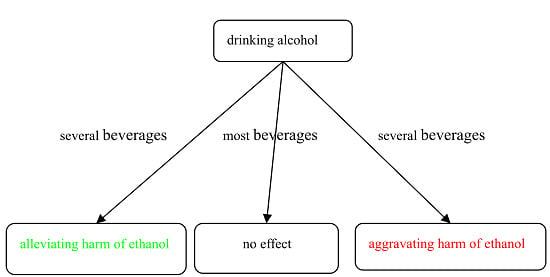
Effects of Beverages on Alcohol Metabolism: Potential Health Benefits and Harmful Impacts
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effects of Beverages on Concentrations of Ethanol and Acetaldehyde in Blood
2.2. Effects of Beverages on Hepatic ADH and ALDH Activities
2.3. Effects of Beverages on ALT and AST Levels in Serum
2.4. Effects of Beverages on SOD and MDA Levels in Liver
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Beverages
3.3. Detection of the Effects of 20 Beveragess on Alcohol Metabolism in Mice
3.3.1. Animal Study for Evaluation of Ethanol and Acetaldehyde Levels in Blood as well as ADH and ALDH Activities in Liver
3.3.2. Determination of Concentrations of Ethanol and Acetaldehyde in Blood
3.3.3. Analyses of ADH and ALDH Activities
3.4. Detection of the Effects of 20 Beveragess on Acute Alcohol-induced Liver Injury in Mice
3.4.1. Animal Study for Evaluation of ALT and AST Activities in Serum as well as SOD and MDA Levels in Liver
3.4.2. Determination of ALT and AST Levels in Serum
3.4.3. Measurement of SOD and MDA Levels in Liver
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, C.; Zhan, J.; Liu, Y.J.; Li, D.J.; Wang, S.Q.; He, Q.Q. Association between alcohol consumption and risk of cardiovascular disease and all-cause mortality in patients with hypertension: A meta-analysis of prospective cohort studies. Mayo Clin. Proc. 2014, 89, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sola, J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat. Rev. Cardiol. 2015, 12, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol consumption and mortality in patients with cardiovascular disease a meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Kouda, K.; Iki, M.; Fujita, Y.; Tamaki, J.; Yura, A.; Kadowaki, E.; Sato, Y.; Moon, J.S.; Morikawa, M.; Tomioka, K.; et al. Alcohol intake and bone status in elderly Japanese men: Baseline data from the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone 2011, 49, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.M.; Persson, E.C.; Weinstein, S.J.; Graubard, B.I.; Freedman, N.D.; Mannisto, S.; Albanes, D.; McGlynn, K.A. Alcohol consumption, one-carbon metabolites, liver cancer and liver disease mortality. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Rosa-e-Silva, L.; Troncon, L.E.; Oliveira, R.B.; Gallo, L.; Foss, M.C. Fecal parameters and gastrointestinal transit in patients with alcohol related chronic pancreatitis with and without chronic diarrhea: Factors associated with this symptom. Gastroenterology 2013, 144, S457. [Google Scholar] [CrossRef]
- Barker, J.M.; Taylor, J.R. Habitual alcohol seeking: Modeling the transition from casual drinking to addiction. Neurosci. Biobehav. R. 2014, 47, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, V.A.; Bosron, W.F.; Li, T.K. Research advances in ethanol metabolism. Pathol. Biol. 2001, 49, 676–682. [Google Scholar] [CrossRef]
- Zimatkin, S.M.; Pronko, S.P.; Vasiliou, V.; Gonzalez, F.J.; Deitrich, R.A. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol. Clin. Exp. Res. 2006, 30, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Li, S.Y.; Brown, R.A.; Ren, J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: From bad to ugly en route to oxidative stress. Alcohol 2004, 32, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.J.P. Genetic-epidemiological evidence for the role of acetaldehyde in cancers related to alcohol drinking. Adv. Exp. Med. Biol. 2015, 815, 41–58. [Google Scholar] [PubMed]
- Chiang, C.P.; Wu, C.W.; Lee, S.P.; Ho, J.L.; Lee, S.L.; Nieh, S.; Yin, S.J. Expression pattern, ethanol-metabolizing activities, and cellular localization of alcohol and aldehyde dehydrogenases in human small intestine. Alcohol. Clin. Exp. Res. 2012, 36, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Nutraceutical strategies for ameliorating the toxic effects of alcohol. Med. Hypotheses 2013, 80, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gan, L.G.; Li, S.G.; Zheng, J.C.; Xu, D.P.; Li, H.B. Effects of herbal infusions, tea and carbonated beverages on alcohol dehydrogenase and aldehyde dehydrogenase activity. Food Funct. 2014, 5, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Swift, R.M. Drug therapy for alcohol dependence. New Engl. J. Med. 1999, 340, 1482–1490. [Google Scholar] [PubMed]
- Nuutinen, H.; Lindros, K.O.; Salaspuro, M. Determinants of blood acetaldehyde level during ethanol oxidation in chronic alcoholics. Alcoholism 1983, 7, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Vaglini, F.; Viaggi, C.; Piro, V.; Pardini, C.; Gerace, C.; Scarselli, M.; Corsini, G.U. Acetaldehyde and parkinsonism: Role of CYP450 2E1. Front. Behav. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Du, J.A.; He, D.; Sun, L.N.; Han, T.; Zhang, H.; Qin, L.P.; Rahman, K. Semen Hoveniae extract protects against acute alcohol-induced liver injury in mice. Pharm. Biol. 2010, 48, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wen, D.C.; Gao, S.D.; Hu, X.Y.; Yi, C. The protective effects of Buzui on acute alcoholism in mice. Evid.-Based Complement Altern. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Wu, X.L.; Zhang, Y.S.; Zhou, L.; Li, L.N.; Yu, Y.L.; Wang, L.Y. Discrepant roles of CpG ODN on acute alcohol-induced liver injury in mice. Int. Immunopharmacol. 2012, 12, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, H.; Li, Y. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. Eur. J. Pharmacol. 2008, 586, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Qi, X.F.; Song, S.B.; Kim, D.H.; Teng, Y.C.; Yoon, Y.S.; Kim, K.Y.; Li, J.H.; Jin, D.; Lee, K.J. Electrolyzed-reduced water inhibits acute ethanol-induced hangovers in Sprague-Dawley rats. Biomed. Res. 2009, 30, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.A.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Pecorari, M.; Villano, D.; Testa, M.F.; Schmid, M.; Serafini, M. Biomarkers of antioxidant status following ingestion of green teas at different polyphenol concentrations and antioxidant capacity in human volunteers. Mol. Nutr. Food Res. 2010, 542, S278–S283. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Kochi, T.; Ideta, T.; Miyazaki, T.; Moriwaki, H. Chemopreventive potential of green tea catechins in hepatocellular carcinoma. Int. J. Mol. Sci. 2015, 16, 6124–6139. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakachi, K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ 1995, 310, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Pennay, A.; Lubman, D.I.; Miller, P. Combining energy drinks and alcohol: A recipe for trouble? Aust. Fam. Physician 2011, 40, 104–107. [Google Scholar] [PubMed]
- Bonar, E.E.; Cunningham, R.M.; Polshkova, S.; Chermack, S.T.; Blow, F.C.; Walton, M.A. Alcohol and energy drink use among adolescents seeking emergency department care. Addict. Biobehav. 2015, 43, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Isse, T.; Matsuno, K.; Oyama, T.; Kitagawa, K.; Kawamoto, T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity shows high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol. Clin. Exp. Res. 2005, 29, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Isse, T.; Kawamoto, T.; Woo, H.; Kim, A.K.; Park, J.Y.; Yang, M. Effects and action mechanisms of Korean pear (Pyrus pyrifolia cv. Shingo) on alcohol detoxification. Phytother. Res. 2012, 26, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Dai, R.J.; Bai, J.M.; Chen, Y.; Yu, Y.H.; Meng, W.W.; Deng, Y.L. Effect of Elaeagnus conferta Roxb (Elaeagnaceae) dry fruit on the activities of hepatic alcohol dehydrogenase and aldehyde dehydrogenase in mice. Trop. J. Pharm. Res. 2011, 10, 761–766. [Google Scholar] [CrossRef]
- Lindahl, R.; Evces, S. Changes in aldehyde dehydrogenase activity during diethylnitrosamine-initiated rat hepatocarcinogenesis. Carcinogenesis 1987, 8, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, S.; Haas, S.; Schneider, A.; Singer, M.V. Animal models in gastrointestinal alcohol research—A short appraisal of the different models and their results. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 519–542. [Google Scholar] [CrossRef]
- Yang, R.K.; Han, X.N.; Delude, R.L.; Fink, M.P. Ethyl pyruvate ameliorates acute alcohol-induced liver injury and inflammation in mice. J. Lab. Clin. Med. 2003, 142, 322–331. [Google Scholar] [CrossRef]
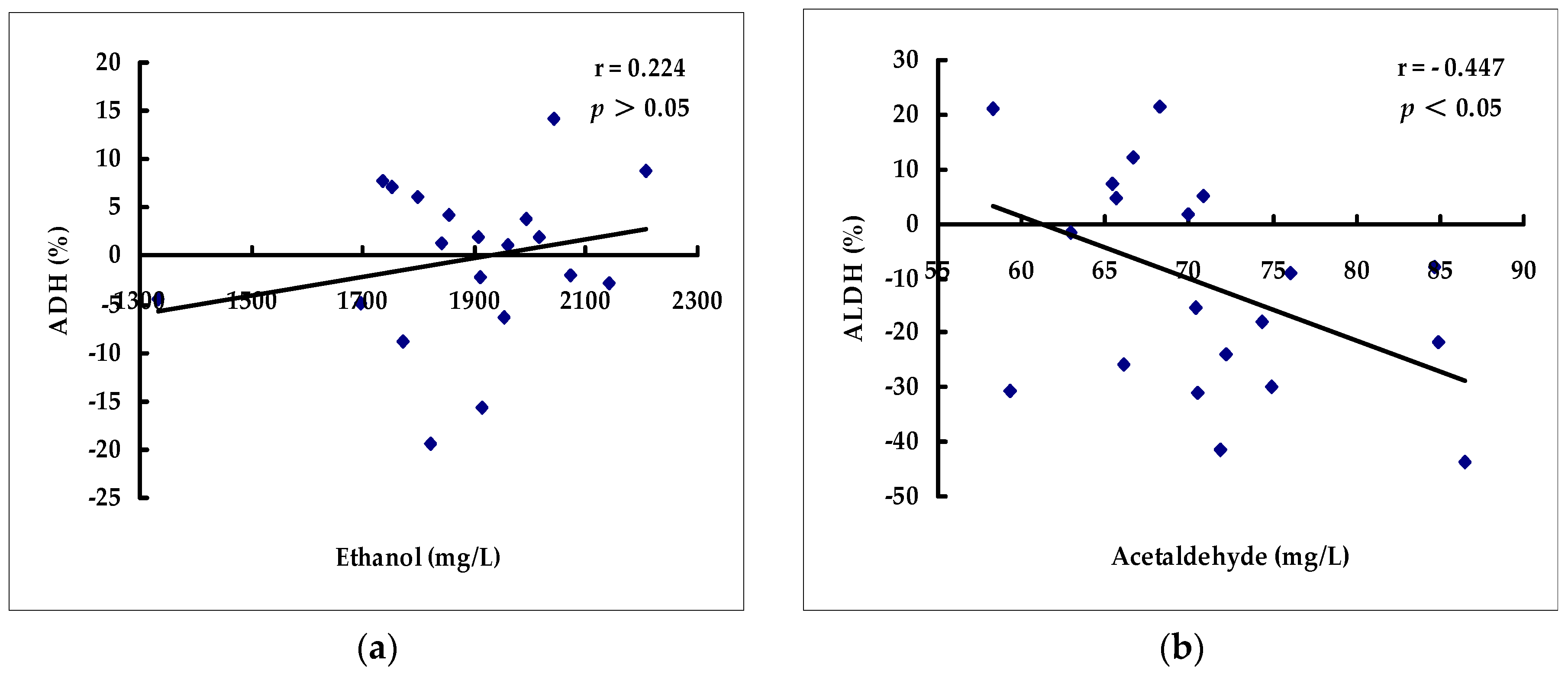
| Group | Ethanol Level (mg/L) | Acetaldehyde Level (mg/L) |
|---|---|---|
| Normal group | 147.11 ± 14.87 | 25.35 ± 4.07 |
| Control group | 1901.17 ± 296.83 ** | 69.17 ± 5.33 ** |
| Ban sha herbal infusion | 1821.83 ± 182.49 | 66.09 ± 15.36 |
| Coca cola | 1909.29 ± 231.45 | 74.99 ± 4.93 |
| Fresh orange juice | 1696.53 ± 170.31 | 86.48 ± 8.27 * |
| Fructus cannabis herbal infusion | 2140.80 ± 467.26 | 74.35 ± 4.96 |
| Green tea | 1862.48 ± 401.14 | 65.39 ± 7.83 |
| He qi zheng herbal infusion | 2208.69 ± 406.42 | 72.22 ± 3.81 |
| Honey chrysanthemum tea | 1734.96 ± 272.68 | 66.70 ± 4.78 |
| Honey citron tea | 1332.72± 249.50 * | 84.88 ± 16.75 * |
| Honey jasmine tea | 1853.08 ± 384.00 | 65.62 ± 5.23 |
| Iced black tea | 1797.09 ± 301.41 | 58.26 ± 4.01 |
| Jasmine tea | 1960.09 ± 220.74 | 71.87 ± 7.57 |
| Jia duo bao herbal infusion | 2073.84 ± 292.95 | 70.82 ± 8.80 |
| Plum juice | 1752.74 ± 214.60 | 84.66 ± 5.37 * |
| Red bull | 1907.48 ± 312.00 | 76.12 ± 2.60 * |
| Rock candy pear juice | 1842.41 ± 148.05 | 62.93 ± 3.63 |
| Soda water | 1951.82 ± 237.80 | 68.22 ± 4.21 |
| Semen coicis herbal infusion | 1773.14 ± 169.46 | 59.32 ± 6.51 |
| Sprite | 1737.21 ± 384.33 | 70.45 ± 4.73 |
| Water chestnut juice | 2014.97 ± 233.86 | 70.51 ± 1.84 |
| Wang lao ji herbal infusion | 1992.95 ± 182.08 | 69.94 ± 3.86 |
| Group | ADH (%) | ALDH (%) |
|---|---|---|
| Ban sha herbal infusion | −19.47 ± 5.40 | −25.81 ± 5.41 |
| Coca cola | −2.09 ± 0.21 | −29.98 ± 10.27 * |
| Fresh orange juice | −4.80 ± 0.26 | −43.71 ± 10.12 * |
| Fructus cannabis herbal infusion | −2.82 ± 0.18 | −18.08 ± 5.33 |
| Green tea | 14.26 ± 1.24 * | 7.42 ± 2.32 |
| He qi zheng herbal infusion | 8.86 ± 0.55 | −23.94 ± 4.95 |
| Honey chrysanthemum tea | 7.78 ± 0.41 * | 12.26 ± 3.18 |
| Honey citron tea | −4.37 ± 0.22 | −21.68 ± 6.62 |
| Honey jasmine tea | 4.20 ± 0.60 | 4.69 ± 0.70 |
| Iced black tea | 6.11 ± 0.08 | 21.20 ± 1.79 * |
| Jasmine tea | 1.14 ± 0.17 | −41.56 ± 17.17 * |
| Jia duo bao herbal infusion | −2.02 ± 0.17 | 5.08 ± 1.53 |
| Plum juice | 1.34 ± 0.22 | −1.56 ± 0.82 |
| Red bull | 1.98 ± 0.24 | −8.89 ± 1.54 |
| Rock candy pear juice | 7.20 ± 0.48 | −7.85 ± 2.48 |
| Soda water | −6.39 ± 1.48 | 21.43 ± 3.16 * |
| Semen coicis herbal infusion | −8.85 ± 0.93 | −30.64 ± 0.79 |
| Sprite | −15.64 ± 3.81 | −15.33± 3.14 |
| Water chestnut juice | 2.05 ± 0.11 | −30.90 ± 3.56 * |
| Wang lao ji herbal infusion | 3.78 ± 0.55 | 1.64 ± 0.20 |
| Group | AST | ALT |
|---|---|---|
| Normal group | 40.11 ± 9.04 | 20.52 ± 7.14 |
| Control group | 55.28 ± 11.23 ** | 35.88 ± 11.04 ** |
| Ban sha herbal infusion | 101.68 ± 35.2 * | 34.10 ± 7.09 |
| Coca cola | 55.20 ± 13.91 | 35.36 ± 11.27 |
| Fresh orange juice | 77.97 ± 13.24 * | 49.01 ± 6.74 * |
| Fructus cannabis herbal infusion | 51.59 ± 14.06 | 19.91 ± 7.06 * |
| Green tea | 20.91 ± 9.95 * | 20.81 ± 1.70 * |
| He qi zheng herbal infusion | 49.54 ± 32.66 | 32.18 ± 8.99 |
| Honey chrysanthemum tea | 10.53 ± 2.43 * | 28.09 ± 1.24 * |
| Honey citron tea | 41.42 ± 10.40 | 38.45 ± 5.89 |
| Honey jasmine tea | 59.65 ± 15.89 | 32.94 ± 12.46 |
| Iced black tea | 46.44 ± 11.50 | 33.14 ± 9.91 |
| Jasmine tea | 32.03 ± 9.04 * | 38.45 ± 11.40 |
| Jia duo bao herbal infusion | 42.03 ± 17.06 | 25.44 ± 6.33 |
| Plum juice | 43.00 ± 14.90 | 36.24 ± 14.39 |
| Red bull | 57.03 ± 24.72 | 63.55 ± 17.87 * |
| Rock candy pear juice | 31.81 ± 11.04 | 28.80 ± 2.18 |
| Soda water | 16.31 ± 15.88 * | 25.11 ± 1.76 * |
| Semen coicis herbal infusion | 55.27 ± 11.49 | 28.06 ± 8.04 |
| Sprite | 78.15 ± 4.41 * | 38.15 ± 15.01 |
| Water chestnut juice | 55.38 ± 11.41 | 31.18 ± 6.21 |
| Wang lao ji herbal infusion | 69.43 ± 15.75 | 40.27 ± 9.81 |
| Group | MDA (nmol/mg protein) | SOD (U/mL) |
|---|---|---|
| Normal group | 0.75 ± 0.07 | 60.51 ± 3.66 |
| Control group | 0.86 ± 0.16 ** | 57.83 ± 7.62 |
| Ban sha herbal infusion | 0.96 ± 0.30 | 56.78 ± 2.75 |
| Coca cola | 0.84 ± 0.18 | 68.34 ± 10.00 |
| Fresh orange juice | 0.83 ± 0.23 | 55.61 ± 3.95 |
| Fructus cannabis herbal infusion | 0.74 ± 0.21 | 53.53 ± 4.45 |
| Green tea | 0.87 ± 0.15 | 60.52 ± 5.40 |
| He qi zheng herbal infusion | 0.55 ± 0.10 * | 51.57 ± 4.19 |
| Honey chrysanthemum tea | 0.68 ± 0.06 * | 61.98 ± 5.34 |
| Honey citron tea | 0.76 ± 0.08 | 55.63 ± 8.50 |
| Honey jasmine tea | 0.75 ± 0.10 | 59.94 ± 2.80 |
| Iced black tea | 0.98 ± 0.29 | 52.33 ± 6.02 |
| Jasmine tea | 0.89 ± 0.13 | 59.49 ± 4.62 |
| Jia duo bao herbal infusion | 0.79 ± 0.32 | 52.90 ± 3.59 * |
| Plum juice | 0.75 ± 0.08 | 58.82 ± 7.43 |
| Red bull | 0.78 ± 0.41 | 57.03 ± 3.20 |
| Rock candy pear juice | 0.66 ± 0.15 | 55.67 ± 4.53 |
| Soda water | 0.54 ± 0.10 * | 58.52 ± 6.29 |
| Semen coicis herbal infusion | 0.56 ± 0.19 * | 51.34 ± 2.87 * |
| Sprite | 0.76 ± 0.14 | 57.61 ± 1.77 |
| Water chestnut juice | 1.15 ± 0.27 | 61.49 ± 7.51 |
| Wang lao ji herbal infusion | 0.77 ± 0.24 | 58.19 ± 9.43 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhang, Y.-J.; Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Zhang, J.-J.; Li, S.; Xu, D.-P.; Li, H.-B. Effects of Beverages on Alcohol Metabolism: Potential Health Benefits and Harmful Impacts. Int. J. Mol. Sci. 2016, 17, 354. https://doi.org/10.3390/ijms17030354
Wang F, Zhang Y-J, Zhou Y, Li Y, Zhou T, Zheng J, Zhang J-J, Li S, Xu D-P, Li H-B. Effects of Beverages on Alcohol Metabolism: Potential Health Benefits and Harmful Impacts. International Journal of Molecular Sciences. 2016; 17(3):354. https://doi.org/10.3390/ijms17030354
Chicago/Turabian StyleWang, Fang, Yu-Jie Zhang, Yue Zhou, Ya Li, Tong Zhou, Jie Zheng, Jiao-Jiao Zhang, Sha Li, Dong-Ping Xu, and Hua-Bin Li. 2016. "Effects of Beverages on Alcohol Metabolism: Potential Health Benefits and Harmful Impacts" International Journal of Molecular Sciences 17, no. 3: 354. https://doi.org/10.3390/ijms17030354
APA StyleWang, F., Zhang, Y.-J., Zhou, Y., Li, Y., Zhou, T., Zheng, J., Zhang, J.-J., Li, S., Xu, D.-P., & Li, H.-B. (2016). Effects of Beverages on Alcohol Metabolism: Potential Health Benefits and Harmful Impacts. International Journal of Molecular Sciences, 17(3), 354. https://doi.org/10.3390/ijms17030354