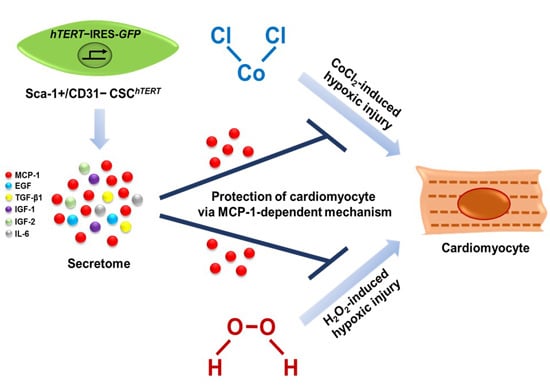
Cardiac Stem Cell Secretome Protects Cardiomyocytes from Hypoxic Injury Partly via Monocyte Chemotactic Protein-1-Dependent Mechanism
Abstract
:1. Introduction
2. Results
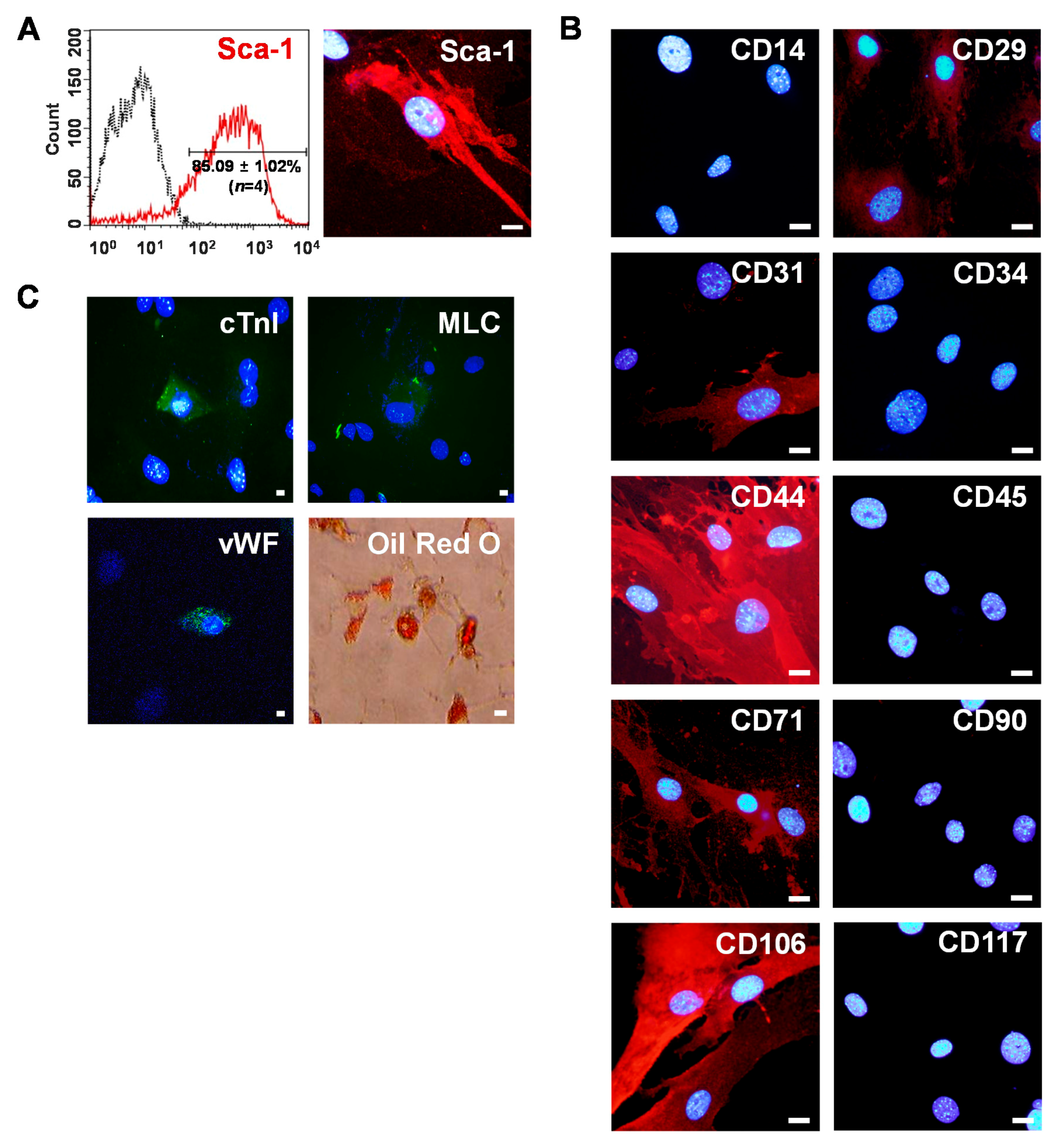
2.1. Isolation of Sca-1+ CSCs from Adult Myocardium
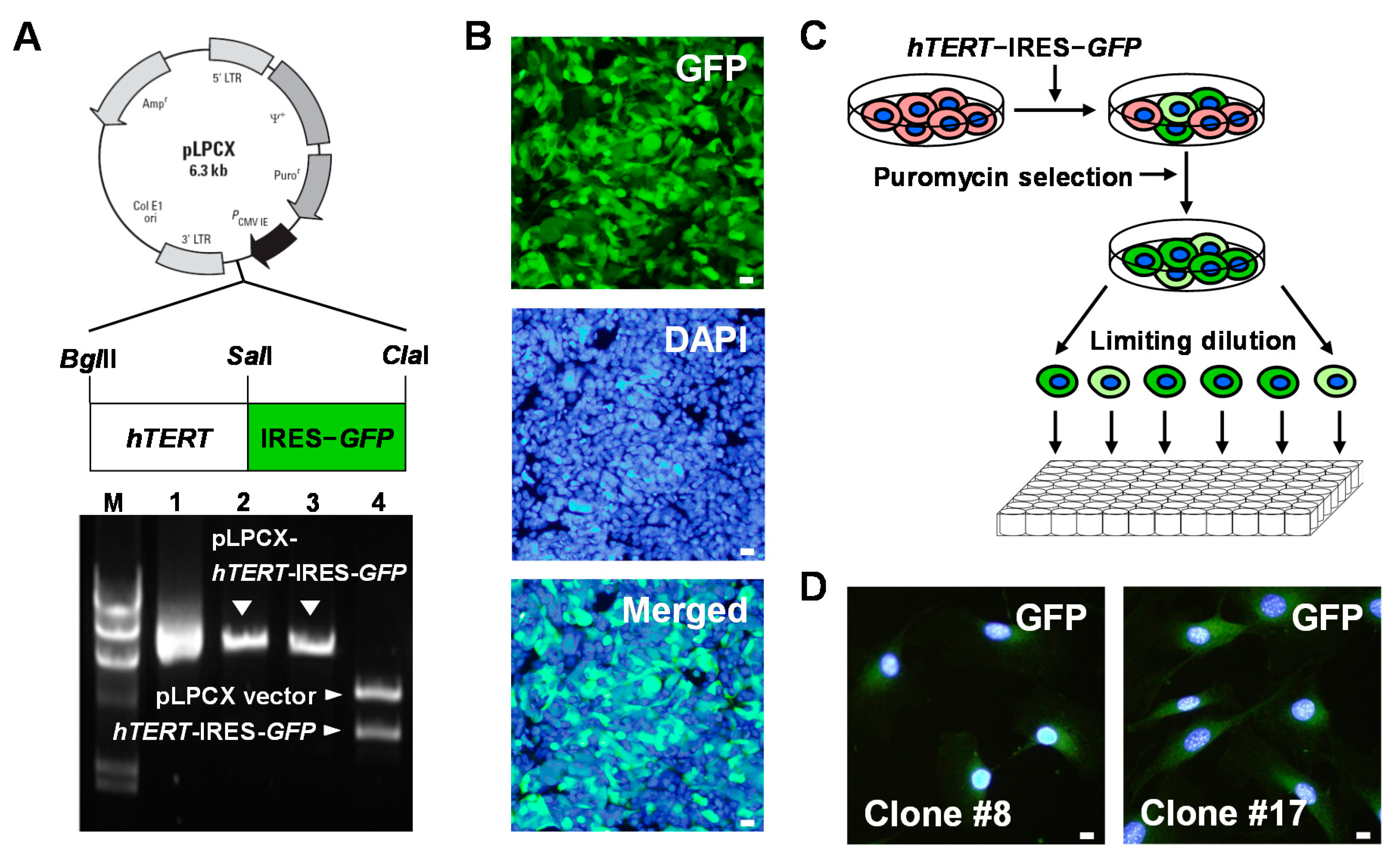
2.2. Establishment of Human TERT (hTERT)-Immortalized Sca-1+ CSC Lines
2.3. Evaluation of Stem Cell Potency of hTERT-Immortalized Sca-1+ CSC Lines
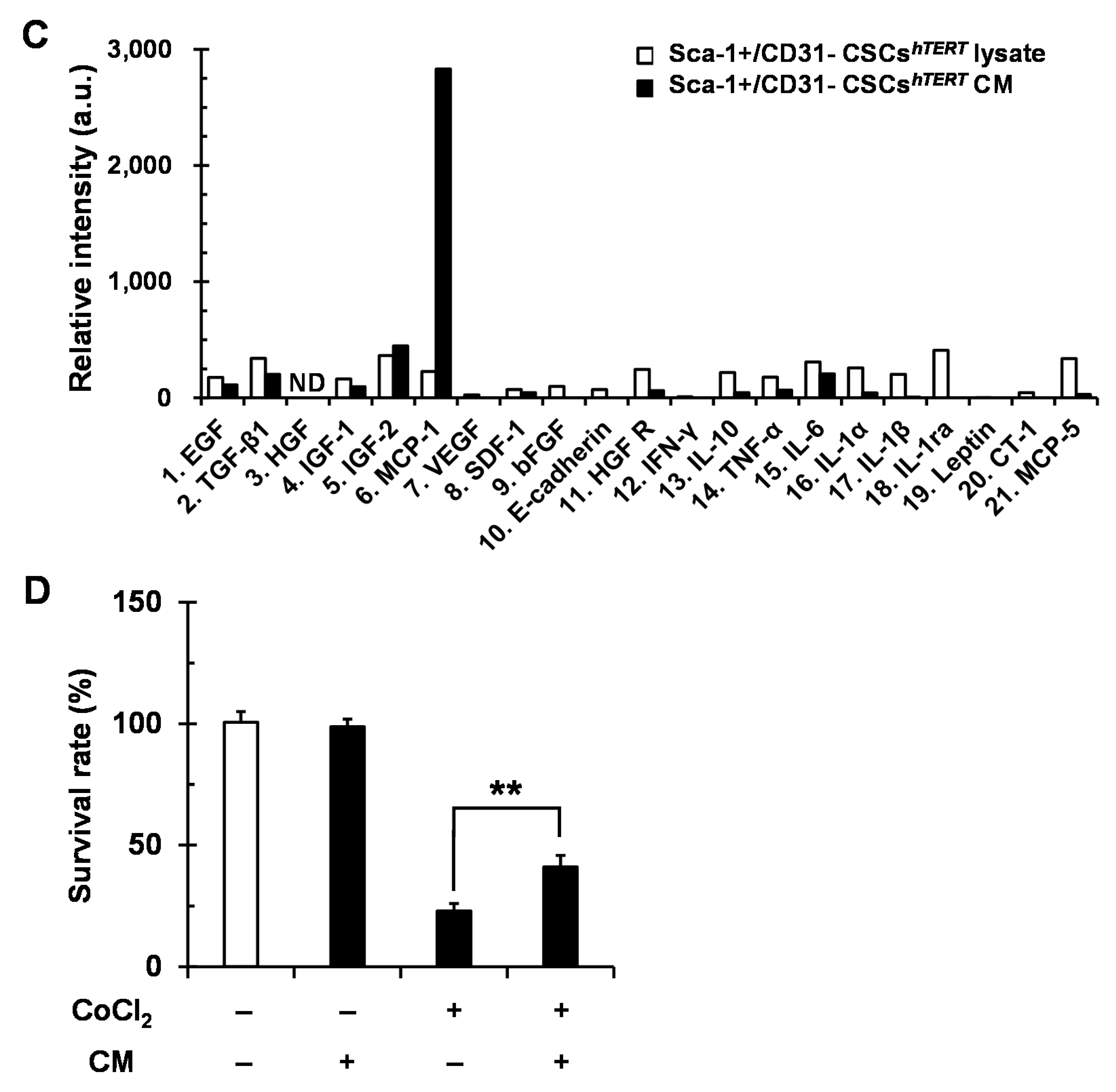
2.4. Sca-1+/CD31− CSCshTERT Conditioned Medium (CM) Protects HL-1 Cardiomyocytes from Cobalt Chloride (CoCl2)-Induced Hypoxic Injury
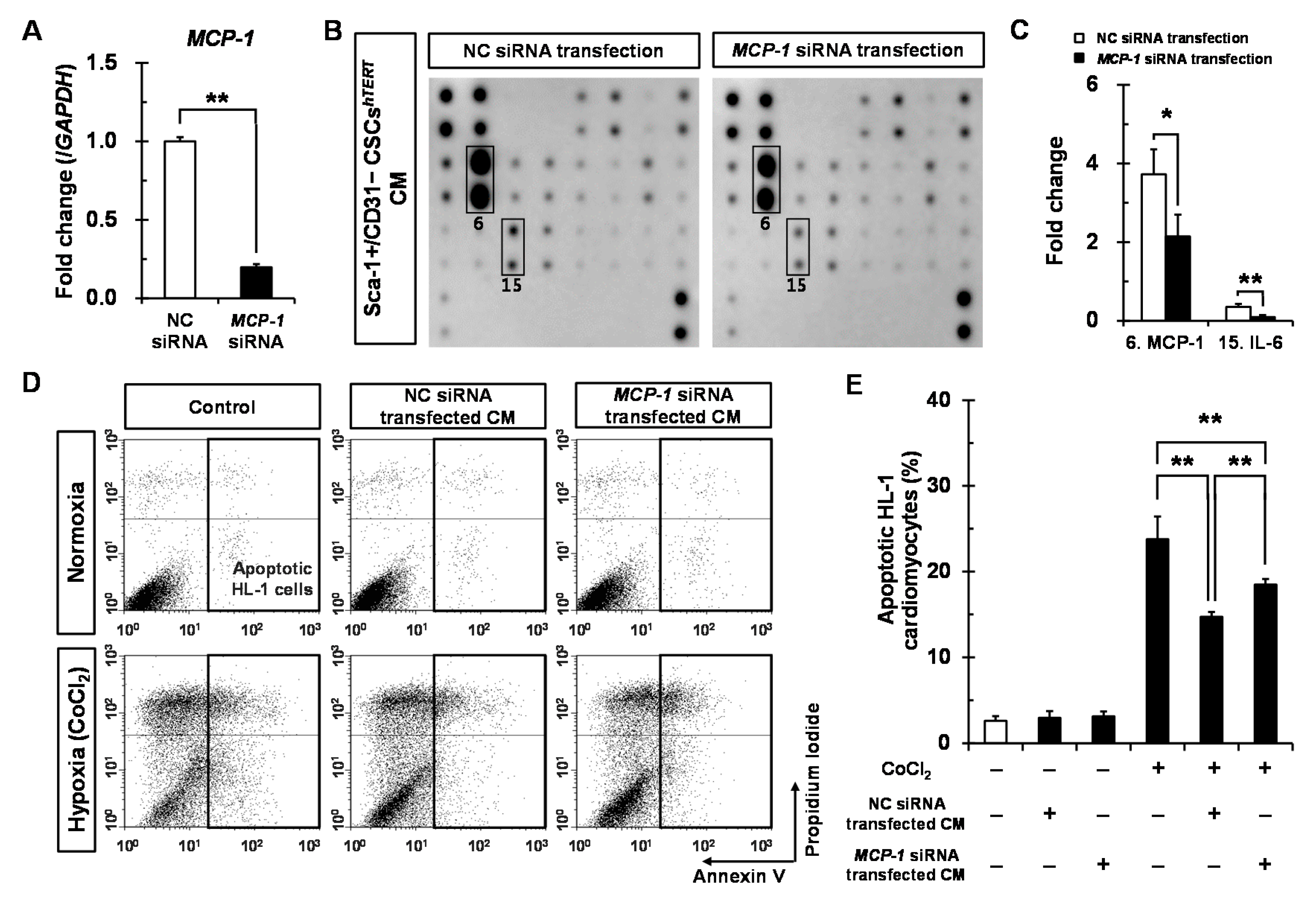
2.5. Sca-1+/CD31− CSCshTERT CM Reduces Hypoxia-Induced Cardiomyocyte Apoptosis Partly via MCP-1-Dependent Mechanism
3. Discussion
4. Material and Methods
4.1. Isolation of Primary Sca-1+ CSCs
4.2. Construction of a Retroviral Vector Encoding hTERT-GFP and Production of Retroviruses
4.3. Generation of Immortalized Sca-1+ CSCs
4.4. Phenotypic Characterization of Sca-1+ CSCs by Immunostaining
4.5. Phenotypic Characterization of Sca-1+ CSCs by Flow Cytometry
4.6. Differentiation Potential of Sca-1+ CSCs
4.7. Real-Time PCR
4.8. WST-1 Proliferation Assay
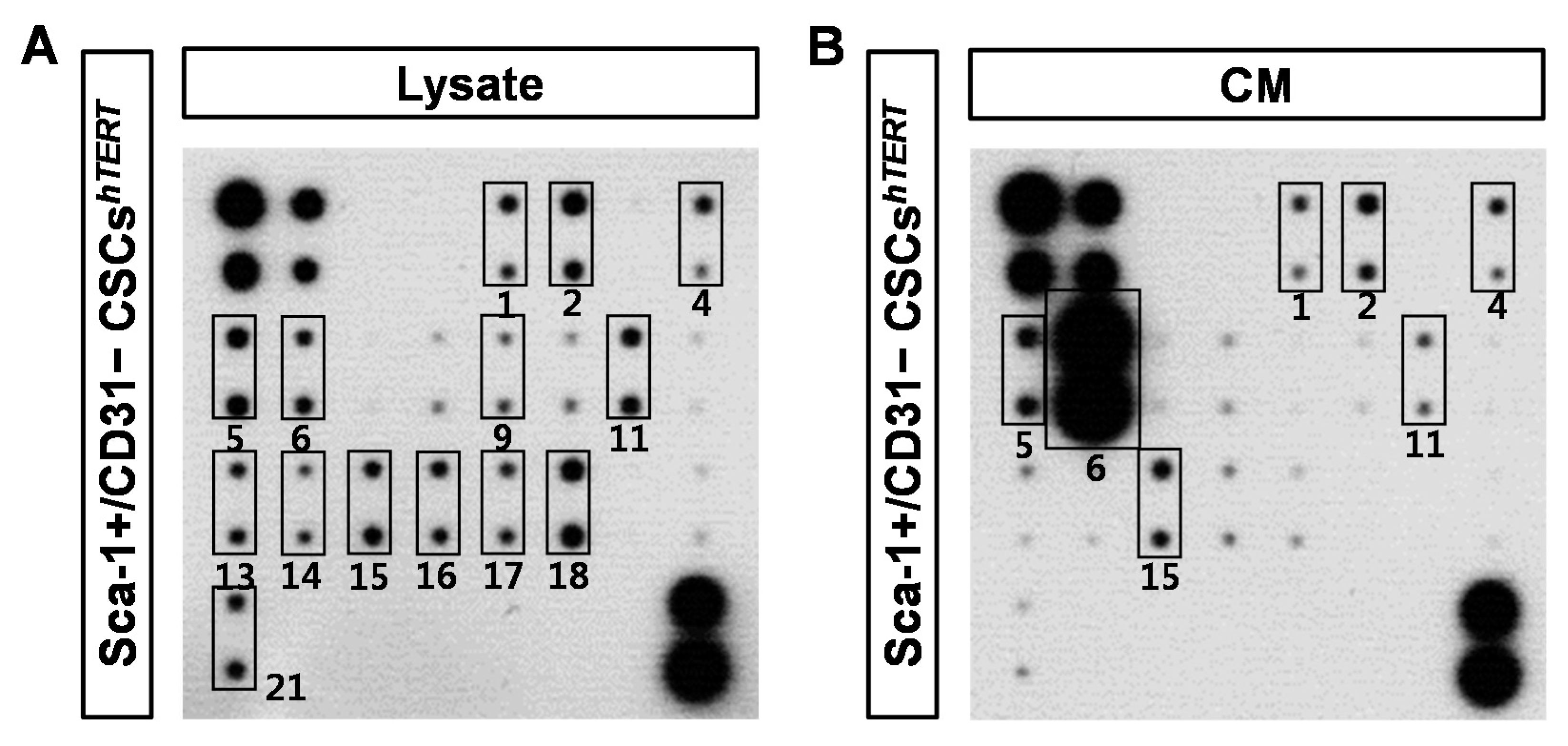
4.9. Antibody Array Detection of Sca-1+ CSCs CM
4.10. Apoptosis Assay of HL-1 Cardiomyocytes
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Nagai, T.; Nishigaki, N.; Oyama, T.; Nishi, J.; Wada, H.; Sano, M.; Toko, H.; Akazawa, H.; Sato, T.; et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 2004, 279, 11384–11391. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Honda, A.; Nagai, T.; Fukushima, N.; Iwanaga, K.; Tokunaga, M.; Shimizu, T.; Okano, T.; Kasanuki, H.; Hagiwara, N.; et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J. Clin. Investig. 2009, 119, 2204–2217. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Haider, K.H.; Ashraf, M. Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration. PLoS ONE 2011, 6, e25265. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.; Feng, B.; Wang, X.; He, X.; Hu, R.; Yin, M.; Wang, W.; Fu, W.; Xu, Z. Isolation and characterization of a Sca-1+/CD31− progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol. 2014, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Ashihara, E.; Takehara, N.; Nomura, T.; Honsho, S.; Nakagami, T.; Morikawa, S.; Takahashi, T.; Ueyama, T.; Matsubara, H.; et al. Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J. Cell Sci. 2007, 120 Pt 10, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Q.; Nakamura, Y.; Lee, J.; Zhang, G.; From, A.H.; Zhang, J. The role of the Sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 2006, 24, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, S.; Goldstein, A.E.; Novitskiy, S.V.; Blackburn, M.R.; Biaggioni, I.; Feoktistov, I. Role of A2B adenosine receptors in regulation of paracrine functions of stem cell antigen 1-positive cardiac stromal cells. J. Pharmacol. Exp. Ther. 2012, 341, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Du, W.; Yu, B. Sca-1-positive cardiac stem cell migration in a cardiac infarction model. Inflammation 2013, 36, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.; Fransioli, J.; Gude, N.A.; Alvarez, R., Jr.; Zhang, X.; Gustafsson, A.B.; Sussman, M.A. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ. Res. 2012, 111, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; de Gaspari, P.; Kostin, S.; Jenniches, K.; Kilic, A.; Izumiya, Y.; Shiojima, I.; Grosse Kreymborg, K.; Renz, H.; Walsh, K.; et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Rep. 2013, 1, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Weinrich, S.L.; Pruzan, R.; Ma, L.; Ouellette, M.; Tesmer, V.M.; Holt, S.E.; Bodnar, A.G.; Lichtsteiner, S.; Kim, N.W.; Trager, J.B.; et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat. Genet. 1997, 17, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Ramboer, E.; de Craene, B.; de Kock, J.; Vanhaecke, T.; Berx, G.; Rogiers, V.; Vinken, M. Strategies for immortalization of primary hepatocytes. J. Hepatol. 2014, 61, 925–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Hu, Q.; Li, X.; Wang, Y.; Lin, C.; Shen, L.; Li, L. Telomerase immortalization of human neural progenitor cells. Neuroreport 2004, 15, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zheng, Q.; Sun, J.; Guo, C.; Yang, J.; Chen, R.; Xu, Y.; Wang, G.; Shen, D.; Pan, Z.; et al. Stabilization of cellular properties and differentiation mutilpotential of human mesenchymal stem cells transduced with hTERT gene in a long-term culture. J. Cell. Biochem. 2008, 103, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, S.C.; Park, C.Y.; Park, J.H.; Choi, J.H.; Joo, H.J.; Hong, S.J.; Lim, D.S. Transplantation of immortalized CD34+ and CD34− adipose-derived stem cells improve cardiac function and mitigate systemic pro-inflammatory responses. PLoS ONE 2016, 11, e0147853. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Drexler, H. Cell therapy for the treatment of coronary heart disease: A critical appraisal. Nat. Rev. Cardiol. 2010, 7, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Damaser, M.S. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Cemborain, A.; Gavira, J.J.; Abizanda, G.; Arana, M.; Casado, M.; Soriano, M.; Hernandez, S.; Moreno, C.; Ecay, M.; et al. Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity. Cell Transplant. 2012, 21, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Jia, L.; Du, J. Heart regeneration, stem cells, and cytokines. Regen. Med. Res. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Boluyt, M.O.; Hipolito, M.L.; Lundberg, M.S.; Zheng, J.S.; O’Neill, L.; Cirielli, C.; Lakatta, E.G.; Crow, M.T. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J. Clin. Investig. 1997, 99, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Okada, S.; Minamino, T.; Iwanaga, K.; Liu, M.L.; Sumida, T.; Nomura, S.; Sahara, N.; Mizoroki, T.; Takashima, A.; et al. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ. Res. 2010, 106, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- An, W.G.; Kanekal, M.; Simon, M.C.; Maltepe, E.; Blagosklonny, M.V.; Neckers, L.M. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 1998, 392, 405–408. [Google Scholar] [PubMed]
- Aikawa, R.; Komuro, I.; Yamazaki, T.; Zou, Y.; Kudoh, S.; Tanaka, M.; Shiojima, I.; Hiroi, Y.; Yazaki, Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J. Clin. Investig. 1997, 100, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Hu, Q.; Suntharalingam, P.; From, A.H.; Zhang, J. Intra-myocardial injection of both growth factors and heart derived Sca-1+/CD31− cells attenuates post-MI LV remodeling more than does cell transplantation alone: Neither intervention enhances functionally significant cardiomyocyte regeneration. PLoS ONE 2014, 9, e95247. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chen, C.L.; Liu, H.C.; Lee, Y.T.; Wang, H.W.; Hou, L.T.; Hung, S.C. Overexpression of hTERT increases stem-like properties and decreases spontaneous differentiation in human mesenchymal stem cell lines. J. Biomed. Sci. 2010, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.B.; Childress, P.; Philip, B.K.; Gerard-O’Riley, R.; Hanlon, M.; Herbert, B.S.; Robling, A.G.; Pavalko, F.M.; Bidwell, J.P. Immortalization and characterization of osteoblast cell lines generated from wild-type and Nmp4-null mouse bone marrow stromal cells using murine telomerase reverse transcriptase (mTERT). J. Cell. Physiol. 2012, 227, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, D.S.; Makhijani, N.S.; Yamaguchi, D.T. Constitutive expression of human telomerase enhances the proliferation potential of human mesenchymal stem cells. BioRes. Open Access 2012, 1, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.G.; Nascimento, D.S.; Forte, G.; Valente, M.; Resende, T.P.; Pagliari, S.; Abreu, C.; Carvalho, I.; di Nardo, P.; Pinto-do, O.P. Stable phenotype and function of immortalized Lin-Sca-1+ cardiac progenitor cells in long-term culture: A step closer to standardization. Stem Cells Dev. 2014, 23, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Cocce, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res. Ther. 2014, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Akimov, S.S.; Ramezani, A.; Hawley, T.S.; Hawley, R.G. Bypass of senescence, immortalization, and transformation of human hematopoietic progenitor cells. Stem Cells 2005, 23, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gu, H.; Yu, Q.; Manukyan, M.C.; Poynter, J.A.; Wang, M. Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS ONE 2011, 6, e29246. [Google Scholar] [CrossRef] [PubMed]
- Pfister, O.; Mouquet, F.; Jain, M.; Summer, R.; Helmes, M.; Fine, A.; Colucci, W.S.; Liao, R. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ. Res. 2005, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Tarzami, S.T.; Cheng, R.; Miao, W.; Kitsis, R.N.; Berman, J.W. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J. Mol. Cell. Cardiol. 2002, 34, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Tarzami, S.T.; Calderon, T.M.; Deguzman, A.; Lopez, L.; Kitsis, R.N.; Berman, J.W. MCP-1/CCL2 protects cardiac myocytes from hypoxia-induced apoptosis by a G(αi)-independent pathway. Biochem. Biophys. Res. Commun. 2005, 335, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Martire, A.; Fernandez, B.; Buehler, A.; Strohm, C.; Schaper, J.; Zimmermann, R.; Kolattukudy, P.E.; Schaper, W. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice mimics ischemic preconditioning through SAPK/JNK1/2 activation. Cardiovasc. Res. 2003, 57, 523–534. [Google Scholar] [CrossRef]
- Yao, H.; Peng, F.; Dhillon, N.; Callen, S.; Bokhari, S.; Stehno-Bittel, L.; Ahmad, S.O.; Wang, J.Q.; Buch, S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J. Neurosci. 2009, 29, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Stroo, I.; Claessen, N.; Teske, G.J.; Butter, L.M.; Florquin, S.; Leemans, J.C. Deficiency for the chemokine monocyte chemoattractant protein-1 aggravates tubular damage after renal ischemia/reperfusion injury. PLoS ONE 2015, 10, e0123203. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, N.; Smith, A.J.; Waring, C.D.; Hasan, M.K.; Miyamoto, S.; Matsuoka, R.; Ellison, G.M. c-kitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling. PLoS ONE 2010, 5, e14297. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Gatti, S.; Sala, V.; Albano, R.; Costelli, P.; Casanova, E.; Comoglio, P.M.; Crepaldi, T. Agonist antibodies activating the Met receptor protect cardiomyoblasts from cobalt chloride-induced apoptosis and autophagy. Cell Death Dis. 2014, 5, e1185. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Frangogiannis, N.G. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007, 74, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Lefer, A.M.; Tsao, P.; Aoki, N.; Palladino, M.A., Jr. Mediation of cardioprotection by transforming growth factor-β. Science 1990, 249, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodriguez, J.; Segarra, M.; Vilardell, C.; Sanchez, M.; Garcia-Martinez, A.; Esteban, M.J.; Grau, J.M.; Urbano-Marquez, A.; Colomer, D.; Kleinman, H.K.; et al. Elevated production of interleukin-6 is associated with a lower incidence of disease-related ischemic events in patients with giant-cell arteritis: Angiogenic activity of interleukin-6 as a potential protective mechanism. Circulation 2003, 107, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, H.; Takahashi, M.; Izawa, A.; Ise, H.; Hongo, M.; Kolattukudy, P.E.; Ikeda, U. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ. Res. 2006, 99, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Das, A.M.; Seideman, J.; Griswold, D.; Afuh, C.N.; Kobayashi, T.; Abe, S.; Fang, Q.; Hashimoto, M.; Kim, H.; et al. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am. J. Respir. Cell Mol. Biol. 2007, 37, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.S.; Neugeboren, B.A.; Mulligan, R.C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 1996, 93, 11400–11406. [Google Scholar] [CrossRef] [PubMed]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-Y.; Choi, S.-C.; Kim, J.-H.; Choi, J.-H.; Joo, H.J.; Hong, S.J.; Lim, D.-S. Cardiac Stem Cell Secretome Protects Cardiomyocytes from Hypoxic Injury Partly via Monocyte Chemotactic Protein-1-Dependent Mechanism. Int. J. Mol. Sci. 2016, 17, 800. https://doi.org/10.3390/ijms17060800
Park C-Y, Choi S-C, Kim J-H, Choi J-H, Joo HJ, Hong SJ, Lim D-S. Cardiac Stem Cell Secretome Protects Cardiomyocytes from Hypoxic Injury Partly via Monocyte Chemotactic Protein-1-Dependent Mechanism. International Journal of Molecular Sciences. 2016; 17(6):800. https://doi.org/10.3390/ijms17060800
Chicago/Turabian StylePark, Chi-Yeon, Seung-Cheol Choi, Jong-Ho Kim, Ji-Hyun Choi, Hyung Joon Joo, Soon Jun Hong, and Do-Sun Lim. 2016. "Cardiac Stem Cell Secretome Protects Cardiomyocytes from Hypoxic Injury Partly via Monocyte Chemotactic Protein-1-Dependent Mechanism" International Journal of Molecular Sciences 17, no. 6: 800. https://doi.org/10.3390/ijms17060800
APA StylePark, C.-Y., Choi, S.-C., Kim, J.-H., Choi, J.-H., Joo, H. J., Hong, S. J., & Lim, D.-S. (2016). Cardiac Stem Cell Secretome Protects Cardiomyocytes from Hypoxic Injury Partly via Monocyte Chemotactic Protein-1-Dependent Mechanism. International Journal of Molecular Sciences, 17(6), 800. https://doi.org/10.3390/ijms17060800