 2596–2604. [Google Scholar] [CrossRef] [PubMed]
| Various Species Expressing TSPO | TSPO Gene Length (bp) | TSPO Protein Length (aa) |
|---|---|---|
| Human | ||
| Homo sapiens | 11,729 bp | 169 aa |
| Mammals | ||
| Rattus norvegicus | 10,253 bp | 169 aa |
| Mus musculus | 10,631 bp | 169 aa |
| Amphibians | ||
| Xenopus tropicalis | 5970 bp | 211 aa |
| Insects | ||
| Drosophila melanogaster | 6569 bp | 185 aa |
| Aedes aegypti | 1236 bp | 176 aa |
| Archea | ||
| Haloferax mediterranei | 486 bp | 161 aa |
| Bacteria | ||
| Bacillus anthracis str. Ames | 456 bp | 151 aa |
| Rhodobacter sphaeroides | 480 bp | 158 aa |
| Rhodobacter capsulatus | 483 bp | 160 aa |
| Plants | ||
| Arabidopsis thaliana | 1044 bp | 196 aa |
| Solanum tuberosum | 895 bp | 203 aa |
| Ricinus communis | 1530 bp | 196 aa |
| Vitis vinifera | 1055 bp | 185 aa |
| Fungi | ||
| Cryptococcus gattii | 865 bp | 174 aa |
| Schizosaccharomyces cryophilus | 480 bp | 164 aa |
| Aspergillus fumigatus | 632 bp | 177 aa |
| Kluyveromyces | 486 bp | 160 aa |
| TSPO-Associated Functions in Animals, Plants, and Bacteria | ||
|---|---|---|
| Animals | Plants | Bacteria |
| Mitochondrial membrane potential transition | Interactions with large membrane channels | |
| Transport of porphyrin intermediates | Translocation of tetrapyrrole intermediates | Transport of porphyrin intermediates |
| Heme metabolism | Tetrapyrrole metabolism | Heme metabolism |
| ROS generation | Oxidative stress | ROS generation |
| Programmed cell death | Cell death | Induction of apoptosis in eukaryotes |
| Mitochondrial protein transport | ||
| Mitochondrial metabolism | Anaerobic and aerobic metabolism | |
| Mitochondrial cholesterol transport | Cholesterol binding | |
| Steroidogenesis | ||
| Nuclear gene expression | Gene expression | |
| Cell cycle | Cell cycle | Cell cycle |
| Cell growth | Cell growth | |
| Cell proliferation | ||
| Cell migration | ||
| Cell adhesion | Adhesion | |
| Cell differentiation | ||
| Embryonic development | Seed and plant development | |
| Endocrinological function | ||
| Reproduction | ||
| Stress response | Stress response | Stress response |
| Immune response | Response to pathogens | |
| Inflammatory response | ||
| Glial activation | ||
| Response to brain disease and injury | ||
| Emotional health | ||
| Mental health | ||
| Cardiovascular health | ||
| Homeostasis | Homeostasis | Homeostasis |
| Life span of multicellular organisms | ||
| TSPO Functions Affected by Tetrapyrroles | ||
|---|---|---|
| Animals | Plants | Bacteria |
| TSPO expression | TSPO expression | |
| Mitochondrial membrane potential transition | ||
| ROS generation | Stress response | |
| Mitochondrial protein transport | ||
| Mitochondrial cholesterol transport | ||
| Regulation of steroidogenesis | ||
| Heme metabolism | ||
| Transport of porphyrin intermediates | ||
| Modulation of nuclear gene expression | Seed and plant development | Photosynthetic gene expression |
| Cell migration | ||
| Programmed cell death | ||
| Mitochondrial metabolism | Switch between anaerobic and aerobic metabolism | |
| Life span | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veenman, L.; Vainshtein, A.; Yasin, N.; Azrad, M.; Gavish, M. Tetrapyrroles as Endogenous TSPO Ligands in Eukaryotes and Prokaryotes: Comparisons with Synthetic Ligands. Int. J. Mol. Sci. 2016, 17, 880. https://doi.org/10.3390/ijms17060880
Veenman L, Vainshtein A, Yasin N, Azrad M, Gavish M. Tetrapyrroles as Endogenous TSPO Ligands in Eukaryotes and Prokaryotes: Comparisons with Synthetic Ligands. International Journal of Molecular Sciences. 2016; 17(6):880. https://doi.org/10.3390/ijms17060880
Chicago/Turabian StyleVeenman, Leo, Alex Vainshtein, Nasra Yasin, Maya Azrad, and Moshe Gavish. 2016. "Tetrapyrroles as Endogenous TSPO Ligands in Eukaryotes and Prokaryotes: Comparisons with Synthetic Ligands" International Journal of Molecular Sciences 17, no. 6: 880. https://doi.org/10.3390/ijms17060880