Cancer Stem Cells: The Potential Targets of Chinese Medicines and Their Active Compounds
Abstract
:1. Introduction
2. Cancer Stem Cells (CSCs) Identification and Isolation
3. Recent Research Progress on the Biological Function of CSCs in Tumor Progression
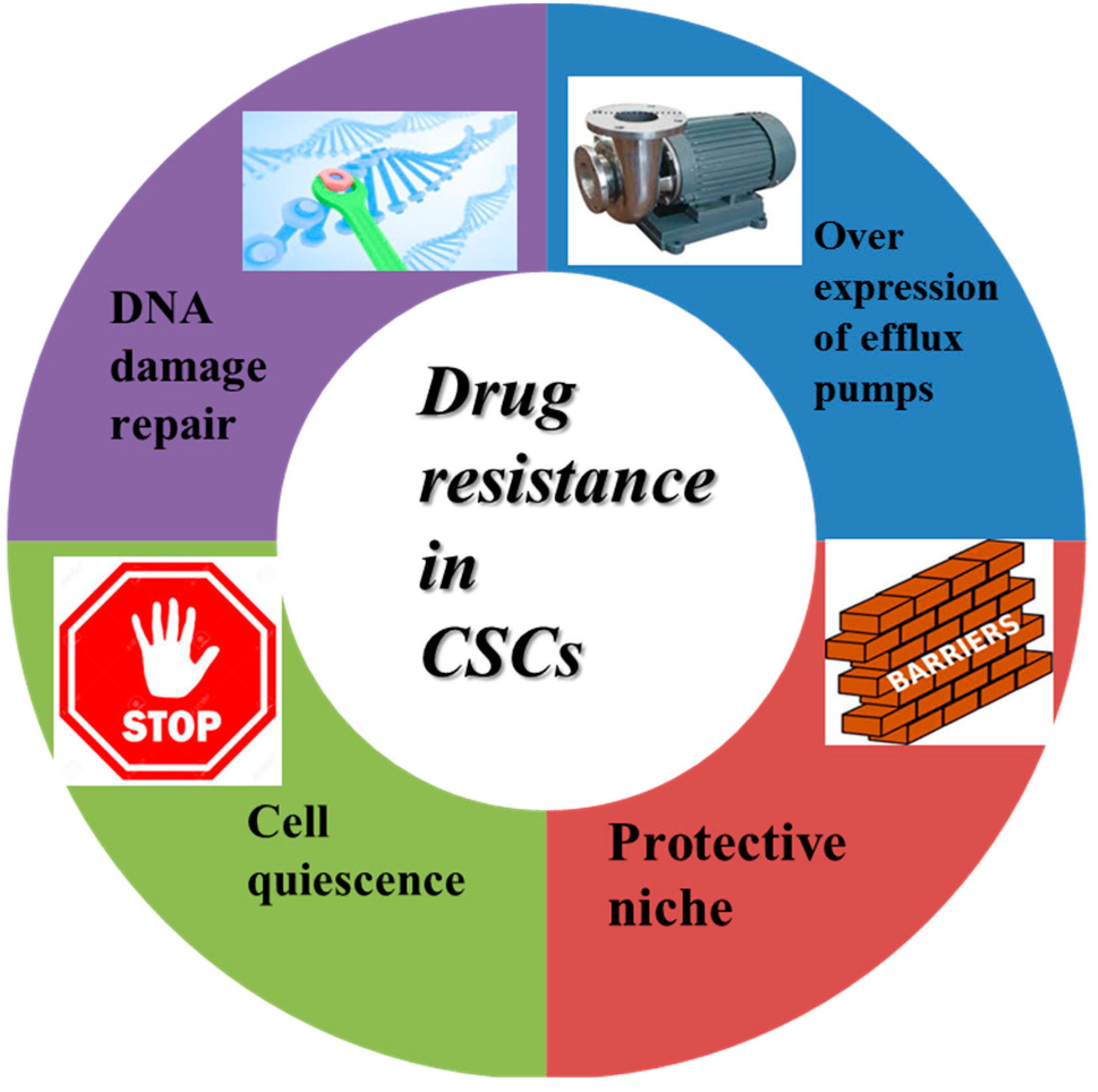
3.1. Drug Resistance-Related Properties of CSCs
3.2. Signaling Pathways Involved in Regulating Proliferation and Cell Death in CSCs
3.3. Inducing Differentiation in CSCs
3.4. CSCs in Tumor Metastasis
3.5. The Role of MicroRNAs in the Regulation of CSCs
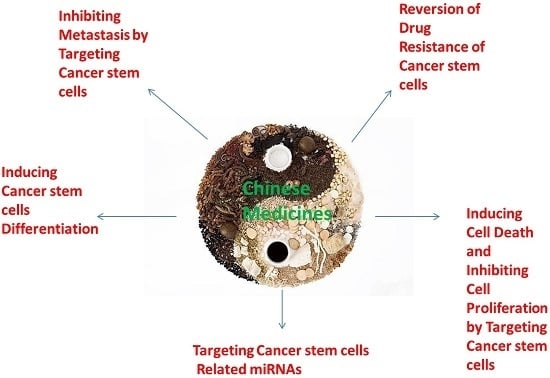
4. Chinese Medicines (CMs) and Their Active Compounds as Potential Therapeutics against CSCs
4.1. CMs and Their Active Compounds for Reversion of Drug Resistance of CSCs
4.2. CMs and Their Active Compounds for Inducing Cell Death and Inhibiting Cell Proliferation by Targeting CSCs
4.3. CMs and Their Active Compounds for Inducing CSCs Differentiation
4.4. CMs and Their Active Compounds for Inhibiting Metastasis by Targeting CSCs
4.5. Targeting CSCs Related miRNAs by CMs and Their Active Compounds
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Matsuo, K.; Fullerton, M.E.; Moeini, A. Treatment patterns and survival outcomes in patients with cervical cancer complicated by complete uterine prolapse: A systematic review of literature. Int. Urogynecol. J. 2016, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.C.; Sousa, J.J.; Pais, A.A. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Schildmann, J.; Baumann, A.; Cakar, M.; Salloch, S.; Vollmann, J. Decisions about limiting treatment in cancer patients: A systematic review and clinical ethical analysis of reported variables. J. Palliat. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rooney, K.P.; McAleese, J.; Crockett, C.; Harney, J.; Eakin, R.L.; Young, V.A.; Dunn, M.A.; Johnston, R.E.; Hanna, G.G. The impact of colleague peer review on the radiotherapy treatment planning process in the radical treatment of lung cancer. Clin. Oncol. 2015, 27, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Livergant, J.; Klein, J.; Witterick, I.; Ringash, J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015, 51, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, H.X. Signal pathways in breast cancer stem cells and the targeted stem cell therapy. Chin. J. Oncol. 2010, 32, 881–885. [Google Scholar]
- Heiden, K.B.; Williamson, A.J.; Doscas, M.E.; Ye, J.; Wang, Y.; Liu, D.; Xing, M.; Prinz, R.A.; Xu, X. The sonic hedgehog signaling pathway maintains the cancer stem cell self-renewal of anaplastic thyroid cancer by inducing snail expression. J. Clin. Endocrinol. Metab. 2014, 99, E2178–E2187. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Seike, M.; Noro, R.; Soeno, C.; Chiba, M.; Zou, F.; Nakamichi, S.; Nishijima, N.; Matsumoto, M.; Miyanaga, A.; et al. Inhibition of ABCB1 overcomes cancer stem cell-like properties and acquired resistance to MET inhibitor in non-small cell lung cancer. Mol. Cancer Ther. 2015, 14, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Hashida, S.; Yamamoto, H.; Shien, K.; Miyoshi, Y.; Ohtsuka, T.; Suzawa, K.; Watanabe, M.; Maki, Y.; Soh, J.; Asano, H.; et al. Acquisition of cancer stem cell-like properties in non-small cell lung cancer with acquired resistance to afatinib. Cancer Sci. 2015, 106, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Di, C.; Zhao, Y. Multiple drug resistance due to resistance to stem cells and stem cell treatment progress in cancer (review). Exp. Ther. Med. 2015, 9, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, E.H. The colon cancer stem cell microenvironment holds keys to future cancer therapy. J. Gastrointest. Surg. 2014, 18, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Heddleston, J.M.; Li, Z.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 2009, 8, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.H.; Gao, X.; Luo, D.; Zhou, X.D.; Xiong, W.; Liu, G.X. EMT and acquisition of stem cell-like properties are involved in spontaneous formation of tumorigenic hybrids between lung cancer and bone marrow-derived mesenchymal stem cells. PLoS ONE 2014, 9, e87893. [Google Scholar] [CrossRef] [PubMed]
- Ansieau, S. EMT in breast cancer stem cell generation. Cancer Lett. 2013, 338, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, L.; Wang, Z.; Wang, L.; Liu, C.; Chen, R.; Zhang, J. MicroRNA expression profile of gastric cancer stem cells in the MKN-45 cancer cell line. Acta Biochim. Biophys. Sin. 2014, 46, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Nan, F.; Ren, F.; Wang, H.; Xu, Y.; Zhang, F. MicroRNA expression profile of colon cancer stem-like cells in HT29 adenocarcinoma cell line. Biochem. Biophys. Res. Commun. 2011, 404, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, G.; Li, X.; Zhang, Y.; Yang, J.; Chang, J.; Sun, X.; Zhou, X.; Guo, Y.; Xu, Y.; et al. Traditional chinese medicine in cancer care: A review of controlled clinical studies published in chinese. PLoS ONE 2013, 8, e60338. [Google Scholar]
- Sotiropoulou, P.A.; Christodoulou, M.S.; Silvani, A.; Herold-Mende, C.; Passarella, D. Chemical approaches to targeting drug resistance in cancer stem cells. Drug Discov. Today 2014, 19, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Marucci, C.; Fumagalli, G.; Calogero, F.; Silvani, A.; Christodoulou, M.S.; Martinet, N.; Passarella, D. Natural products and cancer stem cells. Curr. Pharm. Des. 2015, 21, 5547–5557. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, Y.H.; Chen, J.L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Moghbeli, M.; Moghbeli, F.; Forghanifard, M.M.; Abbaszadegan, M.R. Cancer stem cell detection and isolation. Med. Oncol. 2014, 31. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Rizvanov, A.A.; Cristofanilli, M.; Miftakhova, R.R.; Pestell, R.G. Breast cancer stem cell isolation. Methods Mol. Biol. 2016, 1406, 121–135. [Google Scholar] [PubMed]
- Zhao, Y.; Peng, J.; Zhang, E.; Jiang, N.; Li, J.; Zhang, Q.; Zhang, X.; Niu, Y. CD133 expression may be useful as a prognostic indicator in colorectal cancer, a tool for optimizing therapy and supportive evidence for the cancer stem cell hypothesis: A meta-analysis. Oncotarget 2016, 7, 10023–10036. [Google Scholar] [PubMed]
- Gemei, M.; Mirabelli, P.; di Noto, R.; Corbo, C.; Iaccarino, A.; Zamboli, A.; Troncone, G.; Galizia, G.; Lieto, E.; del Vecchio, L.; et al. CD66c is a novel marker for colorectal cancer stem cell isolation, and its silencing halts tumor growth in vivo. Cancer 2013, 119, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Plesa, A.; Elhamri, M.; Clapisson, G.; Mattei, E.; Gazzo, S.; Hequet, O.; Tigaud, I.; Michallet, M.; Dumontet, C.; Thomas, X. Higher percentage of CD34+ CD38− cells detected by multiparameter flow cytometry from leukapheresis products predicts unsustained complete remission in acute myeloid leukemia. Leuk. Lymphoma 2015, 56, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Yokota, T.; Tanaka, H.; Ichii, M.; Sudo, T.; Satoh, Y.; Doi, Y.; Ueda, T.; Tanimura, A.; Hamanaka, Y.; et al. ESAM is a novel human hematopoietic stem cell marker associated with a subset of human leukemias. Exp. Hematol. 2016, 44, 269.e1–281.e1. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, L.; Zheng, X.; Quan, Y.; Wang, X.; Zhou, X.; Ren, J. A novel molecular marker of breast cancer stem cells identified by cell-selex method. Cancer Biomark. 2015, 15, 163–170. [Google Scholar] [PubMed]
- Cheung, P.F.; Cheung, T.T.; Yip, C.W.; Ng, L.W.; Fung, S.W.; Lo, C.M.; Fan, S.T.; Cheung, S.T. Hepatic cancer stem cell marker granulin-epithelin precursor and β-catenin expression associate with recurrence in hepatocellular carcinoma. Oncotarget 2016. [Google Scholar] [CrossRef]
- Vilchez, V.; Turcios, L.; Zaytseva, Y.; Stewart, R.; Lee, E.Y.; Maynard, E.; Shah, M.B.; Daily, M.F.; Tzeng, C.D.; Davenport, D.; et al. Cancer stem cell marker expression alone and in combination with microvascular invasion predicts poor prognosis in patients undergoing transplantation for hepatocellular carcinoma. Am. J. Surg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Li, Z.J.; Zhang, F.F.; Hou, H.T.; Yu, J.K.; Li, F. Clinical significance of stem cell marker CD133 expression in colorectal cancer. Histol. Histopathol. 2016, 31, 299–306. [Google Scholar] [PubMed]
- Liu, D.; Sun, J.; Zhu, J.; Zhou, H.; Zhang, X.; Zhang, Y. Expression and clinical significance of colorectal cancer stem cell marker EPCAM/CD44 in colorectal cancer. Oncol. Lett. 2014, 7, 1544–1548. [Google Scholar] [PubMed]
- Salnikov, A.V.; Gladkich, J.; Moldenhauer, G.; Volm, M.; Mattern, J.; Herr, I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int. J. Cancer. 2010, 126, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, C.; Wang, Z.; Liu, Z.; Liu, H.; Wang, T. Nanog, a novel prognostic marker for lung cancer. Surg. Oncol. 2013, 22, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, B. The prognostic role of the cancer stem cell marker aldehyde dehydrogenase 1 in head and neck squamous cell carcinomas: A meta-analysis. Oral Oncol. 2014, 50, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Linge, A.; Lock, S.; Gudziol, V.; Nowak, A.; Lohaus, F.; von Neubeck, C.; Jutz, M.; Abdollahi, A.; Debus, J.; Tinhofer, I.; et al. Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV(-) HNSCC after postoperative radiochemotherapy: A multicenter study of the DKTK-ROG. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Selcuk, F.; Rutgen, B.C.; Moulay, M.; Willenbrock, S.; Hammer, S.E.; Sterenczak, K.A.; Junghanss, C.; Hewicker-Trautwein, M.; Nolte, I.; et al. Evaluation of stem cell marker expression in canine B-cell lymphoma cell lines, B-cell lymphoma-generated spheres and primary samples. Anticancer Res. 2015, 35, 2805–2816. [Google Scholar] [PubMed]
- Hardingham, J.E.; Kotasek, D.; Sage, R.E.; Gooley, L.T.; Mi, J.X.; Dobrovic, A.; Norman, J.E.; Bolton, A.E.; Dale, B.M. Significance of molecular marker-positive cells after autologous peripheral-blood stem-cell transplantation for non-Hodgkin’s lymphoma. J. Clin. Oncol. 1995, 13, 1073–1079. [Google Scholar] [PubMed]
- Svachova, H.; Pour, L.; Sana, J.; Kovarova, L.; Raja, K.R.; Hajek, R. Stem cell marker nestin is expressed in plasma cells of multiple myeloma patients. Leuk. Res. 2011, 35, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Robillard, N.; Jego, G.; Pellat-Deceunynck, C.; Pineau, D.; Puthier, D.; Mellerin, M.P.; Barille, S.; Rapp, M.J.; Harousseau, J.L.; Amiot, M.; et al. CD28, a marker associated with tumoral expansion in multiple myeloma. Clin. Cancer Res. 1998, 4, 1521–1526. [Google Scholar] [PubMed]
- Isfoss, B.L.; Busch, C.; Hermelin, H.; Vermedal, A.T.; Kile, M.; Braathen, G.J.; Majak, B.; Berner, A. Stem cell marker-positive stellate cells and mast cells are reduced in benign-appearing bladder tissue in patients with urothelial carcinoma. Virchows Arch. 2014, 464, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Shabahang, M.; Buras, R.R.; Davoodi, F.; Schumaker, L.M.; Nauta, R.J.; Evans, S.R. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. 1993, 53, 3712–3718. [Google Scholar] [PubMed]
- Trepant, A.L.; Bouchart, C.; Rorive, S.; Sauvage, S.; Decaestecker, C.; Demetter, P.; Salmon, I. Identification of OLIG2 as the most specific glioblastoma stem cell marker starting from comparative analysis of data from similar DNA chip microarray platforms. Tumour Biol. 2015, 36, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Richichi, C.; Brescia, P.; Alberizzi, V.; Fornasari, L.; Pelicci, G. Marker-independent method for isolating slow-dividing cancer stem cells in human glioblastoma. Neoplasia 2013, 15, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Wang, H.L.; Lin, Y.S.; Kumar, K.P.; Lin, H.C.; Chang, C.J.; Lu, C.C.; Huang, T.T.; Martel, J.; Ojcius, D.M.; et al. Identification of CD24 as a cancer stem cell marker in human nasopharyngeal carcinoma. PLoS ONE 2014, 9, e99412. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liao, S.; Zhu, G.; Luo, G.; Xiao, S.; He, J.; Pei, Z.; Li, G.; Zhou, Y. CD38 is a putative functional marker for side population cells in human nasopharyngeal carcinoma cell lines. Mol. Carcinog. 2016, 55, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, L.; Rayar, M.; Turlin, B.; Boucher, E.; Bellaud, P.; Desille, M.; Meunier, B.; Clement, B.; Boudjema, K.; Coulouarn, C. Epithelial cell adhesion molecule is a prognosis marker for intrahepatic cholangiocarcinoma. J. Surg. Res. 2014, 192, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Yang, H.G.; Kang, M.C.; Lee, S.; Namkoong, H.; Lee, S.W.; Sung, Y.C. KIAA1114, a full-length protein encoded by the trophinin gene, is a novel surface marker for isolating tumor-initiating cells of multiple hepatocellular carcinoma subtypes. Oncotarget 2014, 5, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Borst, P. Cancer drug pan-resistance: Pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open Biol. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, V.S.; Donnenberg, A.D. Multiple drug resistance in cancer revisited: The cancer stem cell hypothesis. J. Clin. Pharmacol. 2005, 45, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Morozevich, G.E.; Kozlova, N.I.; Ushakova, N.A.; Preobrazhenskaia, M.E.; Berman, A.E. Implication of integrin alpha5beta1 in human breast carcinoma apoptosis and drug resistance. Biomed. Khimiia 2011, 57, 77–84. (In Russian) [Google Scholar] [CrossRef]
- Morozevich, G.E.; Kozlova, N.I.; Susova, O.Y.; Karalkin, P.A.; Berman, A.E. Implication of alpha2beta1 integrin in anoikis of MCF-7 human breast carcinoma cells. Biochemistry 2015, 80, 97–103. [Google Scholar] [PubMed]
- Zobalova, R.; McDermott, L.; Stantic, M.; Prokopova, K.; Dong, L.F.; Neuzil, J. CD133-positive cells are resistant to TRAIL due to up-regulation of FLIP. Biochem. Biophys. Res. Commun. 2008, 373, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 2012, 11, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5. [Google Scholar] [CrossRef]
- Ginestier, C.; Liu, S.; Diebel, M.E.; Korkaya, H.; Luo, M.; Brown, M.; Wicinski, J.; Cabaud, O.; Charafe-Jauffret, E.; Birnbaum, D.; et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Investig. 2010, 120, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo, C.; di Bartolomeo, S.; Cecconi, F. Autophagy in stem and progenitor cells. Cell. Mol. Life Sci. 2016, 73, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Bhattacharyya, S.; Singh, S.K. Autophagy in cancer stem cells: A potential link between chemoresistance, recurrence, and metastasis. Biores. Open Access 2015, 4, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.Y.; Wei, T.Z.; Luo, Q.C.; Wu, Q.W.; Liu, Q.F.; Yang, M.; Ye, G.D.; Wu, J.F.; Chen, Y.Y.; Sun, G.B.; et al. The Wnt-β-catenin pathway represses let-7 microRNA expression through transactivation of Lin28 to augment breast cancer stem cell expansion. J. Cell Sci. 2013, 126, 2877–2889. [Google Scholar] [CrossRef] [PubMed]
- Dodge, M.E.; Lum, L. Drugging the cancer stem cell compartment: Lessons learned from the hedgehog and Wnt signal transduction pathways. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Liu, S.; Wicha, M.S. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Investig. 2011, 121, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. An epigenetic switch involving NF-κB, Lin28, let-7 microRNA, and IL6 links inflammation to cell transformation. Cell 2009, 139, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ginestier, C.; Ou, S.J.; Clouthier, S.G.; Patel, S.H.; Monville, F.; Korkaya, H.; Heath, A.; Dutcher, J.; Kleer, C.G.; et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011, 71, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nanta, R.; Sharma, J.; Gunewardena, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget 2015, 6, 32039–32060. [Google Scholar] [PubMed]
- Xia, P.; Xu, X.Y. PI3k/AKT/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar] [PubMed]
- Campos, B.; Wan, F.; Farhadi, M.; Ernst, A.; Zeppernick, F.; Tagscherer, K.E.; Ahmadi, R.; Lohr, J.; Dictus, C.; Gdynia, G.; et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin. Cancer Res. 2010, 16, 2715–2728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.C.; Zhu, Y.S.; Shi, Y. New hope for cancer treatment: Exploring the distinction between normal adult stem cells and cancer stem cells. Pharmacol. Ther. 2008, 119, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Taddei, A.; Roche, D.; Bickmore, W.A.; Almouzni, G. The effects of histone deacetylase inhibitors on heterochromatin: Implications for anticancer therapy? EMBO Rep. 2005, 6, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Zhou, X.; Xu, W.S.; Scher, H.I.; Rifkind, R.A.; Marks, P.A.; Richon, V.M. The histone deacetylase inhibitor saha arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc. Natl. Acad. Sci. USA 2002, 99, 11700–11705. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, S.G.; Reynolds, B.A.; Zanetti, N.; Lamorte, G.; Binda, E.; Broggi, G.; Brem, H.; Olivi, A.; Dimeco, F.; Vescovi, A.L. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 2006, 444, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, D.; Zhao, N.; Zhao, X. Epithelial-to-endothelial transition and cancer stem cells: Two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Kotiyal, S.; Bhattacharya, S. Epithelial mesenchymal transition and vascular mimicry in breast cancer stem cells. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Abell, A.N.; Johnson, G.L. Implications of mesenchymal cells in cancer stem cell populations: Relevance to EMT. Curr. Pathobiol. Rep. 2014, 2, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Xin, X.; Liu, L.; Girish, G.V.; Lala, P.K. Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. 2014, 105, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Pandit, T.S.; Kennette, W.; Mackenzie, L.; Zhang, G.; Al-Katib, W.; Andrews, J.; Vantyghem, S.A.; Ormond, D.G.; Allan, A.L.; Rodenhiser, D.I.; et al. Lymphatic metastasis of breast cancer cells is associated with differential gene expression profiles that predict cancer stem cell-like properties and the ability to survive, establish and grow in a foreign environment. Int. J. Oncol. 2009, 35, 297–308. [Google Scholar] [PubMed]
- Wakamatsu, Y.; Sakamoto, N.; Oo, H.Z.; Naito, Y.; Uraoka, N.; Anami, K.; Sentani, K.; Oue, N.; Yasui, W. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol. Int. 2012, 62, 112–119. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS is regulated by the let-7 microRNA family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, H.W.; Lu, M.H.; He, X.H.; Li, Y.; Gu, H.; Liu, M.F.; Wang, E.D. MicroRNA-155 functions as an oncomir in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 2010, 70, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Mavrakis, K.J.; Wolfe, A.L.; Oricchio, E.; Palomero, T.; de Keersmaecker, K.; McJunkin, K.; Zuber, J.; James, T.; Khan, A.A.; Leslie, C.S.; et al. Genome-wide RNA-mediated interference screen identifies miR-19 targets in Notch-induced T-cell acute lymphoblastic leukaemia. Nat. Cell Biol. 2010, 12, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Poliseno, L.; Song, M.S.; Ala, U.; Webster, K.; Ng, C.; Beringer, G.; Brikbak, N.J.; Yuan, X.; Cantley, L.C.; et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 2013, 154, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Cheung, K.C.; Cheung, Y.C.; Man, K.; Lui, V.W.; Tsao, S.W.; Feng, Y. Berberine suppresses ID-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim. Biophys. Acta 2015, 1852, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, M.; Wang, X.; Tan, H.Y.; Tsao, S.W.; Feng, Y. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of miR-23a in hepatocellular carcinoma. Biochim. Biophys. Acta 2014, 1839, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Cheung, Y.C.; Lui, V.W.; Yip, Y.L.; Zhang, G.; Lin, V.W.; Cheung, K.C.; Feng, Y.; Tsao, S.W. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer 2013, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Feng, Y.; Zhu, M.; Tsang, C.M.; Man, K.; Tong, Y.; Tsao, S.W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010, 111, 1426–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Feng, Y.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y. Berberine and Coptidis Rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.B.; Ko, E.; Han, W.; Shin, I.; Park, S.Y.; Noh, D.Y. Berberine diminishes the side population and ABCG2 transporter expression in MCF-7 breast cancer cells. Planta Med. 2008, 74, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, J.; Zhang, C.X.; Li, X.Y.; Li, N.; Ju, R.J.; Shi, J.F.; Sun, M.G.; Zhao, W.Y.; Mu, L.M.; et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials 2013, 34, 4452–4465. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.J.; Jin, B.; Chen, X.W.; Xie, J.; Xu, H.M.; Dong, P. Oxymatrine downregulates HPV16E7 expression and inhibits cell proliferation in laryngeal squamous cell carcinoma Hep-2 cells in vitro. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S.; Chen, J.; Ren, P.; Hu, Y.; Cao, Z.; Sun, H.; Ding, Y. Oxymatrine induces mitochondria dependent apoptosis in human osteosarcoma MNNG/HOS cells through inhibition of PI3K/AKT pathway. Tumour Biol. 2014, 35, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, Y.; Xu, G.; Yang, F.; Guan, Z.; Wang, M.; Fang, Y. Oxymatrine extracted from Sophora flavescens inhibited cell growth and induced apoptosis in human osteosarcoma MG-63 cells in vitro. Cell Biochem. Biophys. 2014, 70, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Piao, B.; Zhang, Y.; Hua, B.; Hou, W.; Xu, W.; Qi, X.; Zhu, X.; Pei, Y.; Lin, H. Oxymatrine diminishes the side population and inhibits the expression of β-catenin in MCF-7 breast cancer cells. Med. Oncol. 2011, 28 (Suppl. 1), S99–S107. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.G.; Liu, L.P.; Li, C.Y.; Zhang, M.; Chen, Y.; Qin, J.; Gu, Y.Y.; Li, Z.; Wu, X.L.; Mo, S.L. Scutellaria extract decreases the proportion of side population cells in a myeloma cell line by down-regulating the expression of ABCG2 protein. Asian Pac. J. Cancer Prev. 2013, 14, 7179–7186. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Y.; Liu, L.P.; Qin, J.; Zhang, M.; Chen, Y.; Wang, D.; Li, Z.; Tang, J.Z.; Mo, S.L. Baicalein decreases side population proportion via inhibition of ABCG2 in multiple myeloma cell line RPMI 8226 in vitro. Fitoterapia 2014, 94, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhao, Y.; Zhan, H.; Wei, X.; Liu, T.; Zheng, B. Bufalin inhibits the differentiation and proliferation of human osteosarcoma cell line hMG63-derived cancer stem cells. Tumour Biol. 2014, 35, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sun, H.; Ma, G.; Wang, Z.; Li, E.; Liu, Y.; Liu, Y. Bufalin induces lung cancer cell apoptosis via the inhibition of PI3K/AKT pathway. Int. J. Mol. Sci. 2012, 13, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.H.; Liu, X.; Qiu, Y.Y.; Cai, J.F.; Qin, J.M.; Zhu, H.R.; Li, Q. Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: New hope for cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.H.; Yao, C.A.; Lin, C.H.; Chang, K.J. Safety and efficacy of Tien-Hsien Liquid practical in patients with refractory metastatic breast cancer: A randomized, double-blind, placebo-controlled, parallel-group, phase IIa trial. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Chia, J.S.; Chiang, C.P.; Hsuen, S.P.; Du, J.L.; Wu, C.W.; Wang, W.B. The chinese herbal medicine Tien-Hsien Liquid inhibits cell growth and induces apoptosis in a wide variety of human cancer cells. J. Altern. Complement. Med. 2005, 11, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Chia, J.S.; Wang, W.B.; Chiang, C.P. Immunomodulating effects of “Tien-Hsien liquid” on peripheral blood mononuclear cells and T-lymphocytes from patients with recurrent aphthous ulcerations. Am. J. Chin. Med. 2004, 32, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.; Du, J.L.; Hsu, W.B.; Sun, A.; Chiang, C.P.; Wang, W.B. Inhibition of metastasis, angiogenesis, and tumor growth by chinese herbal cocktail Tien-Hsien Liquid. BMC Cancer 2010, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.J.; Yang, C.M.; Chuang, S.E.; Yan, J.L.; Liu, C.Y.; Chen, S.W.; Yan, K.H.; Lai, T.Y.; Lai, G.M. Targeting PML-RARalpha and oncogenic signaling pathways by chinese herbal mixture Tien-Hsien Liquid in acute promyelocytic leukemia NB4 cells. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.J.; Yeh, C.T.; Lee, L.M.; Chuang, S.E.; Yeh, C.F.; Chao, W.J.; Lai, T.Y.; Lai, G.M. Elimination of cancer stem-like “side population” cells in hepatoma cell lines by Chinese herbal mixture “Tien-Hsien Liquid”. Evid. Based Complement. Altern. Med. 2012, 2012, 617085. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Z.; Huang, J.; Xie, S.; Liu, T.; Mao, Z. Blocking the notch pathway can inhibit the growth of CD133-positive A549 cells and sensitize to chemotherapy. Biochem. Biophys. Res. Commun. 2014, 444, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Chen, Y.; Hong, F.; Lin, J.; Wei, L.; Hong, Z.; Sferra, T.J.; Peng, J. Pien Tze Huang suppresses IL-6-inducible STAT3 activation in human colon carcinoma cells through induction of SOCS3. Oncol. Rep. 2012, 28, 2125–2130. [Google Scholar] [PubMed]
- Zhuang, Q.; Hong, F.; Shen, A.; Zheng, L.; Zeng, J.; Lin, W.; Chen, Y.; Sferra, T.J.; Hong, Z.; Peng, J. Pien Tze Huang inhibits tumor cell proliferation and promotes apoptosis via suppressing the STAT3 pathway in a colorectal cancer mouse model. Int. J. Oncol. 2012, 40, 1569–1574. [Google Scholar] [PubMed]
- Wei, L.; Chen, P.; Chen, Y.; Shen, A.; Chen, H.; Lin, W.; Hong, Z.; Sferra, T.J.; Peng, J. Pien Tze Huang suppresses the stem-like side population in colorectal cancer cells. Mol. Med. Rep. 2014, 9, 261–266. [Google Scholar] [PubMed]
- Wang, S.; Moonasar, N.; Xiao, X.; Yin, T.; Weinreb, R.N.; Sun, X. Effect of resveratrol-based nutritional supplement on choroidal thickness: A pilot study. Curr. Eye Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chen, Y.T.; Chiu, C.C.; Liao, W.T.; Liu, Y.C.; David Wang, H.M. Polygonum cuspidatum extracts as bioactive antioxidaion, anti-tyrosinase, immune stimulation and anticancer agents. J. Biosci. Bioeng. 2015, 119, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Shim, J.H.; Jeon, J.G.; Choi, K.H.; Choi, E.S.; Cho, N.P.; Cho, S.D. Apoptotic effect of polygonum cuspidatum in oral cancer cells through the regulation of specificity protein 1. Oral Dis. 2011, 17, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Nall, D.; Tang, S.N.; Meeker, D.; Passarini, J.; Sharma, J.; Srivastava, R.K. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KRASG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS ONE 2011, 6, e16530. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, S.; Sokolowski, K.; Kunnimalaiyaan, S.; Gamblin, T.C.; Kunnimalaiyaan, M. Curcumin-mediated regulation of Notch1/hairy and enhancer of split-1/survivin: Molecular targeting in cholangiocarcinoma. J. Surg. Res. 2015, 198, 434–440. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, Y.; Li, H.; Li, P.K.; Fuchs, J.; Shibata, H.; Iwabuchi, Y.; Lin, J. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br. J. Cancer 2011, 105, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Vadgama, J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NF-κB signaling. Anticancer Res. 2015, 35, 39–46. [Google Scholar] [PubMed]
- Qi, F.; Wei, L.; Shen, A.; Chen, Y.; Lin, J.; Chu, J.; Cai, Q.; Pan, J.; Peng, J. Pien Tze Huang inhibits the proliferation, and induces the apoptosis and differentiation of colorectal cancer stem cells via suppression of the Notch1 pathway. Oncol. Rep. 2016, 35, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Omene, C.O.; Wu, J.; Frenkel, K. Caffeic Acid Phenethyl Ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Investig. New Drugs 2012, 30, 1279–1288. [Google Scholar]
- Qing, Y.; Hu, H.; Liu, Y.; Feng, T.; Meng, W.; Jiang, L.; Sun, Y.; Yao, Y. Berberine induces apoptosis in human multiple myeloma cell line U266 through hypomethylation of p53 promoter. Cell Biol. Int. 2014, 38, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Yip, N.K.; Ho, W.S. Berberine induces apoptosis via the mitochondrial pathway in liver cancer cells. Oncol. Rep. 2013, 30, 1107–1112. [Google Scholar] [PubMed]
- Yang, X.; Huang, N. Berberine induces selective apoptosis through the ampkmediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol. Med. Rep. 2013, 8, 505–510. [Google Scholar] [PubMed]
- Tang, J.W.; Lin, J. Apoptosis of MDA-MB-231 cells induced by berberine alpha-hydroxy beta-decanoylethyl sulfonate. Acta Pharm. Sin. 2014, 49, 131–135. [Google Scholar]
- Nesaretnam, K.; Lim, E.J.; Reimann, K.; Lai, L.C. Effect of a carotene concentrate on the growth of human breast cancer cells and pS2 gene expression. Toxicology 2000, 151, 117–126. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, Z.; Bai, L.; Shi, Z.; Zhao, W.E.; Zhao, B. β-carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor γ expression and reactive oxygen species production in MCF-7 cancer cells. Eur. J. Cancer 2007, 43, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, J.; Lim, J.W.; Kim, H. β-carotene-induced apoptosis is mediated with loss of Ku proteins in gastric cancer AGS cells. Genes Nutr. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, Y.S.; Kim, K.M.; Min, S.J.; Kim, Y. β-carotene inhibits neuroblastoma tumorigenesis by regulating cell differentiation and cancer cell stemness. Biochem. Biophys. Res. Commun. 2014, 450, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sung, J.H.; Chung, N. Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PACA-2. Mol. Cell. Biochem. 2014, 394, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Teshima, T.; Ito, Y.; Kawaguchi, Y.; Konishi, K.; Takahashi, H.; Ohigashi, H.; Oshima, K.; Araki, N.; Nishiyama, K.; et al. Risk factors for vertebral compression fractures in preoperative chemoradiotherapy with gemcitabine for pancreatic cancer. Radiother. Oncol. 2016, 118, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.L.; Wu, X.J.; Liu, Y.; Xie, J.Q. Berberine counteracts enhanced IL-8 expression of AGS cells induced by evodiamine. Life Sci. 2013, 93, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.B.; Wu, M.F.; Lu, H.F.; Chou, J.; Au, M.K.; Liao, N.C.; Chang, C.H.; Huang, Y.P.; Wu, C.T.; Chung, J.G. Toxicological evaluation of Antrodia cinnamomea in BALB/C mice. In Vivo 2013, 27, 739–745. [Google Scholar] [PubMed]
- Chang, C.W.; Chen, C.C.; Wu, M.J.; Chen, Y.S.; Chen, C.C.; Sheu, S.J.; Lin, T.W.; Chou, S.H.; Lin, S.C.; Liu, C.J.; et al. Active component of Antrodia cinnamomea mycelia targeting head and neck cancer initiating cells through exaggerated autophagic cell death. Evid. Based Complement. Altern. Med. 2013, 2013, 946451. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Wei, P.K.; Yue, B.L. Study on the mechanism of Xiaotan Sanjie Recipe for inhibiting proliferation of gastric cancer cells. J. Tradit. Chin. Med. 2010, 30, 249–253. [Google Scholar] [CrossRef]
- Shi, J.; Wei, P.K. Xiaotan Sanjie decoction inhibits interleukin-8-induced metastatic potency in gastric cancer. World J. Gastroenterol. 2015, 21, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, Y.J.; Wei, P.K. Effects of Xiaotan Sanjie Recipe on vasculogenic mimicry of human gastric cancer xenografts in nude mice. Chin. J. Integr. Tradit. West. Med. 2011, 31, 532–536. [Google Scholar]
- Gui, M.W.; Wei, P.K.; Lu, Y.; Guo, W.; Qin, Z.F.; Sun, D.Z. Effects of Xiaotan Sanjie decoction-containing serum on proliferation and apoptosis of human gastric cancer cells MKN-45. J. Chin. Integr. Med. 2010, 8, 250–255. [Google Scholar] [CrossRef]
- Yan, B.; Liu, L.; Zhao, Y.; Xiu, L.J.; Sun, D.Z.; Liu, X.; Lu, Y.; Shi, J.; Zhang, Y.C.; Li, Y.J.; et al. Xiaotan Sanjie decoction attenuates tumor angiogenesis by manipulating Notch-1-regulated proliferation of gastric cancer stem-like cells. World J. Gastroenterol. 2014, 20, 13105–13118. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhou, Y.; Feng, S.; Lv, C.; Xiu, L.; Zhang, Y.; Shi, J.; Li, Y.; Wei, P.; Qin, Z. β-elemene-attenuated tumor angiogenesis by targeting Notch-1 in gastric cancer stem-like cells. Evid. Based Complement. Altern. Med. 2013, 2013, 268468. [Google Scholar] [CrossRef] [PubMed]
- Rak, J. Is cancer stem cell a cell, or a multicellular unit capable of inducing angiogenesis? Med. Hypotheses 2006, 66, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, H.; Liu, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/AKT/NF-κB signaling pathway. Nutrients 2015, 7, 4383–4402. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Ha, A.W.; Kim, W.K. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr. Res. Pract. 2012, 6, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Huang, Y.L.; Chu, Y.L.; Ho, C.T.; Chung, J.G.; Lai, C.I.; Su, Y.C.; Kuo, Y.H.; Sheen, L.Y. Antcin K, an active triterpenoid from the fruiting bodies of basswood-cultivated Antrodia cinnamomea, inhibits metastasis via suppression of integrin-mediated adhesion, migration, and invasion in human hepatoma cells. J. Agric. Food Chem. 2015, 63, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y.; Wu, C.C.; Chia, C.H.; Hsu, S.L.; Tzeng, Y.M. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Lett. 2016, 373, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Liu, Y.K.; Lan, K.L.; Lee, Y.W.; Tsai, T.H.; Chen, Y.J. Medicinal fungus antrodia cinnamomea inhibits growth and cancer stem cell characteristics of hepatocellular carcinoma. Evid. Based Complement. Altern. Med. 2013, 2013, 569737. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, R.Z.; Zhang, L.; Zhao, X.Y. Berbamine, a novel nuclear factor κB inhibitor, inhibits growth and induces apoptosis in human myeloma cells. Acta Pharmacol. Sin. 2009, 30, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; He, Z.W.; Wu, D.; Xu, R.Z. Berbamine selectively induces apoptosis of human acute promyelocytic leukemia cells via survivin-mediated pathway. Chin. Med. J. 2007, 120, 802–806. [Google Scholar] [PubMed]
- Wang, G.Y.; Zhang, J.W.; Lu, Q.H.; Xu, R.Z.; Dong, Q.H. Berbamine induces apoptosis in human hepatoma cell line SMMC7721 by loss in mitochondrial transmembrane potential and caspase activation. J. Zhejiang Univ. Sci. B 2007, 8, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Nam, S.; Brown, C.E.; Zhao, R.; Starr, R.; Ma, Y.; Xie, J.; Horne, D.A.; Malkas, L.H.; Jove, R.; et al. A novel berbamine derivative inhibits cell viability and induces apoptosis in cancer stem-like cells of human glioblastoma, via up-regulation of miRNA-4284 and JNK/AP-1 signaling. PLoS ONE 2014, 9, e94443. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ahmad, A.; Banerjee, S.; Padhye, S.; Dominiak, K.; Schaffert, J.M.; Wang, Z.; Philip, P.A.; Sarkar, F.H. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010, 70, 3606–3617. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ali, S.; Banerjee, S.; Wang, Z.; Logna, F.; Azmi, A.S.; Kong, D.; Ahmad, A.; Li, Y.; Padhye, S.; et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012, 72, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ahmad, A.; Kong, D.; Ali, S.; Azmi, A.S.; Li, Y.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS ONE 2012, 7, e43726. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ali, S.; Kong, D.; Sarkar, S.H.; Wang, Z.; Banerjee, S.; Aboukameel, A.; Padhye, S.; Philip, P.A.; Sarkar, F.H. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS ONE 2011, 6, e17850. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ahmad, A.; Aboukameel, A.; Bao, B.; Padhye, S.; Philip, P.A.; Sarkar, F.H. Increased Ras GTPase activity is regulated by miRNAs that can be attenuated by CDF treatment in pancreatic cancer cells. Cancer Lett. 2012, 319, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.M.; Huang, C.C.; Lin, T.J.; Fang, J.Y.; Wu, N.L.; Hung, C.F. In vitro and in vivo anti-photoaging effects of an isoflavone extract from soybean cake. J. Ethnopharmacol. 2009, 126, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Suzuki, M.; Kanayama, N.; Terao, T. A soybean Kunitz trypsin inhibitor suppresses ovarian cancer cell invasion by blocking urokinase upregulation. Clin. Exp. Metastasis 2004, 21, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.M.; Park, J.W.; Song, Y.J.; Yun, H.Y.; Park, J.S.; Hyun, T.; Youn, S.J.; Kim, Y.D.; Kang, J.W.; Kim, H. Kimchi and soybean pastes are risk factors of gastric cancer. World J. Gastroenterol. 2005, 11, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Q.; Su, Q.; Ma, D.; An, C.; Ma, L.; Liang, H. Honokiol suppresses renal cancer cells’ metastasis via dual-blocking epithelial-mesenchymal transition and cancer stem cell properties through modulating miR-141/ZEB2 signaling. Mol. Cells 2014, 37, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mei, Q.; Xiong, C.; Zhao, J. Tumor-suppressing effects of miR-141 in human osteosarcoma. Cell Biochem. Biophys. 2014, 69, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Hu, J.; Xu, H. Effects of some Chinese herbal medicine and green tea antagonizing mutagenesis caused by cigarette tar. Chin. J. Prev. Med. 1997, 31, 71–74. [Google Scholar]
- Appari, M.; Babu, K.R.; Kaczorowski, A.; Gross, W.; Herr, I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int. J. Oncol. 2014, 45, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Matsuura, T.; Aoyagi, H.; Matsuda, M.; Hmwe, S.S.; Date, T.; Watanabe, N.; Watashi, K.; Suzuki, R.; Ichinose, S.; et al. Antiviral activity of glycyrrhizin against hepatitis c virus in vitro. PLoS ONE 2013, 8, e68992. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Kim, H.; Lee, J.M. Cancer stem cells in primary liver cancers: Pathological concepts and imaging findings. Korean J. Radiol. 2015, 16, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, C.Y. Natural product-based therapeutics for the treatment of cancer stem cells: A patent review (2010–2013). Expert Opin. Ther. Pat. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Kumar, S. Cancer stem cell: A rogue responsible for tumor development and metastasis. Indian J. Cancer 2014, 51, 282–289. [Google Scholar] [PubMed]
- Kennedy, J.A.; Barabe, F.; Poeppl, A.G.; Wang, J.C.; Dick, J.E. Comment on “tumor growth need not be driven by rare cancer stem cells”. Science 2007, 318. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Dakic, A.; Adams, J.M.; Nutt, S.L.; Strasser, A. Tumor growth need not be driven by rare cancer stem cells. Science 2007, 317. [Google Scholar] [CrossRef] [PubMed]

| Cancer Type | Cell Surface Bio-Markers | Reference |
|---|---|---|
| Acute myeloid leukemia | CD34+ CD38− | [25] |
| Acute lymphoid leukemia | CD34+ CD19− | [26] |
| Breast cancer | CD44+/CD24−/ESA+ | [27] |
| Liver cancer | CD133+/CD44+; EEpCAM+; CD90+ | [28,29] |
| Colorectal cancer | CD133+/CD44+ | [30,31] |
| Prostate cancer | CD44+/CD24−; CD166+; CD151+; p63+ | [32] |
| Lung cancer | CD166+; Sca+/CD45−/Pecam−/CD34+ | [33] |
| Head and Neck cancer | BMI-1+; CD44high/ALDH1+ | [34,35] |
| Non Hodgkin Lymphoma | ABCG2+ | [36,37] |
| Multiple Myeloma | CD138+ | [38,39] |
| Bladder cancer | CD44+; CD47+; CK5+ | [40] |
| Osteosarcoma | CD133+; CD117+ (c-Kit); Stro-1+ | [41] |
| Glioblastoma cancer | CD133+; Sox2+; Nestin+ | [42,43] |
| Nasopharyngeal carcinoma | CD44+; CD38+ | [44,45] |
| Cholangiocarcinoma | CD133+; EpCAM+; CD24+ | [46,47] |
| Chinese Medicines or Their Active Compounds | The Sources of Medicines or Their Active Compounds | Type of Study | Anti-Cancer Target | Molecular Mechanism | Reference |
|---|---|---|---|---|---|
| Berberine | Rhizome of Coptis chinensis Franch | In vitro and in vivo | Reversed drug resistance in CSCs | Inhibited the activity of ABCC1, ABCC2, ABCC3, and ABCG2 in breast cancer cells. | [87] |
| Induced apoptosis in CSCs | Activated the Bax protein while inhibited the Bcl-2 protein, activated the mitochondrial apoptosis pathway in breast cancer cells. | [88] | |||
| Induced CSCs differentiation | Inhibited several stem cell-associated genes such as SOX2, POU5F1, NANOG in pancreatic CSCs. | [128] | |||
| Oxymatrine | Root of Sophora flavescens Aiton | In vitro | Reversed drug resistance in CSCs | Undefined. | [92,166] |
| Radix Scutellariaei | Aqueous extract of the root of Scutellaria baicalensis Georgi | In vitro | Reversed drug resistance in CSCs | Decreased the expression level of ABCG2 protein in multiple myeloma cells. | [94] |
| Baicalein | Root of Scutellaria baicalensis Georgi | In vitro | Reversed drug resistance in CSCs | Inhibited ABCG2 by binding the sites in transmembrane domain in multiple myeloma cells. | [95] |
| Bufalin | Pvarotid venom and skin glands of toads | In vitro | Reversed drug resistance in CSCs | Undefined. | [96] |
| Tien-Hsien Liquid | Undefined mixture consists of extracts from 14 Chinese herbs | In vitro and in vivo | Reversed drug resistance in CSCs | Suppression of CD133 and ABCG2 in hepatoma cells. | [104] |
| Pien Tze Huang | Undefined mixture | In vitro | Reversed drug resistance in CSCs | Suppression of ABCG1 and ABCG2 in colon cancer cell. | [108] |
| In vitro and in vivo | Inhibited proliferation, and inducing apoptosis in CSCs | Suppression of the Notch1 pathway in colon cancer cell. | [118] | ||
| Induced CSCs differentiation | Reduced the mRNA and protein expression of Notch1 and Hes1. | ||||
| Resveratrol | Rhizome of Polygonum cuspidatum Siebold & Zucc. | In vitro and in vivo | Induced apoptosis in CSCs | Inhibited the expression of Bcl-2 and XIAP and activated capase-3/7 in pancreatic cancer cells. | [112] |
| Inhibited migration and invasion by targeting CSCs | Suppression of markers of epithelial-mesenchymal transition (Zeb-1, Slug and Snail) in pancreatic cancer cells. | ||||
| Caffeic acid phenethyl ester | Propolis | In vitro | Inhibited proliferation in CSCs | Undefined. | [119] |
| β-carotene | Root of Daucus carota L. | In vitro and in vivo | Induced CSCs differentiation | Up-regulated differentiation genes peripherin, vimentin and neurofilament in neuroblastoma cells. | [127] |
| YMGKI-1 | Mycelial of Antrodia cinnamomea Chang & Chou | In vitro and in vivo | Induced CSCs differentiation | Decreased the expression of ALDH genes in HNSCC cells. | [132] |
| Antrodia camphorata | Mycelial of Antrodia cinnamomea Chang & Chou | In vitro and in vivo | Inhibited migration and invasion by targeting CSCs | Down-regulated the expression of extracellular VEGF and intracellular HIF1-α in HCC stem-like cells. | [149] |
| Curcumin | Rhizome of Curcuma longa L. | In vitro | Induced CSCs differentiation | Inhibited STAT3 phosphorylation, retained STAT3-NFkB interaction and down-regulated the expression of STAT3-NF-kB target genes in breast cancer cells. | [117] |
| Induced apoptosis in CSCs | |||||
| Inhibited migration and invasion by targeting CSCs | Suppressed beta-catenin nuclear translocation thus inhibited trans-activation of EMT-promoting genes such as Slug in breast cancer cells. | [133] | |||
| Inhibited proliferation, and induced apoptosis by targeting CSCs related miRNAs | Increased expressions of let-7, miR-26a, miR-101 and miR-200, and decreased miR-21 in pancreatic cancer and prostate cancer cells. | [154,155,156,157,158] | |||
| Honokiol | Bark or seed cones of Magnolia officinalis Rehd. et Wils | In vitro | Inhibited migration and invasion by CSCs related miRNAs | Increased miR-141 expression, which targeting ZEB2 and further regulated ZEB2 expression in renal cancer cells. | [162,163] |
| Quercetin | The whole plant of Dysosma veitchii (Hemsl. & E.H.Wilson) L.K.Fu | In vitro | Inhibited migration and invasion by targeting CSCs | Inhibited EMT by inhibiting the expression of snail, nuclear beta-catenin, vimentin and slug, and the activity of LEF-1/TCF responsive reporter in prostate cancer cells. | [134] |
| Induced apoptosis in CSCs | Activated capase-3/7 and inhibited the expressions of Bcl-2, survivin and XIAP. | ||||
| Xiaotan Sanjie decoction | Formula composed of Pinellia tetnata (Thb.) Breit, Arisaema erubescens (Wall.) Schott, Poria cocos (Schw.) Wolf, Citrus aurantium L., Citri reticulatae viride pericarpium, Scorpio, Scolopendra, Galli gigerii endothelium corneum, Fritillariae cirrhosae bulbus, Semen brassicae, and Glycyrrhiza uralensis Fisch | In vitro | Inhibited migration and invasion by targeting CSCs | Inhibited tumor angiogenesis by manipulating Notch-1-regulated proliferation of gastric CSCs. | [140,141] |
| Inhibited the proliferation in CSCs | |||||
| Berbamine | The root of Berberis soulieana Schneid | In vitro | Induced apoptosis by targeting CSCs related miRNAs | Up-regulated the expressions of miR-4284 more than four-fold and an anti-sense inhibitor of miR-4284 activity could partially block the anticancer effects of berbamine on glioblastoma cells. | [153] |
| Genistein | The seed of Glycine max (Linn.) Merr | In vitro | Inhibited proliferation, and induced apoptosis by targeting CSCs related miRNAs | Down-regulated the expressions of let-7b, c, d, e, miR-26a, miR-146a, and miR-200, and up-regulated the expression of miR-21 in pancreatic cancer cells. | [28,88] |
| Green tea catechins | The leaf of Camellia sinensis (L.) O. Ktze | In vitro and in vivo | Induced CSCs differentiation by targeting CSCs related miRNAs | GTC combination with sulforaphane or quercetin activated the expression of miR-let-7a which inhibited K-ras expression and suppressed CSC features in pancreatic ductal adenocarcinoma cells. | [165] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Tan, H.Y.; Li, S.; Cheung, F.; Wang, N.; Nagamatsu, T.; Feng, Y. Cancer Stem Cells: The Potential Targets of Chinese Medicines and Their Active Compounds. Int. J. Mol. Sci. 2016, 17, 893. https://doi.org/10.3390/ijms17060893
Hong M, Tan HY, Li S, Cheung F, Wang N, Nagamatsu T, Feng Y. Cancer Stem Cells: The Potential Targets of Chinese Medicines and Their Active Compounds. International Journal of Molecular Sciences. 2016; 17(6):893. https://doi.org/10.3390/ijms17060893
Chicago/Turabian StyleHong, Ming, Hor Yue Tan, Sha Li, Fan Cheung, Ning Wang, Tadashi Nagamatsu, and Yibin Feng. 2016. "Cancer Stem Cells: The Potential Targets of Chinese Medicines and Their Active Compounds" International Journal of Molecular Sciences 17, no. 6: 893. https://doi.org/10.3390/ijms17060893