Erythropoietin Ameliorates Neonatal Hypoxia-Ischemia-Induced Neurobehavioral Deficits, Neuroinflammation, and Hippocampal Injury in the Juvenile Rat
Abstract
:1. Introduction
2. Results and Discussion
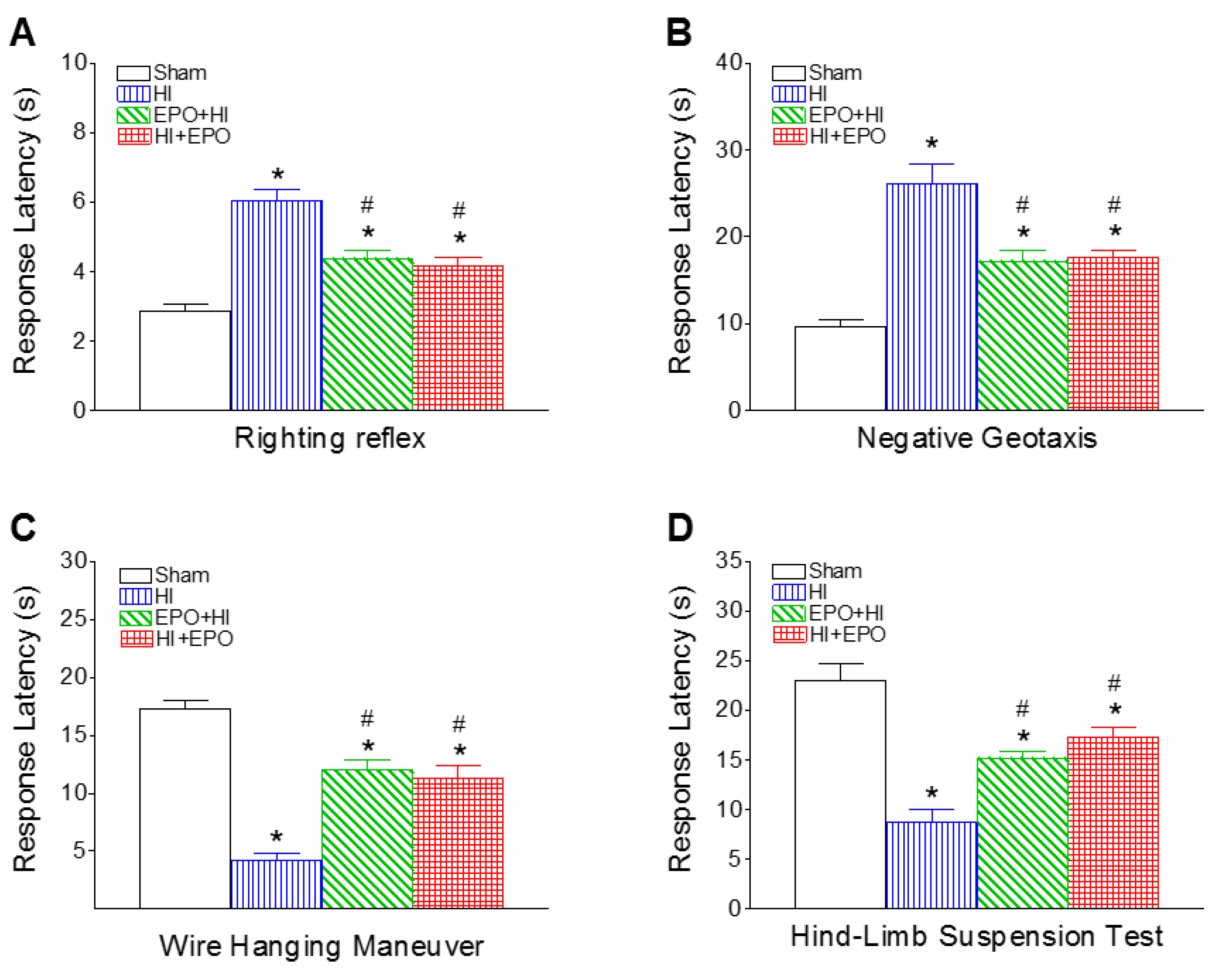
2.1. Recombinant EPO (rEPO) Improved Sensorimotor Deficits Induced by HI
2.1.1. Righting Reflex
2.1.2. Negative Geotaxis
2.1.3. Wire Hanging Maneuver
2.1.4. Hind-Limb Suspension Test
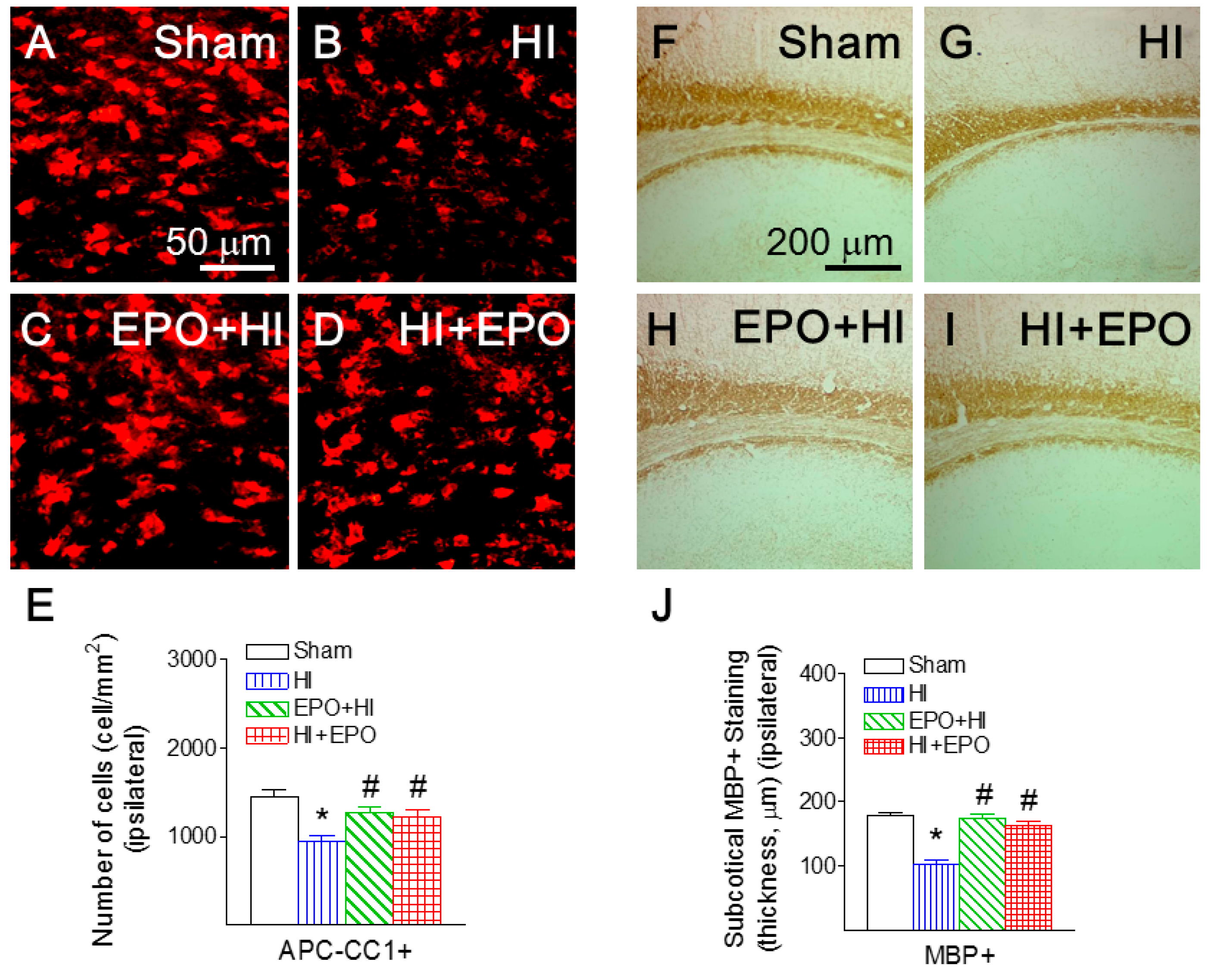
2.2. rEPO Ameliorated HI-Induced Loss of Mature Oligodendrocytes and Myelination in White Matter
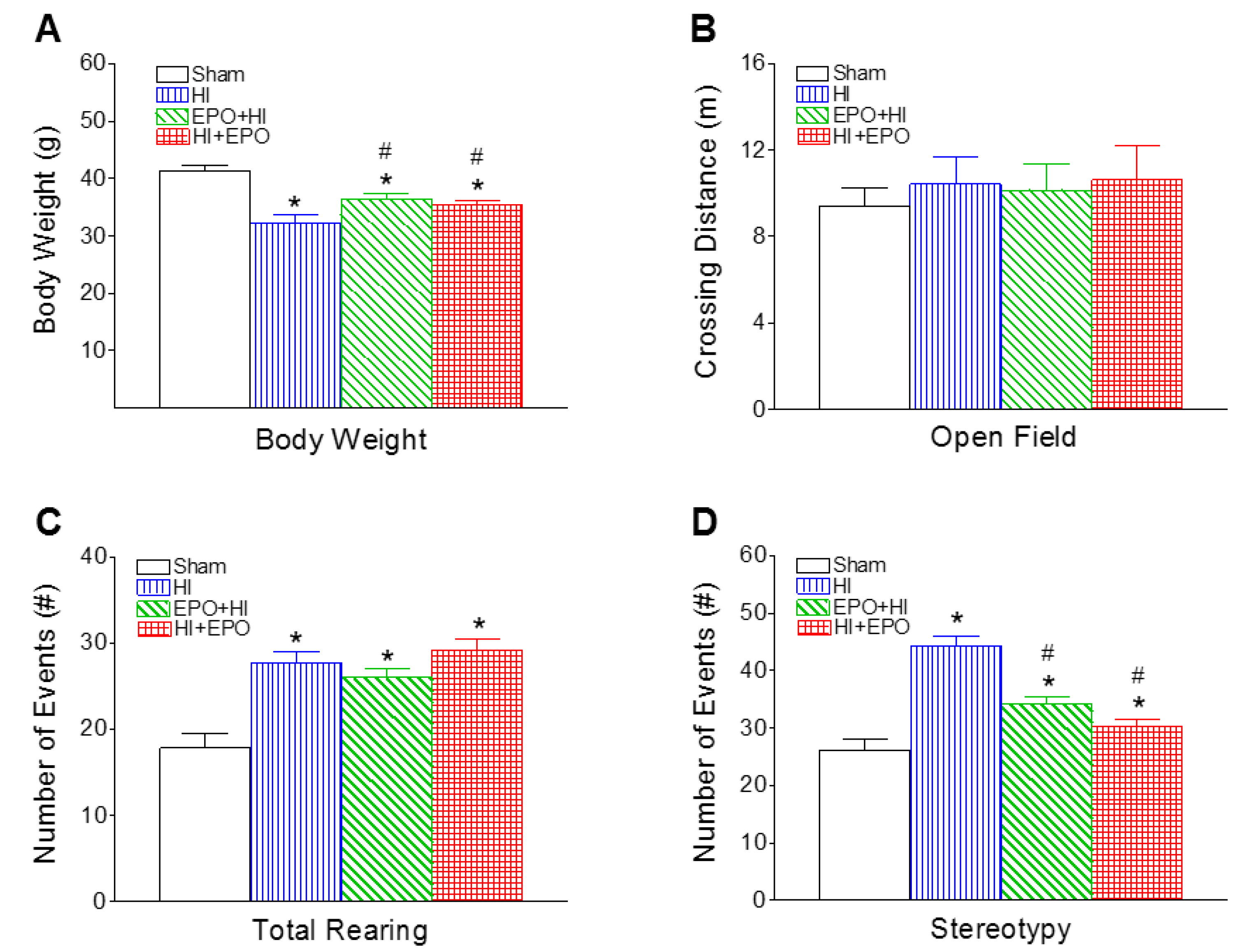
2.3. rEPO Improved Motor Behavioral Deficits Induced by HI
2.3.1. Locomotion and Stereotypy
2.3.2. Vibrissa-Elicited Forelimb-Placing Test
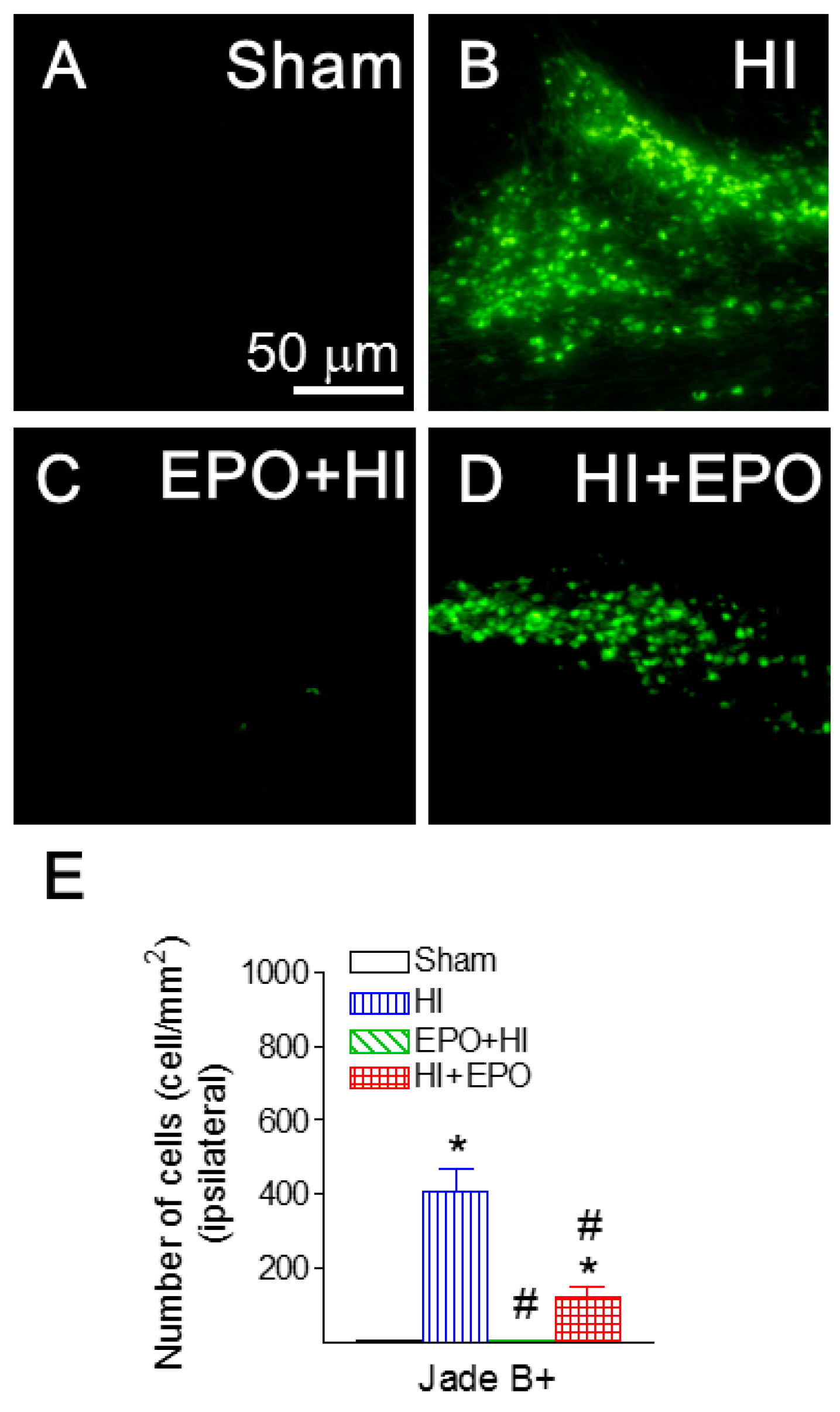
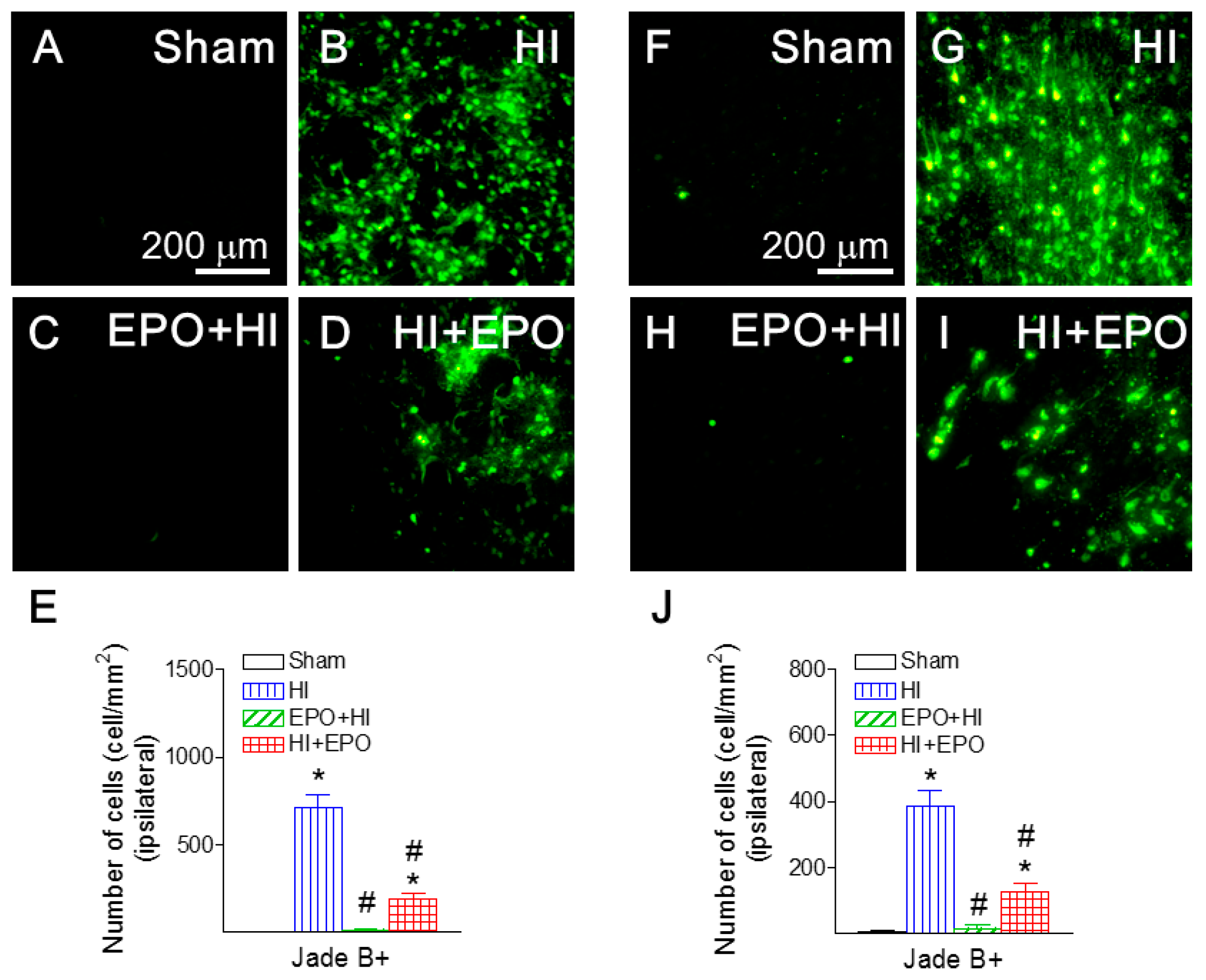
2.4. rEPO Ameliorated HI-Induced Neuronal Death in Striatum and Cortex
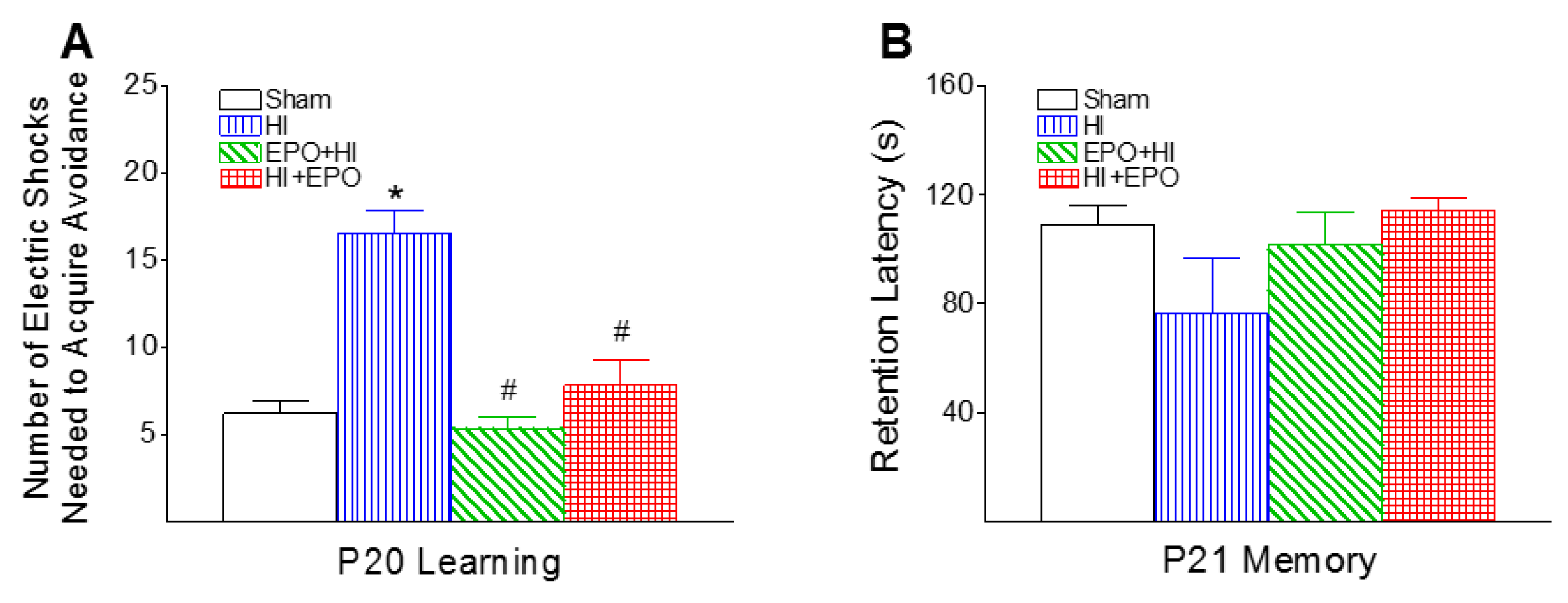
2.5. rEPO Improved Learning Deficits Induced by HI
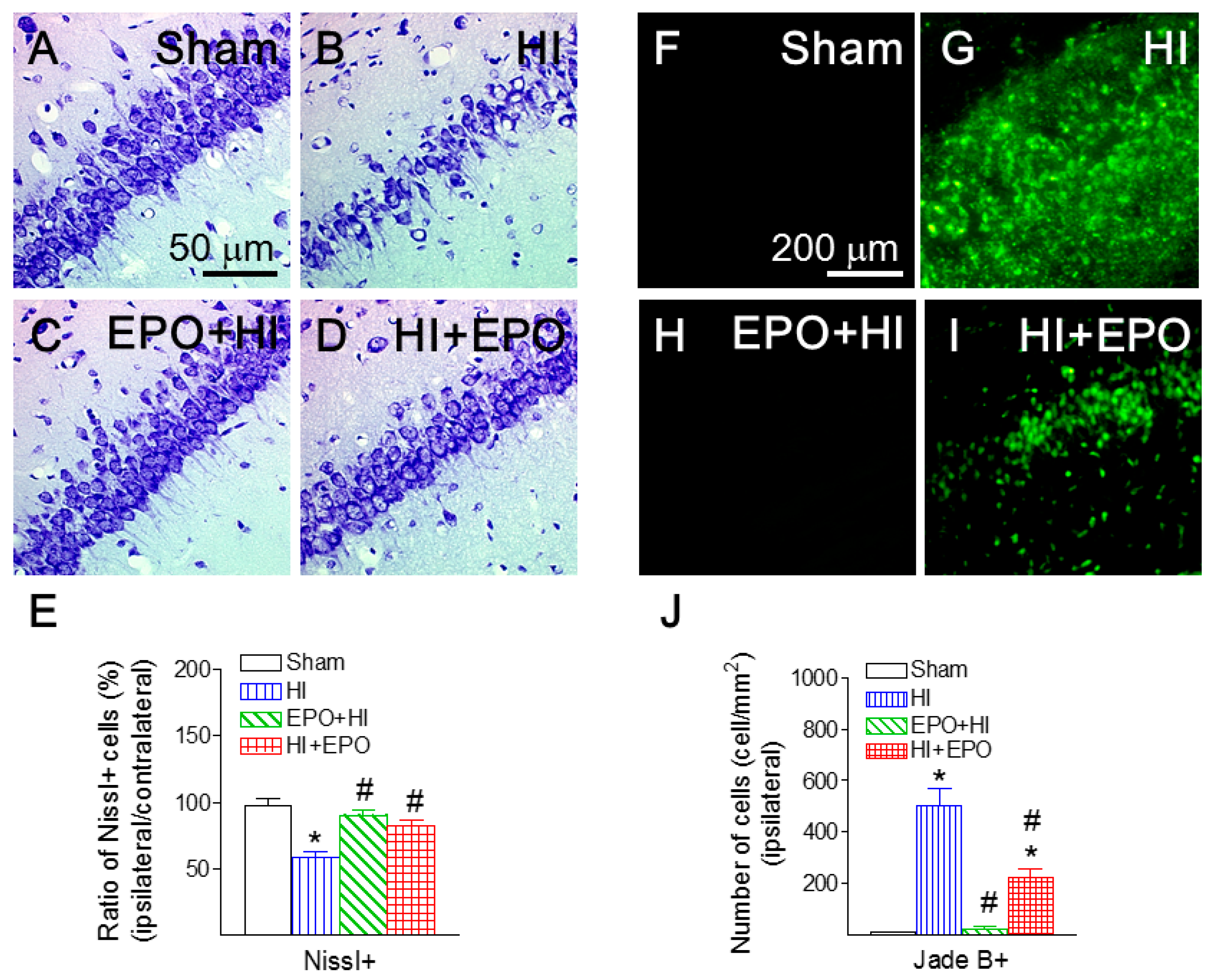
2.6. rEPO Ameliorated HI-Induced Hippocampal Injury
2.7. rEPO Reduced HI-Induced Microglial Activation
3. Experimental Section
3.1. Chemicals
3.2. Animals
3.3. Surgery Procedures and Animal Treatment
3.4. Behavioral Testing
3.4.1. Righting Reflex
3.4.2. Negative Geotaxis
3.4.3. Wire Hanging Maneuver
3.4.4. Hind-Limb Suspension Test
3.4.5. Locomotion and Stereotypy
3.4.6. Vibrissa-Elicited Forelimb-Placing Test
3.4.7. Passive Avoidance Test
3.5. Immunohistochemistry Studies
3.6. Quantification of Staining Data
3.7. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Volpe, J.J. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics 2003, 112, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Lin, S.; Rhodes, P.G. Neuroprotective effects of N-acetylaspartylglutamate in a neonatal rat model of hypoxia-ischemia. Eur. J. Pharmacol. 2002, 437, 139–145. [Google Scholar] [CrossRef]
- Back, S.A.; Miller, S.P. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Ann. Neurol. 2014, 75, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Elitt, C.M.; Rosenberg, P.A. The challenge of understanding cerebral white matter injury in the premature infant. Neuroscience 2014, 276, 216–238. [Google Scholar] [CrossRef] [PubMed]
- Fauchere, J.C.; Koller, B.M.; Tschopp, A.; Dame, C.; Ruegger, C.; Bucher, H.U.; Zeilinger, G.; Pasquier, S.; Bührer, C.; Glanzmann, R.; et al. Safety of Early High-Dose Recombinant Erythropoietin for Neuroprotection in Very Preterm Infants. J. Pediatr. 2015, 167, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E. Erythropoietin as a neonatal neuronprotectant: Basic and clinical studies. Hematol. Meet. Rep. 2006, 2, 108–112. [Google Scholar]
- Juul, S.E.; Ferriero, D.M. Pharmacologic neuroprotective strategies in neonatal brain injury. Clin. Perinatol. 2014, 41, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Messier, A.M.; Ohls, R.K. Neuroprotective effects of erythropoiesis-stimulating agents in term and preterm neonates. Curr. Opin. Pediatr. 2014, 26, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, V.; Juul, S.E. Erythropoietin: Emerging role of erythropoietin in neonatal neuroprotection. Pediatr. Neurol. 2014, 51, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Demers, E.J.; Mcpherson, R.J.; Juul, S.E. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr. Res. 2005, 58, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; van Bel, F.; van der Kooij, M.A.; Heijnen, C.J.; Groenendaal, F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr. Res. 2013, 73, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Heijnen, C.J.; van der Kooij, M.A.; Groenendaal, F.; van Bel, F. Beneficial effect of erythropoietin on sensorimotor function and white matter after hypoxia-ischemia in neonatal mice. Pediatr. Res. 2011, 69, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.F.; Abel, R.; Almli, C.R.; Mu, D.; Wendland, M.; Ferriero, D.M. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev. Neurosci. 2009, 31, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Cao, G.; Yin, W.; Stetler, R.A.; Liu, J.; Chen, J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke 2007, 38, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Kellert, B.A.; McPherson, R.J.; Juul, S.E. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr. Res. 2007, 61, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Wyatt, J.S.; Azzopardi, D.; Ballard, R.; Edwards, A.D.; Ferriero, D.M.; Polin, R.A.; Robertson, C.M.; Thoresen, M.; Whitelaw, A.; et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentr randomized trial. Lancet 2005, 365, 663–670. [Google Scholar] [CrossRef]
- Shankaran, S.; Laptook, A.R. Hypothermia as a treatment for birth asphyxia. Clin. Obstet. Gynecol. 2007, 50, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Han, B.H.; Luo, N.L.; Chricton, C.A.; Xanthoudakis, S.; Tam, J.; Arvin, K.L.; Holtzman, D.M. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. 2002, 22, 455–463. [Google Scholar] [PubMed]
- Back, S.A.; Luo, N.L.; Borensteinm, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [PubMed]
- Craig, A.; Luo, N.L.; Beardsley, D.J.; Wingate-Pearse, N.; Walker, D.W.; Hohimer, A.R.; Back, S.A. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation to human. Exp. Neurol. 2003, 181, 231–240. [Google Scholar] [CrossRef]
- Wen, T.C.; Rogido, M.; Peng, H.; Genetta, T.; Moore, J.; Sola, A. Gender differences in long-term beneficial effects of erythropoietin given after neonatal stroke in postnatal day-7 rats. Neuroscience 2006, 139, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Sudarshan, K.; Das, G.D.; McCormick, N.; Barnes, D. The influence of nutrition on neural and behavioral development: III. Development of some motor, particularly locomotor patterns during infancy. Dev. Psychobiol. 1971, 4, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Hermans, R.H.; Hunter, D.E.; McGivern, R.F.; Cain, C.D.; Longo, L.D. Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol. Teratol. 1992, 14, 119–129. [Google Scholar] [CrossRef]
- Altman, J.; Sudarshan, K. Postnatal development of locomotion in the laboratory rat. Anim. Behav. 1975, 23, 896–920. [Google Scholar] [CrossRef]
- El-Khodor, B.F.; Edgar, N.; Chen, A.; Winberg, M.L.; Joyce, C.; Brunner, D.; Suárez-Fariñas, M.; Heyes, M.P. Identification of a battery of tests for drug candidate evaluation in the SMNDΔ7 neonate model of spinal muscular atrophy. Exp. Neurol. 2008, 212, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Murtie, J.C.; Zhou, Y.X.; Le, T.Q.; Armstrong, R.C. In vivo analysis of oligodendrocyte lineage development in postnatal FGF2 null mice. Glia 2005, 49, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Lin, S.; Pang, Y.; Lei, M.; Zhang, F.; Rhodes, P.G.; Cai, Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav. Brain Res. 2005, 165, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Lin, S.; Pang, Y.; Rhodes, P.G.; Cai, Z. Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. Eur. J. Neurosci. 2006, 24, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade-B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Haroutunian, V.; Katsel, P.; Roussos, P.; Davis, K.L.; Altshuler, L.L.; Bartzokis, G. Myelination, oligodendrocytes, and serious mental illness. Glia 2014, 62, 1856–1877. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.; Marom, R.; Berger, I.; Ben Bashat, D.; Gross-Tsur, V.; Ben-Sira, L.; Artzi, M.; Uliel, S.; Leitner, Y.; Geva, R. Neonatal neuropsychology: Emerging relations of neonatal sensory-motor responses to white matter integrity. Neuropsychologia 2014, 62, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sugawa, M.; Sakurai, Y.; Ishikawa-Ieda, Y.; Suzuki, H.; Asou, H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci. Res. 2002, 22, 391–403. [Google Scholar] [CrossRef]
- Antoniou, K.; Papathanasiou, G.; Panagis, G.; Nomikos, G.G.; Hyphantis, T.; Papadopoulou-Daifoti, Z. Individual responses to novelty predict qualitative differences in d-amphetamine-induced open field but not reward-related behaviors in rats. Neuroscience 2004, 123, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Woodlee, M.T.; Asseo-garcia, A.M.; Zhao, X.; Liu, S.J.; Jones, T.A.; Schallert, T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp. Neurol. 2005, 191, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Bakos, J.; Duncko, R.; Makatsori, A.; Pirnik, Z.; Kiss, A.; Jezova, D. Prenatal immune challenge affects growth, behavior, and brain dopamine in offspring. Ann. N. Y. Acad. Sci. 2004, 1018, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, F.; Zou, X.; Torbey, M. Comparison of unbiased estimation of neuronal number in the rat hippocampus with different staining methods. J. Neurosci. Methods 2015, 254, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Juul, S.E.; Beyer, R.P.; Bammler, T.K.; McPherson, R.J.; Wilkerson, J.; Farin, F.M. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr. Res. 2009, 65, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Calvert, J.W.; Zhang, J.H. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke 2005, 36, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.; Bigini, P.; Mennini, T.; Agnello, D.; Laragione, T.; Cagnotto, A.; Viviani, B.; Marinovich, M.; Cerami, A.; Coleman, T.R.; et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 2003, 198, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Dombrowski, S.C. Prenatal central nervous system development. In Prenatal Exposures: Psychological and Educational Consequences for Children; Springer: New York, NY, USA, 2008; pp. 15–25. [Google Scholar]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Salmeen, K.E.; Jelin, A.C.; Thiet, M.P. Perinatal neuroprotection. F1000Prime Rep. 2014, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Wang, Y.L.; Lo, W.T.; Wu, C.C.; Hsieh, C.W.; Huang, C.F.; Lan, Y.H.; Wang, C.C.; Chang, D.M.; Sytwu, H.K. Erythropoietin enhances endogenous haem oxygenase-1 and represses immune responses to ameliorate experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Wiessner, C.; Allegrini, P.R.; Ekatodramis, D.; Jewell, U.R.; Stallmach, T.; Gassmann, M. Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin. J. Cereb. Blood Flow Metab. 2001, 21, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Wang, L.; Asavaritkrai, P.; Noguchi, C.T. Up-regulation of erythropoietin receptor by nitric oxide mediates hypoxia preconditioning. J. Neurosci. Res. 2010, 88, 3180–3188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Di, L.; Noguchi, C.T. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int. J. Biol. Sci. 2014, 10, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.M.; Yankowitz, J.; Widness, J.A.; Strauss, R.G. Etiology of differences in hematocrit between males and females: Sequence-based polymorphisms in erythropoietin and its receptor. J. Gend. Specif. Med. 2001, 4, 35–40. [Google Scholar] [PubMed]
- Cohen, S.S.; Stonestreet, B.S. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic injury. Exp. Neurol. 2014, 256, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Tin, L.T.; Zheng, B.; Pang, Y.; Rhodes, P.G.; Cai, Z. Interleukin-1β-induced brain injury and neurobehavioral dysfunctions in juvenile rats can be attenuated by α-phenyl-n-tert-butyl-nitrone. Neuroscience 2010, 168, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Kaizaki, A.; Tien, L.T.; Pang, Y.; Cai, Z.; Tanaka, S.; Numazawa, S.; Bhatt, A.J.; Fan, L.W. Celecoxib reduces brain dopaminergic neuronaldysfunction, and improves sensorimotor behavioral performance in neonatal rats exposed to systemic lipopolysaccharide. J. Neuroinflamm. 2013, 45, 10–23. [Google Scholar]
- Fan, L.W.; Pang, Y.; Lin, S.; Tin, L.T.; Ma, T.; Rhodes, P.G.; Cai, Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 2005, 82, 71–82. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, K.-M.; Tien, L.-T.; Cai, Z.; Lin, S.; Pang, Y.; Tanaka, S.; Rhodes, P.G.; Bhatt, A.J.; Savich, R.D.; Fan, L.-W. Erythropoietin Ameliorates Neonatal Hypoxia-Ischemia-Induced Neurobehavioral Deficits, Neuroinflammation, and Hippocampal Injury in the Juvenile Rat. Int. J. Mol. Sci. 2016, 17, 289. https://doi.org/10.3390/ijms17030289
Lan K-M, Tien L-T, Cai Z, Lin S, Pang Y, Tanaka S, Rhodes PG, Bhatt AJ, Savich RD, Fan L-W. Erythropoietin Ameliorates Neonatal Hypoxia-Ischemia-Induced Neurobehavioral Deficits, Neuroinflammation, and Hippocampal Injury in the Juvenile Rat. International Journal of Molecular Sciences. 2016; 17(3):289. https://doi.org/10.3390/ijms17030289
Chicago/Turabian StyleLan, Kuo-Mao, Lu-Tai Tien, Zhengwei Cai, Shuying Lin, Yi Pang, Sachiko Tanaka, Philip G. Rhodes, Abhay J. Bhatt, Renate D. Savich, and Lir-Wan Fan. 2016. "Erythropoietin Ameliorates Neonatal Hypoxia-Ischemia-Induced Neurobehavioral Deficits, Neuroinflammation, and Hippocampal Injury in the Juvenile Rat" International Journal of Molecular Sciences 17, no. 3: 289. https://doi.org/10.3390/ijms17030289





