Molecular Selection, Modification and Development of Therapeutic Oligonucleotide Aptamers
Abstract
:1. Introduction
2. Monoclonal Antibodies versus Oligonucleotide Aptamers
2.1. Advantages of Oligonucleotide Aptamers
2.2. Limitations of Oligonucleotide Aptamers
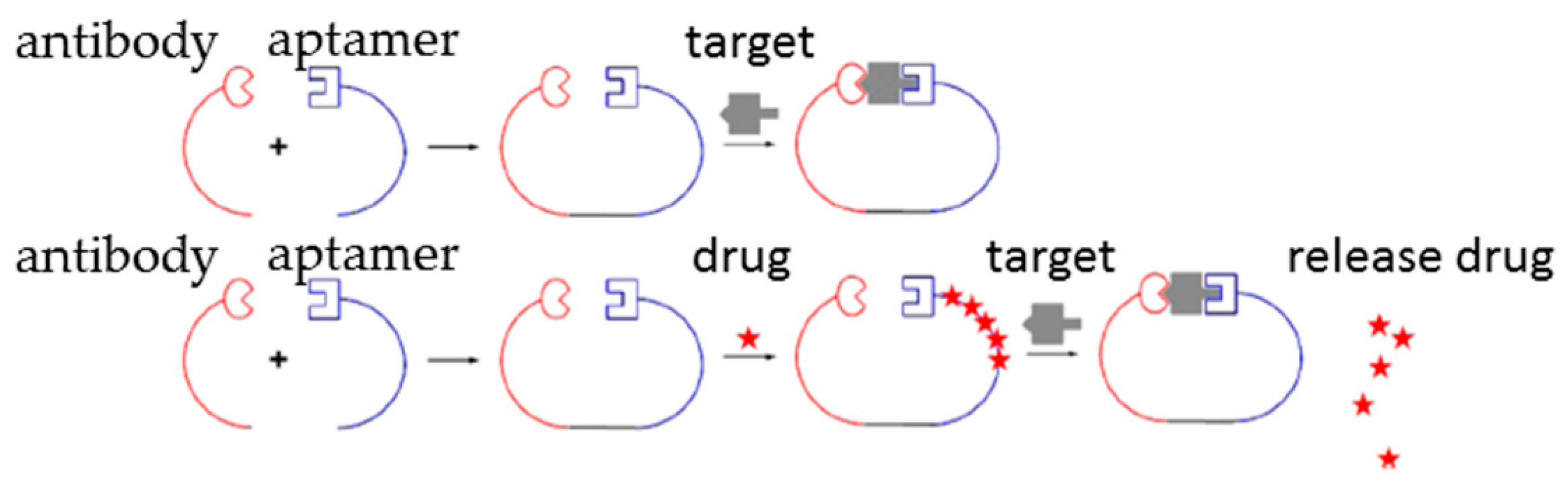
2.3. Aptamer-Antibody Conjugation
3. Aptamer Selection and Modifications
3.1. Systematic Evolution of Ligands by EXponential Enrichment (SELEX)
3.1.1. Conventional SELEX
3.1.2. Modified SELEX
3.2. Modifications of Aptamers for Preclinical Studies
3.2.1. Modifications on Linkage
3.2.2. Modifications on Sugar Ring or Bases
4. Aptamers for Skeletal Diseases Therapy in Preclinical Studies
5. Aptamers in On-Going or Completed Clinical Trials for Therapeutics
5.1. Aptamers against Macular Degeneration
5.1.1. Pegaptanib
5.1.2. ARC1905
5.1.3. E10030
5.2. Aptamers against Cancer
5.2.1. AS1411
5.2.2. NOX-A12
5.3. Aptamers against Coagulation
5.3.1. REG1
5.3.2. ARC1779
5.3.3. NU172
5.3.4. BAX499
5.4. Aptamers against Inflammation
5.4.1. NOX-H94
5.4.2. NOX-E36
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SELEX | Systematic Evolution of Ligands by EXponential enrichment |
| AMD | Age-related Macular Degeneration |
| AAPs | Antibody-aptamer pincers |
| FACS | Fluorescence-Activated Cell Sorting |
| cholODN | cholesteryl-oligonucleotide |
| LDL | low density lipoprotein |
| VEGF | vascular endothelial growth factor |
| PDGF | Platelet-Derived Growth Factor |
| vWF | von Willebrand factor |
| CD | Cluster of Differentiation |
| IL | InterLeukin |
| EGFR | Epidermal Growth Factor Receptor |
| sulfo-SMCC | sulfosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate |
| SATA | N-succinimidyl-S-acetylthioacetate |
| CXCL-12 | The chemokine (C–X–C motif) ligand 12 |
References
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.R.; Sidhu, S.; Dubel, S.; McCafferty, J. Beyond natural antibodies: The power of in vitro display technologies. Nat. Biotechnol. 2011, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage t4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [PubMed]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Toledo, J.; McKeague, M.; Zhang, X.; Giamberardino, A.; McConnell, E.; Francis, T.; DeRosa, M.C.; Dumontier, M. Aptamer base: A collaborative knowledge base to describe aptamers and SELEX experiments. Database 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, D.; Green, L.S.; Bell, C.; Janjic, N. Inhibition of receptor binding by high-affinity RNA ligands to vascular endothelial growth factor. Biochemistry 1994, 33, 10450–10456. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Ambati, B.K.; Yoo, S.H.; Ianchulev, S.; Adamis, A.P. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 2003, 48, 257–293. [Google Scholar] [CrossRef]
- Que-Gewirth, N.S.; Sullenger, B.A. Gene therapy progress and prospects: RNA aptamers. Gene Ther. 2007, 14, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Ruckman, J.; Green, L.S.; Beeson, J.; Waugh, S.; Gillette, W.L.; Henninger, D.D.; Claesson-Welsh, L.; Janjic, N. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). J. Biol. Chem. 1998, 273, 20556–20567. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Verma, A.; Visintin, A.; Gong, M.; Sirois, C.M.; Klein, D.C.; Monks, B.G.; McKnight, C.J.; Lamphier, M.S.; Duprex, W.P.; et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat. Immunol. 2007, 8, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Temsamani, J.; Iadarola, P.L.; Jiang, Z.; Agrawal, S. Effect of different chemically modified oligodeoxynucleotides on immune stimulation. Biochem. Pharmacol. 1996, 51, 173–182. [Google Scholar] [CrossRef]
- Yu, D.; Wang, D.; Zhu, F.G.; Bhagat, L.; Dai, M.; Kandimalla, E.R.; Agrawal, S. Modifications incorporated in CpG motifs of oligodeoxynucleotides lead to antagonist activity of Toll-like receptors 7 and 9. J. Med. Chem. 2009, 52, 5108–5114. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J. Antibodies are challenged. Indian J. Med. Sci. 2010, 64, 144–147. [Google Scholar] [PubMed]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.H.; Phua, K.K.; Leong, K.W. Aptamer nanomedicine for cancer therapeutics: Barriers and potential for translation. ACS Nano 2015, 9, 2235–2254. [Google Scholar] [CrossRef] [PubMed]
- Jo, N.; Mailhos, C.; Ju, M.; Cheung, E.; Bradley, J.; Nishijima, K.; Robinson, G.S.; Adamis, A.P.; Shima, D.T. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am. J. Pathol. 2006, 168, 2036–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhao, N.; Zeng, Z.; Chang, C.C.; Zu, Y. Combination of an aptamer probe to CD4 and antibodies for multicolored cell phenotyping. Am. J. Clin. Pathol. 2010, 134, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hah, S.S. Improved ligand binding by antibody-aptamer pincers. Bioconjug. Chem. 2014, 25, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef] [PubMed]
- Shum, K.T.; Chan, C.; Leung, C.M.; Tanner, J.A. Identification of a DNA aptamer that inhibits sclerostin’s antagonistic effect on Wnt signalling. Biochem. J. 2011, 434, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Guo, B.; Wu, H.; Shao, N.; Li, D.; Liu, J.; Dang, L.; Wang, C.; Li, H.; Li, S.; et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 2015, 21, 288–294. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Rusconi, C.; Scardino, E.; Wolberg, A.; Lawson, J.; Hoffman, M.; Sullenger, B. Generation of species cross-reactive aptamers using “toggle“ SELEX. Mol. Ther. 2001, 4, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Graner, A.; Waugh, R.; Grosse, I.; Close, T.J.; Stein, N. Evidence and evolutionary analysis of ancient whole-genome duplication in barley predating the divergence from rice. BMC Evol. Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Brown, D.; He, Y.; Shtatland, T.; Singer, B.S.; Wu, Y. From oligonucleotide shapes to genomic SELEX: Novel biological regulatory loops. Proc. Natl. Acad. Sci. USA 1997, 94, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Shtatland, T.; Gill, S.C.; Javornik, B.E.; Johansson, H.E.; Singer, B.S.; Uhlenbeck, O.C.; Zichi, D.A.; Gold, L. Interactions of Escherichia coli RNA with bacteriophage MS2 coat protein: Genomic SELEX. Nucleic Acids Res. 2000, 28. [Google Scholar] [CrossRef]
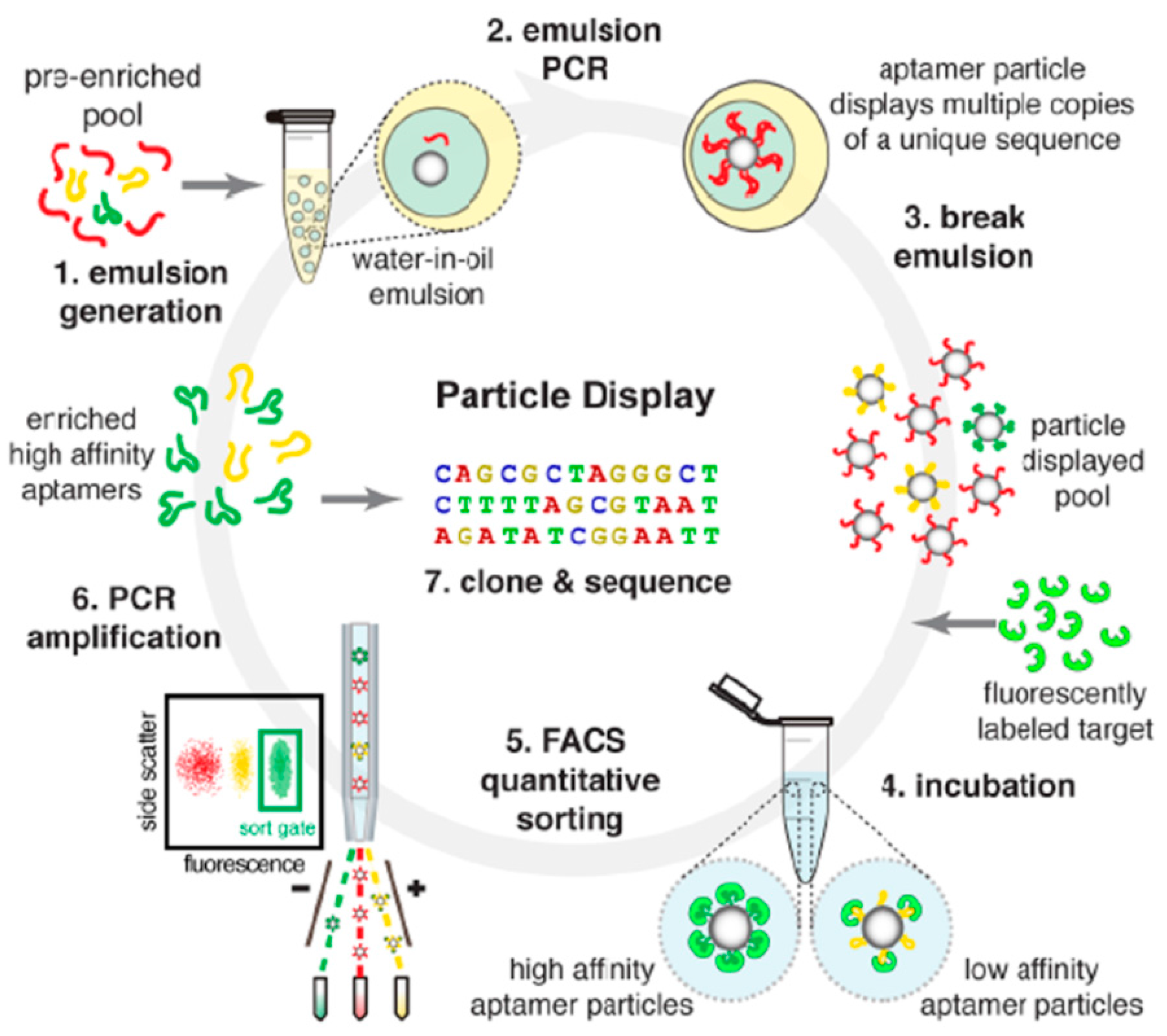
- Wang, J.; Gong, Q.; Maheshwari, N.; Eisenstein, M.; Arcila, M.L.; Kosik, K.S.; Soh, H.T. Particle display: A quantitative screening method for generating high-affinity aptamers. Angew. Chem. Int. Ed. 2014, 53, 4796–4801. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.E.; Wu, H.; Niu, Y.; Cai, J. Improving the stability of aptamers by chemical modification. Curr. Med. Chem. 2011, 18, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, I.; Shafer, R.H. Effect of loop sequence and size on DNA aptamer stability. Biochemistry 2000, 39, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Martino, L.; Virno, A.; Randazzo, A.; Virgilio, A.; Esposito, V.; Giancola, C.; Bucci, M.; Cirino, G.; Mayol, L. A new modified thrombin binding aptamer containing a 5′–5′ inversion of polarity site. Nucleic Acids Res. 2006, 34, 6653–6662. [Google Scholar] [CrossRef] [PubMed]
- Pagano, B.; Martino, L.; Randazzo, A.; Giancola, C. Stability and binding properties of a modified thrombin binding aptamer. Biophys. J. 2008, 94, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Seliger, H.; Frohlich, A.; Groger, G.; Krist, B.; Montenarh, M.; Rosch, H.; Rosch, R.; Ortigao, F.R. Synthetic oligonucleotides for biomedical applications. Nucleic Acids Symp. Ser. 1991, 193–196. [Google Scholar]
- Shaw, J.P.; Kent, K.; Bird, J.; Fishback, J.; Froehler, B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991, 19, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Dougan, H.; Lyster, D.M.; Vo, C.V.; Stafford, A.; Weitz, J.I.; Hobbs, J.B. Extending the lifetime of anticoagulant oligodeoxynucleotide aptamers in blood. Nucl. Med. Biol. 2000, 27, 289–297. [Google Scholar] [CrossRef]
- De Smidt, P.C.; Le Doan, T.; de Falco, S.; van Berkel, T.J. Association of antisense oligonucleotides with lipoproteins prolongs the plasma half-life and modifies the tissue distribution. Nucleic Acids Res. 1991, 19, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Sacca, B.; Lacroix, L.; Mergny, J.L. The effect of chemical modifications on the thermal stability of different G-quadruplex-forming oligonucleotides. Nucleic Acids Res. 2005, 33, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, M.; Kaluzhny, D.; Shchyolkina, A.; Borisova, O.; Smirnov, I.; Pozmogova, G. Conformation and thermostability of oligonucleotide d(GGTTGGTGTGGTTGG) containing thiophosphoryl internucleotide bonds at different positions. Biophys. Chem. 2010, 146, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pozmogova, G.E.; Zaitseva, M.A.; Smirnov, I.P.; Shvachko, A.G.; Murina, M.A.; Sergeenko, V.I. Anticoagulant effects of thioanalogs of thrombin-binding DNA-aptamer and their stability in the plasma. Bull. Exp. Biol. Med. 2010, 150, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, P.; Kurniawan, H.; Byrne, M.E.; Wower, J. Therapeutic RNA aptamers in clinical trials. Eur. J. Pharm. Sci. 2013, 48, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Purschke, W.G.; Radtke, F.; Kleinjung, F.; Klussmann, S. A DNA spiegelmer to staphylococcal enterotoxin B. Nucleic Acids Res. 2003, 31, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Wlotzka, B.; Leva, S.; Eschgfaller, B.; Burmeister, J.; Kleinjung, F.; Kaduk, C.; Muhn, P.; Hess-Stumpp, H.; Klussmann, S. In vivo properties of an anti-GnRH spiegelmer: An example of an oligonucleotide-based therapeutic substance class. Proc. Natl. Acad. Sci. USA 2002, 99, 8898–8902. [Google Scholar] [CrossRef] [PubMed]
- Leva, S.; Lichte, A.; Burmeister, J.; Muhn, P.; Jahnke, B.; Fesser, D.; Erfurth, J.; Burgstaller, P.; Klussmann, S. Gnrh binding RNA and DNA spiegelmers: A novel approach toward GnRH antagonism. Chem. Biol. 2002, 9, 351–359. [Google Scholar] [CrossRef]
- Eulberg, D.; Klussmann, S. Spiegelmers: Biostable aptamers. Chembiochem 2003, 4, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille, F.; Hansen, J.B.; Orum, H.; Di Primo, C.; Toulme, J.J. LNA/DNA chimeric oligomers mimic RNA aptamers targeted to the TAR RNA element of HIV-1. Nucleic Acids Res. 2004, 32, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.S.; Borkowski, S.; Kurreck, J.; Stephens, A.W.; Bald, R.; Hecht, M.; Friebe, M.; Dinkelborg, L.; Erdmann, V.A. Application of locked nucleic acids to improve aptamer in vivo stability and targeting function. Nucleic Acids Res. 2004, 32, 5757–5765. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, A.; Hernandez, F.J.; Rasmussen, L.M.; Vester, B.; Wengel, J. Improved thrombin binding aptamer by incorporation of a single unlocked nucleic acid monomer. Nucleic Acids Res. 2011, 39, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A. Development of highly nuclease-resistant chemically-modified oligonucleotides. Yakugaku Zasshi 2011, 131, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Ostendorf, T.; Janssen, U.; Burg, M.; Radeke, H.H.; Vargeese, C.; Gill, S.C.; Green, L.S.; Janjic, N. Novel approach to specific growth factor inhibition in vivo: Antagonism of platelet-derived growth factor in glomerulonephritis by aptamers. Am. J. Pathol. 1999, 154, 169–179. [Google Scholar] [CrossRef]
- Li, C.J.; Cheng, P.; Liang, M.K.; Chen, Y.S.; Lu, Q.; Wang, J.Y.; Xia, Z.Y.; Zhou, H.D.; Cao, X.; Xie, H.; et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Invest. 2015, 125, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.T.; SchAfer, R.; Paul, A.; Gerber, A.; Ziemer, G.; Wendel, H.P. A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells 2006, 24, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Ardjomandi, N.; Niederlaender, J.; Aicher, W.K.; Reinert, S.; Schweizer, E.; Wendel, H.P.; Alexander, D. Identification of an aptamer binding to human osteogenic-induced progenitor cells. Nucleic Acid Ther. 2013, 23, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.; Adamis, A.P. Anti-VEGF aptamer (pegaptanib) therapy for ocular vascular diseases. Ann. NY Acad. Sci. 2006, 1082, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Damico, L.; Shams, N.; Lowman, H.; Kim, R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006, 26, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Tunon, J.; Ruiz-Moreno, J.M.; Martin-Ventura, J.L.; Blanco-Colio, L.M.; Lorenzo, O.; Egido, J. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv. Ophthalmol. 2009, 54, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Friberg, T.R.; Tolentino, M.; Weber, P.; Patel, S.; Campbell, S.; Goldbaum, M. Pegaptanib sodium as maintenance therapy in neovascular age-related macular degeneration: The level study. Br. J. Ophthalmol. 2010, 94, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, G.; Dihel, L.; Enney, K.; Bendele, R.A. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology 1999, 42, 219–230. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc. 2006, 81, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Hicke, B.J.; Stephens, A.W.; Gould, T.; Chang, Y.F.; Lynott, C.K.; Heil, J.; Borkowski, S.; Hilger, C.S.; Cook, G.; Warren, S.; et al. Tumor targeting by an aptamer. J. Nucl. Med. 2006, 47, 668–678. [Google Scholar] [PubMed]
- Xiang, D.; Zheng, C.; Zhou, S.F.; Qiao, S.; Tran, P.H.; Pu, C.; Li, Y.; Kong, L.; Kouzani, A.Z.; Lin, J.; et al. Superior performance of aptamer in tumor penetration over antibody: Implication of aptamer-based theranostics in solid tumors. Theranostics 2015, 5, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.J.; Vaske, D.; Killoran, C.E.; Ning, Y.; Wargowski, D.; Hudgins, L.; Tifft, C.J.; Meck, J.; Blancato, J.K.; Rosenbaum, K.; et al. Detection of chromosomal aberrations by a whole-genome microsatellite screen. Am. J. Hum. Genet. 2000, 66, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Vater, A.; Klussmann, S. Toward third-generation aptamers: Spiegelmers and their therapeutic prospects. Curr. Opin. Drug Discov. Devel. 2003, 6, 253–261. [Google Scholar] [PubMed]
- Marasca, R.; Maffei, R. NOX-A12: Mobilizing CLL away from home. Blood 2014, 123, 952–953. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, C.P.; Scardino, E.; Layzer, J.; Pitoc, G.A.; Ortel, T.L.; Monroe, D.; Sullenger, B.A. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 2002, 419, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.K.; Steinhubl, S.R.; Kleiman, N.S.; Cannon, R.O.; Aberle, L.G.; Lin, M.; Myles, S.K.; Melloni, C.; Harrington, R.A.; Alexander, J.H.; et al. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: A phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation 2006, 114, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Cohen, M.G.; Dyke, C.K.; Myles, S.K.; Aberle, L.G.; Lin, M.; Walder, J.; Steinhubl, S.R.; Gilchrist, I.C.; Kleiman, N.S.; et al. Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation 2008, 117, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Rusconi, C.P.; Alexander, J.H.; Tonkens, R.M.; Harrington, R.A.; Becker, R.C. A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J. Thromb. Haemost. 2008, 6, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.G.; Purdy, D.A.; Rossi, J.S.; Grinfeld, L.R.; Myles, S.K.; Aberle, L.H.; Greenbaum, A.B.; Fry, E.; Chan, M.Y.; Tonkens, R.M.; et al. First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation 2010, 122, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Mehran, R.; Povsic, T.J.; Zelenkofske, S.L.; Huang, Z.; Armstrong, P.W.; Steg, P.G.; Bode, C.; Cohen, M.G.; Buller, C.; et al. Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): A randomised clinical trial. Lancet 2015, 387, 349–356. [Google Scholar] [CrossRef]
- Diener, J.L.; Daniel Lagasse, H.A.; Duerschmied, D.; Merhi, Y.; Tanguay, J.F.; Hutabarat, R.; Gilbert, J.; Wagner, D.D.; Schaub, R. Inhibition of von willebrand factor—mediated platelet activation and thrombosis by the anti-von willebrand factor A1—domain aptamer ARC1779. J. Thromb. Haemost. 2009, 7, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Lillicrap, D. Genotype/phenotype association in von willebrand disease: Is the glass half full or empty? J. Thromb. Haemost. 2009, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E.; Budde, U.; Eikenboom, J.C.; Favaloro, E.J.; Hill, F.G.; Holmberg, L.; Ingerslev, J.; Lee, C.A.; Lillicrap, D.; Mannucci, P.M.; et al. Update on the pathophysiology and classification of von willebrand disease: A report of the subcommittee on von willebrand factor. J. Thromb. Haemost. 2006, 4, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Jilma, B.; Paulinska, P.; Jilma-Stohlawetz, P.; Gilbert, J.C.; Hutabarat, R.; Knobl, P. A randomised pilot trial of the anti-von willebrand factor aptamer ARC1779 in patients with type 2b von willebrand disease. Thromb. Haemost. 2010, 104, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.K.; Genga, R.M.; Schwartz, M.C.; Nelson, J.A.; Schaub, R.G.; Olson, K.A.; Kurz, J.C.; McGinness, K.E. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood 2011, 117, 5514–5522. [Google Scholar] [CrossRef] [PubMed]
- Schwoebel, F.; van Eijk, L.T.; Zboralski, D.; Sell, S.; Buchner, K.; Maasch, C.; Purschke, W.G.; Humphrey, M.; Zollner, S.; Eulberg, D.; et al. The effects of the anti-hepcidin spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood 2013, 121, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, O.; Pawar, R.D.; Purschke, W.; Eulberg, D.; Selve, N.; Buchner, K.; Ninichuk, V.; Segerer, S.; Vielhauer, V.; Klussmann, S.; et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 2007, 18, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Ninichuk, V.; Clauss, S.; Kulkarni, O.; Schmid, H.; Segerer, S.; Radomska, E.; Eulberg, D.; Buchner, K.; Selve, N.; Klussmann, S.; et al. Late onset of Ccl2 blockade with the spiegelmer mNOX-E36-3′PEG prevents glomerulosclerosis and improves glomerular filtration rate in db/db mice. Am. J. Pathol. 2008, 172, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]


| Name | Property | Advantages |
|---|---|---|
| Counter SELEX | Introduce negative selection to exclude aptamers bind to negative target | Could discriminate highly similar structure to increase specificity |
| Toggle SELEX | Multiple positive selection targets | Could select aptamers bind to multiple targets |
| Capillary electrophoresis-SELEX | Separate aptamer–target complexes from free aptamers according to their electrophoretic mobility with capillary electrophoresis | Could effectively identify high affinity aptamers in four rounds |
| Cell SELEX | Select against whole cells | No prior target knowledge required |
| In vivo SELEX | generate aptamers in living organisms | No prior target knowledge required Suitable for cancer therapy as tumors have high varieties and in vitro selection may not work |
| In silico SELEX | Employ computational docking | Could be used to predict aptamer affinity, specificity, 3D structure and aptamer-target interaction by computer prior to experimental characterization |
| SELEX with high-throughput sequencing | Could use high-through sequencing after each round of selection. | Could be used for selection of a large number of aptamers. Could identify aptamers in two to three rounds of SELEX and could perform comprehensive characterization of identified aptamers. |
| Therapeutic Purpose | Name | Target | Form | Modification | Status | Section |
|---|---|---|---|---|---|---|
| Macular degeneration | Pegaptanib | Vascular endothelial growth factor (VEGF) | RNA | 2′-fluoro pyrimidines, 2′-O-methyl purines, 3′-inverted dT, PEGylated | Approved for age-related macular degeneration (wet AMD) | 5.1.1 |
| ARC1905 | Complement component 5 | RNA | 3′-inverted dT, PEGylated | Phase I completed | 5.1.2 | |
| E10030 | Platelet-derived growth factor (PDGF) | DNA | 2′-fluoro pyrimidines, 2′-O-methyl purines 3′-inverted dT | Phase III await | 5.1.3 | |
| Cancer | AS1411 | Nucleolin | RNA | G-rich, PEGylated | Phase II on-going | 5.2.1 |
| NOX-A12 | The chemokine (C–X–C motif) ligand 12 (CXCL-12) | l-RNA | l-form, PEGylated | Phase II on-going | 5.2.2 | |
| Coagulation | REG1 | Coagulation factor IXa | RNA | 3′-inverted dT, PEGylated | Phase III await | 5.3.1 |
| ARC1779 | von Willebrand factor (vWF) A1 domain | DNA | 3′-inverted dT, PEGylated | Phase II on-going | 5.3.2 | |
| NU172 | Thrombin | DNA | Unmodified DNA | Phase II on-going | 5.3.3 | |
| BAX499 | Tissue factor pathway | RNA | 3′-inverted dT, PEGylated | Phase I on-going | 5.3.4 | |
| Inflammation | NOX-H94 | Hepcidlin | l-RNA | l-form, PEGylated | Phase II on-going | 5.4.1 |
| NOX-E36 | The chemokine (C–C motif) ligand 2 (CCL2) | l-RNA | l-form, PEGylated | Phase II on-going | 5.4.2 |
| Antibody | Trade Name | Target | Approved Indication |
|---|---|---|---|
| Muromomab | Orthoclone | CD3 | Allograft rejection in allogeneic renal transplantation |
| Abciximab | ReoPro | Glycoprotein IIb/IIIa | Percutaneous coronary intervention |
| Rituximab | Rituxan | CD20 | RA, Wegner granulomatosis, microscopic polyangiitis |
| Daclizumab | Zenapax | CD25 (II2r) | Allograft rejection |
| Basiliximab | Simulect | CD25 (II2r) | Allograft rejection |
| Palivizumab | Synagis | Protein F | Respiratory syncytial virus (RSV inhibitor) in children |
| Infliximab | Remicade | TNFα | Crohn’s disease and rheumatoid arthritis |
| Trastuzumab | Herceptin | HER2/Neu | Metastatic breast cancer |
| Etanercept | Enbrel | TNFα and β | Autoimmune diseases such as ankylosing spondylitis |
| Gemtuzumab | Mylotarg | CD33 | CD33-positive acute myeloid leukemia |
| Alemtuzumab | Mabcampath | CD52 | B-cell chronic lymphocytic leukemia |
| Ibritomomab | Zevalin 90Y | CD20 | B-cell non-Hodgkin’s lymphoma |
| Adalimumab | Trudexa | TNFα | Crohn’s disease and rheumatoid arthritis |
| Alefacept | Amevive | CD2 | Chronic plaque psoriasis |
| Omalizumab | Xolair | IgE | asthema |
| Tositumomab | Bexxar | CD20 | CD20-positive B-cell non-Hodgkin’s lymphoma |
| Efalizumab | Raptiva | CD11a | Moderate to severe plaque psoriasis |
| Cetuximab | Erbitus | EGFR | Metastatic colorectal and head and neck carcinoma |
| Bevacizumab | Avastin | VEGF-A | Metastatic colorectal and non-small cell lung carcinoma |
| Natalizumab | Tysabri | Integrin-α4 | Multiple sclerosis |
| Ranibizumab | Lucentis | VEGF-A | Wet type age-related macular degeneration |
| Panitumumab | Vectibid | EGFR | Metastatic colorectal carcinoma |
| Eculizumab | Soliris | C5 | Paroxysmal nocturnal haemoglobinuria |
| Certolizumab | Cimzia | TNFα | Crohn’s disease |
| Daratumumab | Darzalex | CD38 | Multiple myeloma |
| Elotuzumab | EMPLICITI | CS1 | In combination with lenalidomide and dexamethasone for Multiple myeloma |
| Mepolizumab | Nucala | IL-5 | Asthma |
| Denosumab | Prolia/Xgeva | Nuclear factor kappa B ligand | Bone matastases, osteoporosis, giant cell tumor of bone |
| Secukinumab | Cosentyx | IL-17 | Psoriasis |
| Sirukumab | (CNTO 136) | IL-6 | Rheumatoid arthritis (soon) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Liang, C.; Lv, Q.; Li, D.; Xu, X.; Liu, B.; Lu, A.; Zhang, G. Molecular Selection, Modification and Development of Therapeutic Oligonucleotide Aptamers. Int. J. Mol. Sci. 2016, 17, 358. https://doi.org/10.3390/ijms17030358
Yu Y, Liang C, Lv Q, Li D, Xu X, Liu B, Lu A, Zhang G. Molecular Selection, Modification and Development of Therapeutic Oligonucleotide Aptamers. International Journal of Molecular Sciences. 2016; 17(3):358. https://doi.org/10.3390/ijms17030358
Chicago/Turabian StyleYu, Yuanyuan, Chao Liang, Quanxia Lv, Defang Li, Xuegong Xu, Baoqin Liu, Aiping Lu, and Ge Zhang. 2016. "Molecular Selection, Modification and Development of Therapeutic Oligonucleotide Aptamers" International Journal of Molecular Sciences 17, no. 3: 358. https://doi.org/10.3390/ijms17030358







