Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids
Abstract
:1. Introduction
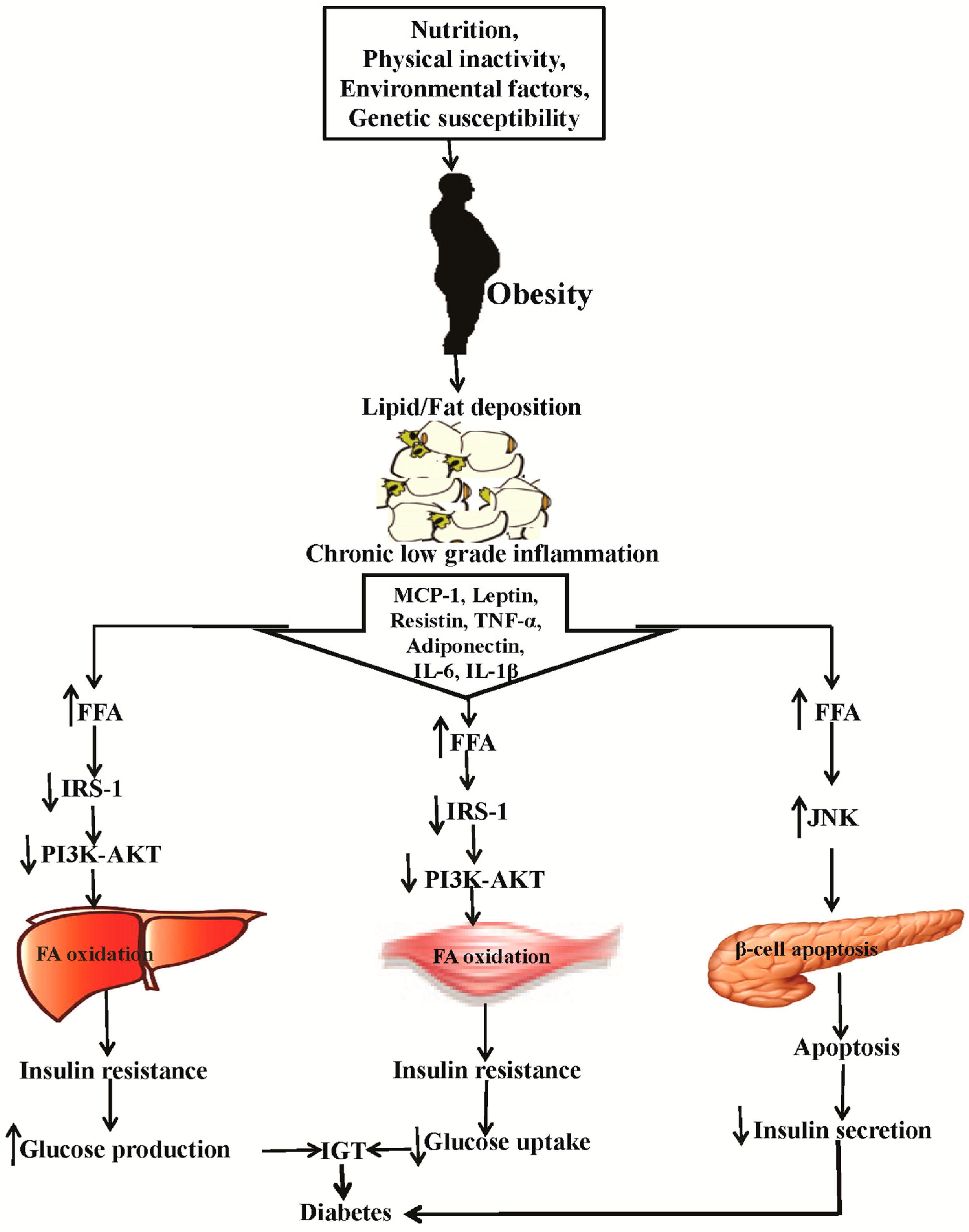
2. Causes of Obesity and Diabetes and the Related Patho-Physiology
3. Flavonoids: Classification and Their Biological Properties
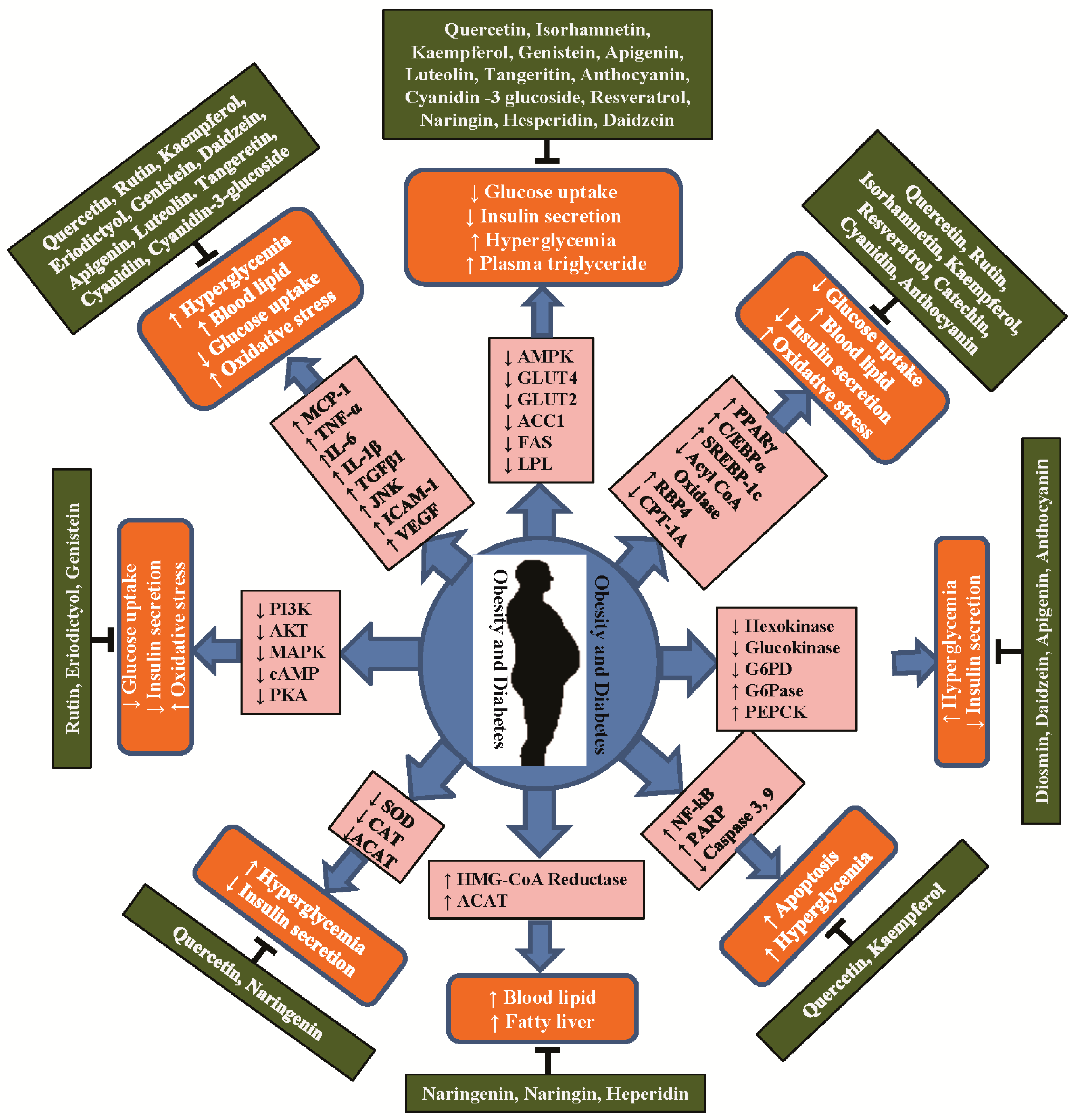
4. Anti-Obesity and Anti-Diabetic Properties of Flavonoids and Their Molecular Functions
4.1. Flavonol
4.2. Flavanones
4.3. Isoflavones
4.4. Flavones
4.5. Flavan-3-ols
4.6. Anthocyanidins and Other Flavonoids
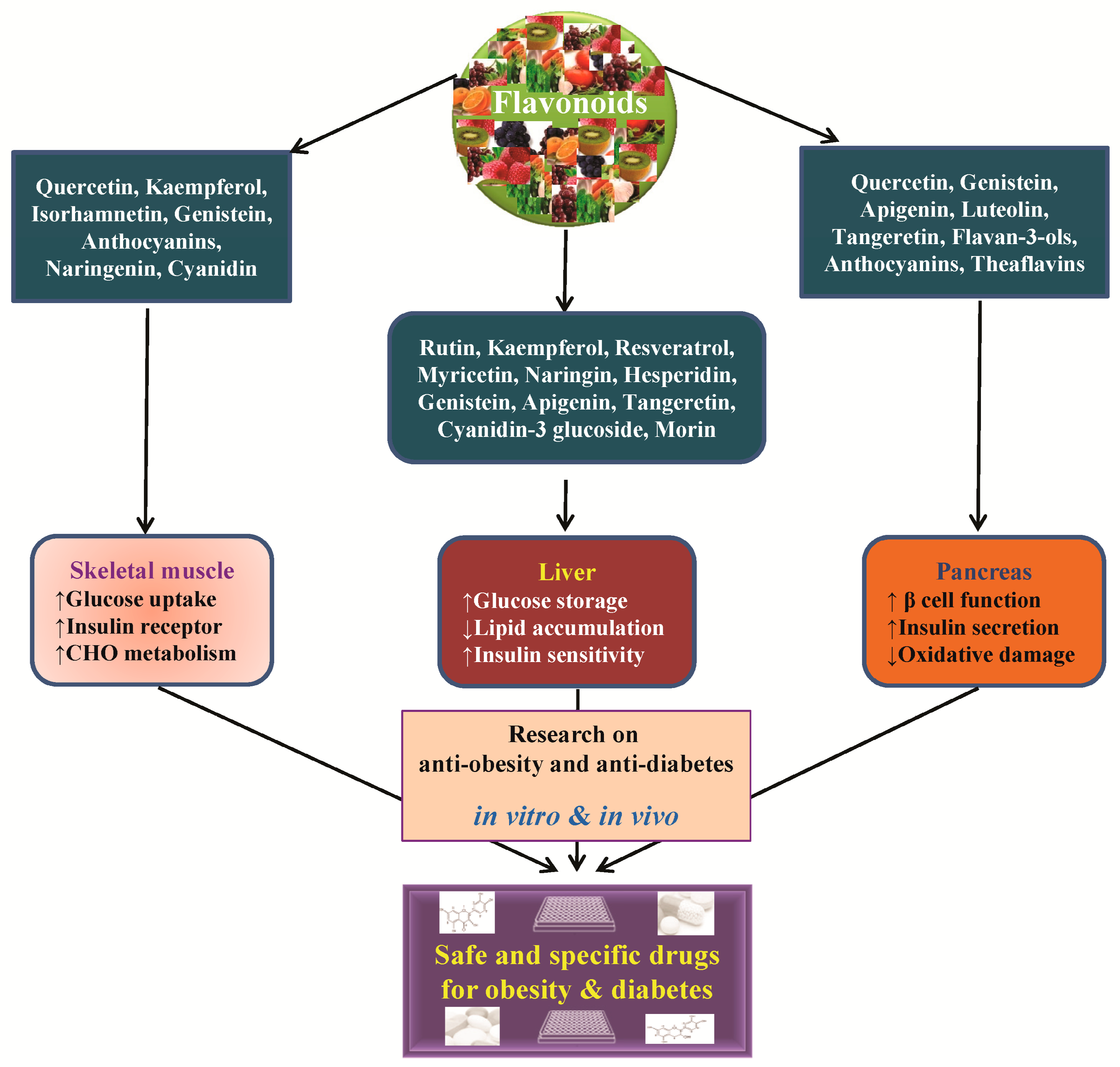
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desai, M.Y.; Dalal, D.; Santos, R.D.; Carvalho, J.A.; Nasir, K.; Blumenthal, R.S. Association of body mass index, metabolic syndrome, and leukocyte count. Am. J. Cardiol. 2006, 97, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Ahirwar, D.; Jhade, D.; Jain, V.K. In-vitro anti-obesity assay of alcoholic and aqueous extracts of camellia sinensis leaves. Int. J. Pharm. Sci. Res. 2012, 3, 1863–1866. [Google Scholar]
- Mokdad, A.H.; Bowman, B.A.; Ford, E.S.; Vinicor, F.; Marks, J.S.; Koplan, J.P. The continuing epidemics of obesity and diabetes in the united states. JAMA 2001, 286, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Devendra, D.; Liu, E.; Eisenbarth, G.S. Type 1 diabetes: Recent developments. BMJ 2004, 328, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, F.B. The global implications of diabetes and cancer. Lancet 2014, 383, 1947–1948. [Google Scholar] [CrossRef]
- WHO. About Diabetes; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Recent advances in disease modeling and drug discovery for diabetes mellitus using induced pluripotent stem cells. Int. J. Mol. Sci. 2016, 17, 256. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian. Pac. J. Trop. Biomed. 2012, 2, 411–420. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Faubion, W.A. Clinical pharmacology of inflammatory bowel disease therapies. Curr. Gastroenterol. Rep. 2000, 2, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Phromnoi, K.; Yadav, V.R.; Chaturvedi, M.M.; Aggarwal, B.B. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010, 76, 1044–1063. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Di Gaspero, G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Waters, M.J.; Schirra, H.J. Investigating potential mechanisms of obesity by metabolomics. Biomed. Res. Int. 2012. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Barnes, G.T.; Yang, Q.; Tan, Q.; Yang, D.S.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (at): Implication of macrophages resident in the at. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Kim, C.S.; Kwon, B.S.; Kawada, T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity 2006, 14, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Ehala-Aleksejev, K.; Guiot, Y.; Detry, R.; Vandenhooft, A.; Brichard, S.M. Adipokines oversecreted by omental adipose tissue in human obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E656–E665. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Khorami, S.A.H.; Movahedi, A.; Khaza’ai, H.; Mutalib, A.; Sokhini, M. PI3K/AKT pathway in modulating glucose homeostasis and its alteration in diabetes. AMBS 2015, 1, 46–55. [Google Scholar]
- Bouzakri, K.; Roques, M.; Gual, P.; Espinosa, S.; Guebre-Egziabher, F.; Riou, J.P.; Laville, M.; le Marchand-Brustel, Y.; Tanti, J.F.; Vidal, H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 2003, 52, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Cho, J.M.; Kim, S.; Baek, S.H.; Lee, M.K.; Kim, K.W.; Yu, S.W.; Solinas, G.; Kim, S.S.; Lee, M.S. Role of jnk activation in pancreatic beta-cell death by streptozotocin. Mol. Cell Endocrinol. 2010, 321, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Barnes, S.; Prasain, J. Current progress in the use of traditional medicines and nutraceuticals. Curr. Opin. Plant Biol. 2005, 8, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.; Carlson, S.; Wyss, J. Flavonoids and age-related disease: Risk, benefits and critical windows. Maturitas 2010, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr. Effect of Plant Flavonoids on Immune and Inflammatory Cell Function; Springer: Berlin, Germany, 1998; pp. 175–182. [Google Scholar]
- Hossain, M.K.; Choi, H.Y.; Hwang, J.-S.; Dayem, A.A.; Kim, J.-H.; Kim, Y.B.; Poo, H.; Cho, S.-G. Antiviral activity of 3, 4’-dihydroxyflavone on influenza a virus. J. Microbiol. 2014, 52, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.-G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar]
- Lee, E.R.; Kang, Y.J.; Choi, H.Y.; Kang, G.H.; Kim, J.H.; Kim, B.W.; Han, Y.S.; Nah, S.Y.; Paik, H.D.; Park, Y.S.; et al. Induction of apoptotic cell death by synthetic naringenin derivatives in human lung epithelial carcinoma a549 cells. Biol. Pharm. Bull. 2007, 30, 2394–2398. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed c57bl/6 mice by anthocyanins and ursolic acid in cornelian cherry (cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, A.; Pandey, A.K. Calotropis procera root extract has the capability to combat free radical mediated damage. ISRN Pharmacol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; Samman, S. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Kang, G.H.; Cho, S.G. Effect of flavonoids on human health: Old subjects but new challenges. Recent Pat Biotechnol 2007, 1, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Lee, E.R.; Min, H.M.; Jeong, H.S.; Ahn, J.Y.; Kim, J.H.; Choi, H.Y.; Choi, H.; Kim, E.Y.; Park, S.P.; et al. Sustained erk activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-d culture condition. Cancer Biol. Ther. 2008, 7, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, M.; Kang, G.H.; Lee, E.R.; Choi, H.Y.; Lee, C.; Kim, Y.; Koo, B.N.; Cho, S.G. Combined treatment of 3-hydroxyflavone and imatinib mesylate increases apoptotic cell death of imatinib mesylate-resistant leukemia cells. Leuk. Res. 2012, 36, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Kim, J.H.; Choi, H.Y.; Jeon, K.; Cho, S.G. Cytoprotective effect of eriodictyol in uv-irradiated keratinocytes via phosphatase-dependent modulation of both the p38 mapk and akt signaling pathways. Cell Physiol. Biochem. 2011, 27, 513–524. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Dzomba, P.; Musekiwa, C. Anti-obesity and antioxidant activity of dietary flavonoids from dioscorea steriscus tubers. JCLM 2014, 2, 465–470. [Google Scholar]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar]
- Yang, J.Y.; Della-Fera, M.A.; Rayalam, S.; Ambati, S.; Hartzell, D.L.; Park, H.J.; Baile, C.A. Enhanced inhibition of adipogenesis and induction of apoptosis in 3t3-l1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008, 82, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Oue, E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2006, 70, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and O-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Park, Y.; Choi, H.; Lee, E.H. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet. Biofactors 2006, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Kim, S.; Huh, S.; Kim, Y.; Byun, S.Y.; Kim, Y.S.; Park, D. Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity 2009, 17, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, C.; Torres, N.; Gutiérrez-Uribe, J.A.; Noriega, L.G.; Torre-Villalvazo, I.; Leal-Díaz, A.M.; Antunes-Ricardo, M.; Márquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.A. The effect of isorhamnetin glycosides extracted from opuntia ficus-indica in a mouse model of diet induced obesity. Food Funct. 2015, 6, 805–815. [Google Scholar] [PubMed]
- Zanatta, L.; Rosso, A.; Folador, P.; Figueiredo, M.S.; Pizzolatti, M.G.; Leite, L.D.; Silva, F.R. Insulinomimetic effect of kaempferol 3-neohesperidoside on the rat soleus muscle. J. Nat. Prod. 2008, 71, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhen, W.; Maechler, P.; Liu, D. Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB. J. Nutr. Biochem. 2013, 24, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate amp-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic ldl receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Floyd, Z.E.; Wang, Z.Q.; Kilroy, G.; Cefalu, W.T. Modulation of peroxisome proliferator-activated receptor gamma stability and transcriptional activity in adipocytes by resveratrol. Metabolism 2008, 57, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2009, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, Y.B.; Bae, K.H.; Bok, S.H.; Kwon, Y.K.; Lee, E.S.; Choi, M.S. Cholesterol-lowering activity of naringenin via inhibition of 3-hydroxy-3-methylglutaryl coenzyme a reductase and acyl coenzyme A: Cholesterol acyltransferase in rats. Ann. Nutr. Metab. 1999, 43, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.W.; Patel, Y.M. Naringenin inhibits phosphoinositide 3-kinase activity and glucose uptake in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2003, 305, 229–234. [Google Scholar] [CrossRef]
- Hasanein, P.; Fazeli, F. Role of naringenin in protection against diabetic hyperalgesia and tactile allodynia in male wistar rats. J. Physiol. Biochem. 2014, 70, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Bok, S.H.; Lee, S.H.; Park, Y.B.; Bae, K.H.; Son, K.H.; Jeong, T.S.; Choi, M.S. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: Cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 1999, 129, 1182–1185. [Google Scholar] [PubMed]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Kang, M.A.; Choi, M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Lee, J.-J.; Kim, Y.; Kim, I.-S.; Han, J.-H.; Lee, S.-G.; Ahn, M.-J.; Jung, S.-H.; Myung, C.-S. Effect of eriodictyol on glucose uptake and insulin resistance in vitro. J. Agric. Food Chem. 2012, 60, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Demonty, I.; Lamarche, B.; Deshaies, Y.; Jacques, H. Role of soy isoflavones in the hypotriglyceridemic effect of soy protein in the rat. J. Nutr. Biochem. 2002, 13, 671–677. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Si, H.; Fu, Z.; Zhen, W.; Liu, D. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J. Nutr. 2012, 142, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Pfeiffer, L.; Zhang, Y.; Fu, Y.; Liu, D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl. Physiol. Nutr. Metab. 2012, 37, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Dongare, S.; Mathur, R.; Mohanty, I.R.; Srivastava, S.; Mathur, S.; Nag, T.C. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol. Cell. Biochem. 2015, 408, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Park, I.J.; Shin, J.I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating amp-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Rauter, A.P.; Martins, A.; Borges, C.; Mota-Filipe, H.; Pinto, R.; Sepodes, B.; Justino, J. Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother. Res. 2010, 24, S133–S138. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Oh, S.; Woo, J.-T.; Kim, S.-W.; Kim, J.-W.; Kim, Y.S.; Chon, S. Apigenin attenuates 2-deoxy-d-ribose-induced oxidative cell damage in HIT-T15 pancreatic. β-cells. Biol. Pharm. Bull. 2012, 35, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Jin, D.; Chen, X. Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J. Nutr. Biochem. 2010, 21, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, X.; Shuai, X.; Xu, Y.; Liu, Y.; Liang, X.; Wei, D.; Su, D. Luteolin prevents uric acid-induced pancreatic β-cell dysfunction. J. Biomed. Res. 2014, 28, 292. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hur, H.J.; Kwon, D.Y.; Hwang, J.T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell. Endocrinol. 2012, 358, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Huang, H.-C.; Lin, J.-K. Theaflavins attenuate hepatic lipid accumulation through activating ampk in human HEPG2 cells. J. Lipid Res. 2007, 48, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Park, P.J.; Shin, H.J.; Kim, Y.K.; Shin, D.W.; Shin, E.S.; Lee, H.H.; Lee, B.G.; Baik, J.H.; Lee, T.R. (−)-catechin suppresses expression of kruppel-like factor 7 and increases expression and secretion of adiponectin protein in 3T3-L1 cells. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1166–E1172. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [PubMed]
- Tsuda, T.; Ueno, Y.; Yoshikawa, T.; Kojo, H.; Osawa, T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006, 71, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-o-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a camp–pka-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Induction of cell apoptosis in 3T3-L1 pre-adipocytes by flavonoids is associated with their antioxidant activity. Mol. Nutr. Food Res. 2006, 50, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Miranda, J.; Lasa, A.; Churruca, I.; Portillo, M.P. Doses of quercetin in the range of serum concentrations exert delipidating effects in 3T3-L1 preadipocytes by acting on different stages of adipogenesis, but not in mature adipocytes. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Takahashi, Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol. Nutr. Food Res. 2009, 53, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hu, J.; Zhao, W.; Gao, X.; Jiang, C.; Liu, K.; Liu, B.; Huang, F. Quercetin differently regulates insulin-mediated glucose transporter 4 translocation under basal and inflammatory conditions in adipocytes. Mol. Nutr. Food Res. 2014, 58, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. C 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kang, M.J.; Choi, H.N.; Kim, J.H.; Kim, J.I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Martinez-Castano, M.G.; Portillo, M.P. Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation. Genes Nutr. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Q.; Ding, Y.; Zhang, Z.F.; Cai, X.X.; Li, Y. Quercetin and quercitrin protect against cytokine-induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int. J. Mol. Med. 2013, 31, 265–271. [Google Scholar] [PubMed]
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (fagopyrum esculentummoench) seeds and determination by capillary electrophoresis. J. Agric. Food Chem. 1999, 47, 4649–4652. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kappel, V.D.; Cazarolli, L.H.; Pereira, D.F.; Postal, B.G.; Zamoner, A.; Reginatto, F.H.; Silva, F.R. Involvement of glut-4 in the stimulatory effect of rutin on glucose uptake in rat soleus muscle. J. Pharm. Pharmacol. 2013, 65, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Niture, N.T.; Ansari, A.A.; Naik, S.R. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: An effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J. Exp. Biol. 2014, 52, 720–727. [Google Scholar] [PubMed]
- Wang, Y.B.; Ge, Z.M.; Kang, W.Q.; Lian, Z.X.; Yao, J.; Zhou, C.Y. Rutin alleviates diabetic cardiomyopathy in a rat model of type 2 diabetes. Exp. Ther. Med. 2015, 9, 451–455. [Google Scholar] [PubMed]
- Ola, M.S.; Ahmed, M.M.; Ahmad, R.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J. Mol. Neurosci. 2015, 56, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Choi, J.S.; Chung, H.Y. Antioxidant effects of isorhamnetin 3, 7-di-O-β-d-glucopyranoside isolated from mustard leaf (brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, S.; Lee, H.S.; Kim, B.K.; Ohuchi, K.; Shin, K.H. Inhibitory effects of isorhamnetin-3-O-β-d-glucoside from salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol. Pharm. Bull. 2005, 28, 916–918. [Google Scholar] [CrossRef] [PubMed]
- An, G.H.; Gallegos, J.; Morris, M.E. The bioflavonoid kaempferol is an ABCG2 substrate and inhibits ABCG2-mediated quercetin efflux. Drug Metab. Dispos. 2011, 39, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, P.; Ramanathan, M. Effect of kaempferol on lipid peroxidation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur. J. Pharmacol. 2011, 654, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.P.; Horst, H.; de Sousa, E.; Pizzolatti, M.G.; Silva, F.R. Insulinomimetic effects of kaempferitrin on glycaemia and on 14c-glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2004, 149, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, Y.; Dai, X.; Wang, J.; Li, Y. Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway. Eur. J. Pharmacol. 2011, 670, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Abo-Salem Osama, M. Kaempferol attenuates the development of diabetic neuropathic pain in mice: Possible anti-inflammatory and anti-oxidant mechanisms. Maced. J. Med. Sci. 2014, 7, 424. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic β-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.M.; Chen, J.K.; Huang, S.S.; Lee, R.S.; Su, M.J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000, 47, 549–555. [Google Scholar] [CrossRef]
- Atten, M.J.; Godoy-Romero, E.; Attar, B.M.; Milson, T.; Zopel, M.; Holian, O. Resveratrol regulates cellular pkc alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Investig. New Drugs 2005, 23, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spuy, W.J.; Pretorius, E. Is the use of resveratrol in the treatment and prevention of obesity premature? Nutr. Res. Rev. 2009, 22, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Seo, S.G.; Yue, S.; Cheng, J.X.; Lee, K.W.; Kim, K.H. An inhibitory effect of resveratrol in the mitotic clonal expansion and insulin signaling pathway in the early phase of adipogenesis. Nutr. Res. 2012, 32, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, L.; Chen, J. Physiologically achievable doses of resveratrol enhance 3T3-L1 adipocyte differentiation. Eur. J. Nutr. 2015, 54, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Cho, I.; Kim, S.; Kwon, D.; Ha, T. Dietary resveratrol alters lipid metabolism-related gene expression of mice on an atherogenic diet. J. Hepatol. 2008, 49, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, G.V.; Paglialonga, G. Resveratrol inhibits fatty acid and triacylglycerol synthesis in rat hepatocytes. Eur. J. Clin. Investig. 2009, 39, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M.; Hasan, S.T. Dietary polyphenols and obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, G.; Rodriguez, V.M.; Macarulla, M.T.; Miranda, J.; Churruca, I.; Portillo, M.P. Hepatic lipid metabolic pathways modified by resveratrol in rats fed an obesogenic diet. Nutrition 2013, 29, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zorita, S.; Treguer, K.; Mercader, J.; Carpene, C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J. Physiol. Biochem. 2013, 69, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Hiermann, A.; Schramm, H.; Laufer, S. Anti-inflammatory activity of myricetin-3-O-β-d-glucuronide and related compounds. Inflamm. Res. 1998, 47, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Hollman, P.C.; van de Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Tzeng, T.F.; Liou, S.S.; Liu, I.M. Myricetin ameliorates defective post-receptor insulin signaling via beta-endorphin signaling in the skeletal muscles of fructose-fed rats. Evid. Complement. Altern. 2011, 2011, 150752. [Google Scholar]
- Liu, I.-M.; Tzeng, T.-F.; Liou, S.-S.; Lan, T.-W. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007, 81, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, N.; Ashokkumar, N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin-cadmium induced diabetic nephrotoxic rats. Toxicol. Appl. Pharmacol. 2014, 279, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Khoo, H.-E. Insulinomimetic effects of myricetin on lipogenesis and glucose transport in rat adipocytes but not glucose transporter translocation. Biochem. Pharmacol. 1996, 51, 423–429. [Google Scholar] [CrossRef]
- Choi, H.N.; Kang, M.J.; Lee, S.J.; Kim, J.I. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr. Res. Pract. 2014, 8, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.-K.; Jeong, K.-S.; Choi, M.-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [PubMed]
- Kim, H.J.; Oh, G.T.; Park, Y.B.; Lee, M.K.; Seo, H.J.; Choi, M.S. Naringin alters the cholesterol biosynthesis and antioxidant enzyme activities in ldl receptor-knockout mice under cholesterol fed condition. Life Sci. 2004, 74, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via ampk. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Kim, H.J.; Lee, J.S.; Lee, M.K.; Kim, H.O.; Park, E.J.; Kim, H.K.; Jeong, T.S.; Choi, M.S. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003, 22, 561–568. [Google Scholar] [CrossRef]
- Huong, D.T.; Takahashi, Y.; Ide, T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition 2006, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.E.; Ganjam, G.K.; Steger, J.; Legler, K.; Stohr, S.; Schumacher, D.; Hoggard, N.; Heldmaier, G.; Tups, A. The dietary flavonoids naringenin and quercetin acutely impair glucose metabolism in rodents possibly via inhibition of hypothalamic insulin signalling. Br. J. Nutr. 2013, 109, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Do, K.M.; Park, Y.S.; Jeon, S.M.; Jeong, T.S.; Lee, Y.K.; Lee, M.K.; Bok, S.H. Effect of naringin supplementation on cholesterol metabolism and antioxidant status in rats fed high cholesterol with different levels of vitamin e. Ann. Nutr. Metab. 2001, 45, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.J.; Lee, M.K.; Jeon, S.M.; Do, G.M.; Kwon, E.Y.; Cho, Y.Y.; Kim, D.J.; Jeong, K.S.; Park, Y.B.; et al. Naringin time-dependently lowers hepatic cholesterol biosynthesis and plasma cholesterol in rats fed high-fat and high-cholesterol diet. J. Med. Food 2006, 9, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Gao, D.-M.; Mohamed, S.; Chen, J.; Zhang, J.; Zhou, X.-Y.; Zhou, N.-J.; Xie, J.; Jiang, H. Naringin ameliorates metabolic syndrome by activating amp-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2012, 518, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Cho, Y.S.; Kim, I.; Anno, T.; Rahman, S.M.; Yanagita, T. Effect of hesperetin, a citrus flavonoid, on the liver triacylglycerol content and phosphatidate phosphohydrolase activity in orotic acid-fed rats. Plant Foods Hum. Nutr. 2001, 56, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Bilheimer, D.W.; Grundy, S.M.; Brown, M.S.; Goldstein, J.L. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc. Natl. Acad. Sci. USA 1983, 80, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.C.; Kim, H.S.; Jeong, T.S.; Bok, S.H.; Park, Y.B. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J. Cardiovasc. Pharmacol. 2001, 38, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Ojha, S.; Upadhya, H.M.; Arya, D.S.; Goyal, S.N. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS ONE 2014, 9, e111212. [Google Scholar] [CrossRef] [PubMed]
- Byington, R.P.; Jukema, J.W.; Salonen, J.T.; Pitt, B.; Bruschke, A.V.; Hoen, H.; Furberg, C.D.; Mancini, G.B. Reduction in cardiovascular events during pravastatin therapy. Pooled analysis of clinical events of the pravastatin atherosclerosis intervention program. Circulation 1995, 92, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Delsing, D.J.; Offerman, E.H.; van Duyvenvoorde, W.; van Der Boom, H.; de Wit, E.C.; Gijbels, M.J.; van Der Laarse, A.; Jukema, J.W.; Havekes, L.M.; Princen, H.M. Acyl-CoA:Cholesterol acyltransferase inhibitor avasimibe reduces atherosclerosis in addition to its cholesterol-lowering effect in apoe*3-leiden mice. Circulation 2001, 103, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Kim, M.; Han, J. Stereospecific microbial production of isoflavanones from isoflavones and isoflavone glucosides. Appl. Microbiol. Biotechnol. 2011, 91, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Song, T.T.; Lee, S.O.; Murphy, P.A.; Hendrich, S. Soy protein with or without isofleivones, soy germ and soy germ extract, and daidzein lessen plasma cholesterol levels in golden syrian hamsters. Exp. Biol. Med. 2003, 228, 1063–1068. [Google Scholar]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in l6 myocytes and improves glucose homeostasis in type 2 diabetic model mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Genistein affects lipogenesis and lipolysis in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2000, 75, 265–271. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T.; Nogowski, L. Daidzein, coumestrol and zearalenone affect lipogenesis and lipolysis in rat adipocytes. Phytomedicine 2002, 9, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Nogowski, L.; Maćkowiak, P.; Kandulska, K.; Szkudelski, T.; Nowak, K.W. Genistein-induced changes in lipid metabolism of ovariectomized rats. Ann. Nutr. Metab. 1998, 42, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Isoflavonoids of the leguminosae. Nat. Prod. Rep. 2007, 24, 417–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, S.; Li, L.; Liang, Z.; Wang, L. Genistein reduces hyperglycemia and islet cell loss in a high-dosage manner in rats with alloxan-induced pancreatic damage. Pancreas 2011, 40, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Mezei, O.; Banz, W.J.; Steger, R.W.; Peluso, M.R.; Winters, T.A.; Shay, N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the ppar pathways in obese zucker rats and murine raw 264.7 cells. J. Nutr. 2003, 133, 1238–1243. [Google Scholar] [PubMed]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kuhne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W.; et al. Dietary phytoestrogens activate amp-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, M.J.; Choi, H.N.; Jeong, S.M.; Lee, Y.M.; Kim, J.I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J. Nutr. Biochem. 2005, 16, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.Q.; Wang, Y.W.; Misra, H.; Liu, D.M. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, H.E.; Choi, S.E.; Shin, H.C.; Kwag, W.J.; Lee, B.K.; Cho, K.W.; Kang, Y. Involvement of Ca2+/calmodulin kinase II (CAMK II) in genistein-induced potentiation of leucine/glutamine-stimulated insulin secretion. Mol. Cells 2009, 28, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kwon, K.B.; Song, M.Y.; Seo, S.W.; Park, S.J.; Ka, S.O.; Na, L.; Kim, K.A.; Ryu, D.G.; So, H.S.; et al. Genistein protects pancreatic β cells against cytokine-mediated toxicity. Mol. Cell. Endocrinol. 2007, 278, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Jayagopal, V.; Kilpatrick, E.S.; Chapman, T.; Atkin, S.L. Effects of isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care 2007, 30, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Apigenin (4′,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; de Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.; Lan, N.; Li, S.; Zhang, J.; Wang, S.; Li, C.; Shang, Y.; Huang, T.; Zhang, L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav. Brain Res. 2014, 267, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Tanaka, H.; Shimada, A.; Sato, T.; Ito, A.; Yamanouchi, T.; Kosano, H. Regulation of adipocytokine secretion and adipocyte hypertrophy by polymethoxyflavonoids, nobiletin and tangeretin. Life Sci. 2011, 88, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Chen, L.; Lee, M.-J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomark. Prev. 1998, 7, 351–354. [Google Scholar]
- Sartippour, M.R.; Shao, Z.-M.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002, 132, 2307–2311. [Google Scholar] [PubMed]
- Kavanagh, K.T.; Hafer, L.J.; Kim, D.W.; Mann, K.K.; Sherr, D.H.; Rogers, A.E.; Sonenshein, G.E. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J. Cell. Biochem. 2001, 82, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Osada, K.; Takahashi, M.; Hoshina, S.; Nakamura, M.; Nakamura, S.; Sugano, M. Tea catechins inhibit cholesterol oxidation accompanying oxidation of low density lipoprotein in vitro. Comp. Biochem. Physiol. C 2001, 128, 153–164. [Google Scholar] [CrossRef]
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000, 141, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S. Effects of green tea and egcg on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007, 26, 373S–388S. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.F.; Kusumoto, A.; Abe, K.; Hosoda, K.; Kiso, Y.; Wang, M.F.; Yamamoto, S. Polyphenol-enriched oolong tea increases fecal lipid excretion. Eur. J. Clin. Nutr. 2006, 60, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Raederstorff, D.; Wang, Y.; Teixeira, S.R.; Elste, V.; Weber, P. Teavigo (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann. Nutr. Metab. 2005, 49, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S.; Pultz, S.; Thone-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Harwood, H.J., Jr. Acetyl-coenzyme a carboxylases: Versatile targets for drug discovery. J. Cell. Biochem. 2006, 99, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.T.; Hung, P.F.; Chen, H.C.; Huang, R.N.; Chang, H.H.; Kao, Y.H. The apoptotic effect of green tea (−)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the CDK2 pathway. J. Agric. Food Chem. 2005, 53, 5695–5701. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Lin, J.K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating irs2 and ampk signaling in rat pancreatic beta cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef] [PubMed]
- Withers, D.J.; Gutierrez, J.S.; Towery, H.; Burks, D.J.; Ren, J.M.; Previs, S.; Zhang, Y.; Bernal, D.; Pons, S.; Shulman, G.I.; et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998, 391, 900–904. [Google Scholar] [PubMed]
- Kim, S.J.; Winter, K.; Nian, C.; Tsuneoka, M.; Koda, Y.; McIntosh, C.H. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic β-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor foxo1, and down-regulation of bax expression. J. Biol. Chem. 2005, 280, 22297–22307. [Google Scholar] [PubMed]
- Buteau, J.; Accili, D. Regulation of pancreatic beta-cell function by the forkhead protein foxo1. Diabetes Obes. Metab. 2007, 9 (Suppl. 2), 140–146. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; La Fauci, L.; Vitaglione, P.; Fogliano, V.; Vanella, L.; Felgines, C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann. Ist. Super. Sanita 2007, 43, 382–393. [Google Scholar] [PubMed]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200. [Google Scholar] [PubMed]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008, 582, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, Y.; Shibayama, Y.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of amp-activated protein kinase in diabetic mice. J. Agric. Food Chem. 2013, 61, 5558–5564. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor foxo1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.D.; Zhang, B.; Zhang, J.K.; Xu, C.J.; Wu, Y.L.; Li, X.; Chen, K.S. Cyanidin-3-glucoside-rich extract from chinese bayberry fruit protects pancreatic beta cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J. Med. Food 2012, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Judd, J.T.; Baer, D.J.; Clevidence, B.A.; Paul, D.R.; Edwards, A.J.; Wiseman, S.A.; Muesing, R.A.; Chen, S.C. Black tea consumption reduces total and ldl cholesterol in mildly hypercholesterolemic adults. J. Nutr. 2003, 133, 3298S–3302S. [Google Scholar] [PubMed]
- Kobayashi, M.; Ichitani, M.; Suzuki, Y.; Unno, T.; Sugawara, T.; Yamahira, T.; Kato, M.; Takihara, T.; Sagesaka, Y.; Kakuda, T.; et al. Black-tea polyphenols suppress postprandial hypertriacylglycerolemia by suppressing lymphatic transport of dietary fat in rats. J. Agric. Food Chem. 2009, 57, 7131–7136. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, K.F.S.; de Oliveira, T.T.; Nagem, T.J.; Pinto, A.D.; Oliveira, M.G.A.; Soares, J.F. Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Braz. Arch. Biol. Technol. 2001, 44, 263–267. [Google Scholar] [CrossRef]
- Sreedharan, V.; Venkatachalam, K.K.; Namasivayam, N. Effect of morin on tissue lipid peroxidation and antioxidant status in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Investig. New Drugs 2009, 27, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Abuohashish, H.M.; Al-Rejaie, S.S.; Al-Hosaini, K.A.; Parmar, M.Y.; Ahmed, M.M. Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetol. Metab. Syndr. 2013, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.M.; Gu, T.T.; Ding, X.Q.; Fan, C.Y.; Zhu, Q.; Shi, Y.W.; Hong, Y.; Kong, L.D. Morin reduces hepatic inflammation-associated lipid accumulation in high fructose-fed rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Biochem. Pharmacol. 2013, 86, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.; Cirri, P.; Caselli, A.; Ranaldi, F.; Bruschi, G.; Santi, A.; Camici, G. The insulin-mimetic effect of morin: A promising molecule in diabetes treatment. Biochim. Biophys. Acta 2013, 1830, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Gunasekaran, P.; Sivasubramanian, S.; Ramkumar, K.M. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and beta-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharmacol. 2014, 37, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Bak, E.-J.; Kim, J.; Choi, Y.H.; Kim, J.-H.; Lee, D.-E.; Woo, G.-H.; Cha, J.-H.; Yoo, Y.-J. Wogonin ameliorates hyperglycemia and dyslipidemia via pparα activation in db/db mice. Clin. Nutr. 2014, 33, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Bae, J.S. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015, 48, 519–524. [Google Scholar] [CrossRef] [PubMed]

| Name of Flavonoids | Structures | Plant Sources | Anti-Obesity and Anti-Diabetic Effect in in Vitro/in Vivo Model | Molecular Mechanism in Obesity and Diabetes | References |
|---|---|---|---|---|---|
| Quercetin |  | Apples, berries, red onions, cherries, broccoli, coriander, etc. | ↑ Apoptosis in 3T3-L1 preadipocytes | ↓ PARP, ↑ AMPK, ↑ Caspase 3 and 9 | [45] |
| ↑ Glucose uptake in rat adipocyte, C2C12 muscle cells | ↑ GLUT4 | [46] | |||
| ↑ Glucose uptake, ↓ Fat accumulation in 3T3-L1 preadipocytes | ↓ PPARγ1 | [47] | |||
| ↓ Hyperglycemia, ↑ Insulin in STZ-induced diabetic rats, db/db mice | ↓ NF-κB, ↓ Caspase 3, ↓ MDA levels, ↑ SOD and CAT | [48] | |||
| Rutin |  | Buckwheat, oranges, grapes, lemons, limes, peaches and berries | ↓ Blood lipids, ↓ Fatty liver in DIO mice and rat | ↓ PPAR and ↓ C/EBP, ↓ TNF-α, ↓ IL-6 | [49] |
| ↑ Glucose uptake in the rat soleus muscle | ↑ PI3K, ↑ MAPK | [50] | |||
| Isorhamnetin |  | Ginkgo biloba L., Hippophae rhamnoides L. and Oenanthe javanica (Blume) | ↓ Hyperglycemia and oxidative stress in STZ-induced diabetic rat, Inhibition adipogenesis in 3 T3-L1 cells | ↓ PPARγ, ↓ C/EBPα | [51] |
| ↑ Insulin secretion in HFD-induced C57BL/6 mice | ↑ GLUT2, ↑ PPARγ | [52] | |||
| Kaempferol |  | Grapefruit, tea, cruciferous vegetables | ↓ Hyperglycemia, ↑ Glucose uptake in rat soleus muscle | ↓ Caspase 3 | [53] |
| ↑ β-cell survival in INS-1E cells | ↑ GLUT4, ↑ AMPK | [54] | |||
| ↑ Antioxidant defense and body weight gain in diabetic rats and HFD-obese mice | ↓ PPARγ, ↓ SREBP-1c, ↓ TNF-α, ↓ IL-6 | [55] | |||
| Resveratrol |  | Red grapes, red wine, peanuts, and ground nuts | ↑ Glucose uptake | ↑ GLUT4 | [56] |
| ↓ Lipid accumulation 3T3-L1 | ↓ PPARγ | [57] | |||
| ↑ Lipolytic activity in adipocytes | ↑ cAMP | [58] | |||
| Naringenin |  | Spreng, Grapefruits, oranges and tomatoes | ↓ Blood lipids, ↓ Fatty liver in Hypercholesterolemic rats | ↓ HMG-CoA, ↓ ACAT | [59] |
| ↓ Glucose uptake in 3T3-L1 adipocytes | ↓ PI3K, ↓ AKT | [60] | |||
| ↓ Hyperglycemia in STZ-induced rat | ↑ Antioxidant enzyme (SOD) | [61] | |||
| Naringin |  | Citrus fruits and Grapefruit | ↓ Hyperglycemia, ↑ Plasma insulin, ↑ Leptin in STZ-induced diabetic mice and db/db mice | ↑ GLUT4, ↑ PPARγ | [59] |
| ↓ Blood lipids, ↓ Fatty liver in db/db Mice | ↓ HMG-CoA, ↓ ACAT | [62] | |||
| Hesperidin |  | Lemons and oranges | ↓ Blood glucose level, ↓ Blood lipids in STZ-induced type 1 diabetic rats | ↑ Glucokinase | [63] |
| ↓ Oxidative stress, apoptosis | ↑ GLUT4, ↓ HMG-CoA, ↓ ACAT | [64] | |||
| Eriodictyol |  | Lemon fruits | ↓ Adipocyte-specific fatty acid binding protein in differentiated 3 T3-L1 adipocytes | ↑ PPARγ | [65] |
| ↑ Glucose uptake, ↑ Insulin resistance in HepG2 cells | ↑ AKT | [65] | |||
| ↓ Diabetes-related lipid peroxidation | ↓ TNFα, ↓ ICAM-1, ↓ VEGF | [66] | |||
| Genistein |  | Soy foods | ↓ Plasma triglycerides in Sprague-Dawley rats | ↑ GLUT4 | [67,68,69] |
| ↑ Insulin-positive β cell in HG-induced diabetic mice | ↑ cAMP signaling, ↑ PKA activation | [70] | |||
| ↓ Blood glucose, ↓ Blood HbA1c in STZ-induced diabetic mice, ↓ Adipocyte differentiation | ↓ TNF-α, ↓ TGFβ1, ↓ NF-κB, ↑ AMPK, ↑ ACC | [71] | |||
| Daidzein |  | Soy foods and nuts | ↓ Blood glucose, ↓ Urinary glucose | ↓TNF-α, ↓ TGFβ1, ↓ NF-κB | [70] |
| ↓ Plasma triglycerides in Sprague-Dawley rats | ↑ GLUT4, ↓ G6Pase, ↓ PEPCK | [67] | |||
| Apigenin |  | Passion flower and chamomile | ↓ Hyperglycemia, ↓ Oxidative stress in STZ-induced diabetic rats and mice | ↓ NF-κB, ↓ TNF-α, ↓ IL-1β | [72] |
| ↑ Glucose uptake, ↑ Insulin secretion in alloxan-induced diabetic mice and INS-1E cells, ↓ Lipid accumulation, ↓ Hyperglycemia in HepG2 hepatocytes | ↓ G6Pase, ↑ GLUT4, ↑ AMPK, ↓ MCP-1, ↑ AMPK, ↑ ACC | [73] | |||
| Luteolin |  | Celery, parsley, broccoli, onion leaves, carrots, peppers, cabbages, apple skins, and chrysanthemum flowers | ↑ Insulin secretion in 3T3-L1 hepatocyte | ↑ GLUT4, ↑ Leptin | [74] |
| ↑ Insulin secretion in uric acid damaged pancreatic β-cells | ↓ MAFA, ↓ NF-κB, ↓ CREB-B | [75] | |||
| Tangeretin |  | Citrus fruit rinds, mandarin orange | ↑ Insulin secretion, ↑ Glycogen, ↓ Total cholesterol in HFD-induced obese mice | ↓ TNF-α, ↓ IL-6, ↓ IL-1β | [76] |
| ↓ Plasma glucose level, ↓ Plasma HbA1c in diabetic rats | ↑ AMPK | [77] | |||
| Epicatechin Gallate |  | Tea, grapes and seeds of certain leguminous plants | ↓ Hepatic lipid accumulation in HepG2 cells | ↓ Fatty acid synthase, ↓ ACC1 | [78] |
| (−)-Catechin |  | Tea, grapes and seeds of certain leguminous plants | ↓ Insulin-dependent glucose uptake, ↑ Adiponectin protein | ↓ KLF7, ↓ PPARγ, ↓ C/EBPα | [79] |
| (−)-Epigallo catechin gallate |  | Tea, grapes and seeds of certain leguminous plants | ↑ Insulin secretion, protect insulin-producing β-cells | ↑ FOXO1, ↑ PDX-1, ↑ IRS2, ↑ AKT, ↑ NeuroD, ↑ MAFA | [80] |
| Cyanidin |  | Plants with purple corn color (PCC) | ↓ White and brown adipose tissue weights, ↓ Hyperglycemia | ↓ TNF-α, ↓ SREBP-1 | [81] |
| Anthocyanins |  | Black soybean seed coats bilberries | ↓ Hyperglycemia, ↑ Insulin sensitivity, ↑ GLUT4 (WAT and muscle) in T2DM mice | ↑ AMPK, ↓ PEPCK, ↓ G6Pase, ↓ ACC1, ↓ PPARα, ↑ Acyl-CoA oxidase, ↑ CPT-1A, ↓ RBP4 | [82] |
| Cyanidin-3-glucoside |  | Plant bayberry fruit | Protect hepatocytes ↓ HG-stimulated damage | ↑ AKT, ↓ JNK, ↓ TNF-α, ↓ IL-6, ↓ MCP-1 | [83] |
| ↑ Insulin secretion in oxidative stress-induced pancreatic β damage | ↑ GLUT4, ↑ LPL, ↑ FAS, ↑ AMPK | [84] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. https://doi.org/10.3390/ijms17040569
Kawser Hossain M, Abdal Dayem A, Han J, Yin Y, Kim K, Kumar Saha S, Yang G-M, Choi HY, Cho S-G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. International Journal of Molecular Sciences. 2016; 17(4):569. https://doi.org/10.3390/ijms17040569
Chicago/Turabian StyleKawser Hossain, Mohammed, Ahmed Abdal Dayem, Jihae Han, Yingfu Yin, Kyeongseok Kim, Subbroto Kumar Saha, Gwang-Mo Yang, Hye Yeon Choi, and Ssang-Goo Cho. 2016. "Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids" International Journal of Molecular Sciences 17, no. 4: 569. https://doi.org/10.3390/ijms17040569
APA StyleKawser Hossain, M., Abdal Dayem, A., Han, J., Yin, Y., Kim, K., Kumar Saha, S., Yang, G.-M., Choi, H. Y., & Cho, S.-G. (2016). Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. International Journal of Molecular Sciences, 17(4), 569. https://doi.org/10.3390/ijms17040569










