Plasma Monocyte Chemoattractant Protein-1 Level as a Predictor of the Severity of Community-Acquired Pneumonia
Abstract
:1. Introduction
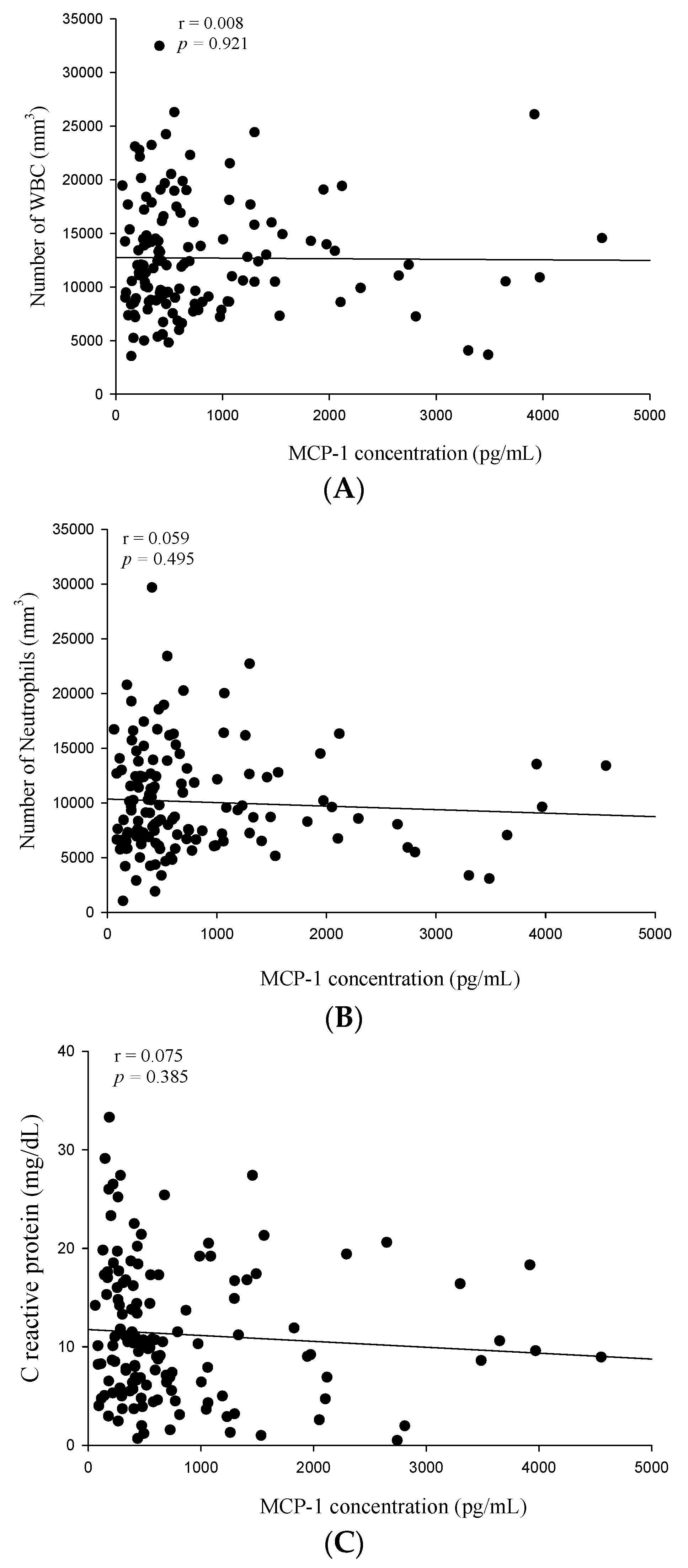
2. Results
| Clinical Variable | Controls (n = 74) Median (Range) | Before Antibiotic Treatment (n = 137) Median (Range) | After Antibiotic Treatment (n = 137) Median (Range) | p Value UT/C b | p Value UT/T c |
|---|---|---|---|---|---|
| Age | 59.89 ± 11.04 d | 64.89 ± 20.89 d | - | p = 0.057 | - |
| Gender | - | - | - | - | |
| Male | 49 (66.2%) | 87 (63.5%) | - | p = 0.694 | - |
| Female | 25 (33.8%) | 50 (36.5%) | - | - | - |
| CRP (mg/dL) | 0.40 (0.2–1.50) | 10.1 (0.50–33.30) | 2.10 (0.10–17.20) | p < 0.001 | p < 0.001 |
| WBCs (cells/mm3) | 5900 (2980–13,700) | 12,030 (3560–32,480) | 8280 (3300–28,180) | p < 0.001 | p < 0.001 |
| Neutrophils (cells/mm3) | 3580 (1078–9946) | 8715 (1032–29,686) | 5616 (1518–25,841) | p < 0.001 | p < 0.001 |
| PSI score | - | 83.89 ± 36.63 d | - | - | - |
| CURB-65 score | - | 1.12 ± 0.94 d | - | - | - |
| APACHE II score | - | 10.20 ± 5.27 d | - | - | - |
| Hospital length of stay (Days) | - | 10.37 ±14.54 d | - | - | - |


| Variable | WBC (n = 137) | CRP (n = 137) | MCP-1 (n = 137) | |||
|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | |
| PSI score | −0.034 | 0.690 | −0.007 | 0.931 | 0.509 | <0.001 |
| CURB-65 score | 0.036 | 0.674 | 0.027 | 0.758 | 0.468 | <0.001 |
| APACHE II score | 0.050 | 0.563 | 0.015 | 0.859 | 0.360 | <0.001 |
| Length of hospital stay | −0.040 | 0.641 | 0.024 | 0.779 | 0.049 | 0.567 |
3. Discussion
4. Materials and Methods
4.1. Participants and Diagnoses
4.2. Patients and Blood Sample Collection
4.3. Measurements of the White Blood Cell (WBC) and Neutrophil Counts and C-Reactive Protein (CRP) Level
4.4. Measurement of Plasma MCP-1 Levels
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious diseases society of america/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl. S2), S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; Anzueto, A.R. Changing needs of community-acquired pneumonia. J. Antimicrob. Chemother. 2011, 66 (Suppl. S3), iii3–iii9. [Google Scholar] [CrossRef] [PubMed]
- Department of Statistics of Ministry of Health and Welfare in Taiwan. Causes of Death in Taiwan, 2012; Ministry of Health and Welfare in Taiwan: Taipei, Taiwan, 2013. [Google Scholar]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011, 17 (Suppl. S6), E1–E59. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.; Bolibar, I.; Toran, P.; Pera, G.; Boquet, X.; Balanzo, X.; Sauca, G. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest 2004, 125, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Nexoe, J.; Bistrup, L.A.; Pedersen, S.S.; Obel, N.; Nielsen, L.P.; Pedersen, C. Aetiology and prediction of pneumonia in lower respiratory tract infection in primary care. Br. J. Gen. Pract. 2007, 57, 547–554. [Google Scholar] [PubMed]
- Huang, D.T.; Weissfeld, L.A.; Kellum, J.A.; Yealy, D.M.; Kong, L.; Martino, M.; Angus, D.C.; Gen, I.M.S.I. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann. Emerg. Med. 2008, 52, 48–58 e42. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Harbarth, S.; Stolz, D.; Bingisser, R.; Mueller, C.; Leuppi, J.; Nusbaumer, C.; Tamm, M.; Christ-Crain, M. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect. Dis. 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Standiford, T.J.; Kunkel, S.L.; Phan, S.H.; Rollins, B.J.; Strieter, R.M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J. Biol. Chem. 1991, 266, 9912–9918. [Google Scholar] [PubMed]
- Lundien, M.C.; Mohammed, K.A.; Nasreen, N.; Tepper, R.S.; Hardwick, J.A.; Sanders, K.L.; van Horn, R.D.; Antony, V.B. Induction of MCP-1 expression in airway epithelial cells: Role of CCR2 receptor in airway epithelial injury. J. Clin. Immunol. 2002, 22, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.K.; Kwon, H.; Khil, L.Y.; Zhang, L.; Jun, H.S.; Yoon, J.W. IL-18 induces monocyte chemotactic protein-1 production in macrophages through the phosphatidylinositol 3-kinase/Akt and MEK/ERK1/2 pathways. J. Immunol. 2005, 175, 8280–8286. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.A.; Standiford, T.J. Cytokine immunotherapy during bacterial pneumonia: From benchtop to bedside. Semin. Respir. Infect. 2001, 16, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Balamayooran, G.; Batra, S.; Balamayooran, T.; Cai, S.; Jeyaseelan, S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during escherichia coli infection. Infect. Immun. 2011, 79, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.M.; Baggiolini, M.; Charo, I.F.; Hebert, C.A.; Horuk, R.; Matsushima, K.; Miller, L.H.; Oppenheim, J.J.; Power, C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000, 52, 145–176. [Google Scholar] [PubMed]
- Kurihara, T.; Warr, G.; Loy, J.; Bravo, R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 1997, 186, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Kohro, T.; Kodama, T.; Nagata, S.; Fukunaga, R. Identification of CCR2, flotillin, and gp49B genes as new G-CSF targets during neutrophilic differentiation. J. Leukoc. Biol. 2005, 78, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Mai, J.; Cai, S.; Jeyaseelan, S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 2009, 77, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, M.F.; Azak, A.; Denizli, N.; Huddam, B.; Kocak, G.; Gucun, M.; Tatlisu, M.A.; Demirci, R.; Yilmaz, B.; Dikec, M.; et al. MCP-1 and soluble tweak levels are independently associated with coronary artery disease severity in patients with chronic kidney disease. Ren. Fail. 2015, 37, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Liou, L.B.; Tsai, W.P.; Chang, C.J.; Chao, W.J.; Chen, M.H. Blood monocyte chemotactic protein-1 (MCP-1) and adapted disease activity score28-MCP-1: Favorable indicators for rheumatoid arthritis activity. PLoS ONE 2013, 8, e55346. [Google Scholar]
- Zhang, X.W.; Qin, X.; Qin, C.Y.; Yin, Y.L.; Chen, Y.; Zhu, H.L. Expression of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in non-small cell lung cancer and its significance. Cancer Immunol. Immunother. 2013, 62, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Lebrecht, A.; Hefler, L.; Tempfer, C.; Koelbl, H. Serum cytokine concentrations in patients with cervical cancer: Interleukin-4, interferon-γ, and monocyte chemoattractant protein-1. Gynecol. Oncol. 2001, 83, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Hefler, L.; Tempfer, C.; Heinze, G.; Mayerhofer, K.; Breitenecker, G.; Leodolter, S.; Reinthaller, A.; Kainz, C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br. J. Cancer 1999, 81, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Griese, M.; Nicolai, T.; Zissel, G.; Prell, C.; Reinhardt, D.; Schendel, D.J.; Krauss-Etschmann, S. A role for MCP-1/CCR2 in interstitial lung disease in children. Respir. Res. 2005, 6, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.P.; Lipworth, B.J. C-reactive protein in simple community-acquired pneumonia. Chest 1995, 107, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Flanders, S.A.; Stein, J.; Shochat, G.; Sellers, K.; Holland, M.; Maselli, J.; Drew, W.L.; Reingold, A.L.; Gonzales, R. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am. J. Med. 2004, 116, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ortqvist, A.; Hedlund, J.; Wretlind, B.; Carlstrom, A.; Kalin, M. Diagnostic and prognostic value of interleukin-6 and C-reactive protein in community-acquired pneumonia. Scand. J. Infect. Dis. 1995, 27, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hohenthal, U.; Hurme, S.; Helenius, H.; Heiro, M.; Meurman, O.; Nikoskelainen, J.; Kotilainen, P. Utility of C-reactive protein in assessing the disease severity and complications of community-acquired pneumonia. Clin. Microbiol. Infect. 2009, 15, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, F.M.; Al-Nawas, B.; Krummenauer, F.; Forycki, Z.F.; Shah, P.M. Procalcitonin, C-reactive protein and apache II score for risk evaluation in patients with severe pneumonia. Clin. Microbiol. Infect. 2002, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Thiem, U.; Niklaus, D.; Sehlhoff, B.; Stuckle, C.; Heppner, H.J.; Endres, H.G.; Pientka, L. C-reactive protein, severity of pneumonia and mortality in elderly, hospitalised patients with community-acquired pneumonia. Age Ageing 2009, 38, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Polzin, A.; Pletz, M.; Erbes, R.; Raffenberg, M.; Mauch, H.; Wagner, S.; Arndt, G.; Lode, H. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Eur. Respir. J. 2003, 21, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Tateda, K.; Kusano, E.; Matsumoto, T.; Kimura, K.; Uchida, K.; Nakata, K.; Yamaguchi, K. Semi-quantitative analysis of streptococcus pneumoniae urinary antigen: Kinetics of antigen titers and severity of diseases. Scand. J. Infect. Dis. 2006, 38, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, J.; Riches, P.; Gooding, R.; Soni, N.; Hobbs, J.R. C-reactive protein and its cytokine mediators in intensive-care patients. Clin. Chem. 1993, 39, 147–150. [Google Scholar] [PubMed]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Whicher, J.; Bienvenu, J.; Monneret, G. Procalcitonin as an acute phase marker. Ann. Clin. Biochem. 2001, 38, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.; Welte, T. Biomarkers in community-acquired pneumonia. Expert Rev. Respir. Med. 2012, 6, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.J.; Yang, H.W.; Tsao, S.M.; Cheng, C.W.; Bien, M.Y.; Yu, M.C.; Bai, K.J.; Yang, S.F.; Chien, M.H. Plasma long pentraxin 3 (PTX3) concentration is a novel marker of disease activity in patients with community-acquired pneumonia. Clin. Chem. Lab. Med. 2013, 51, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Hsiao, P.C.; Tsai, H.T.; Yeh, C.B.; Yang, S.F. Usefulness of plasma YKL-40 in management of community-acquired pneumonia severity in patients. Int. J. Mol. Sci. 2013, 14, 22817–22825. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Ugajin, M.; Suzuki, M.; Noguchi, S.; Tanaka, W.; Yoshihara, H.; Kawakami, M.; Kichikawa, Y.; Sakamoto, Y. Diagnostic and prognostic value of procalcitonin in community-acquired pneumonia. Am. J. Med. Sci. 2012, 343, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Holub, M.; Lawrence, D.A.; Andersen, N.; Davidova, A.; Beran, O.; Maresova, V.; Chalupa, P. Cytokines and chemokines as biomarkers of community-acquired bacterial infection. Mediat. Inflamm. 2013, 2013, 190145. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. Apache II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.I.; Lewis, S.A.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, K.-K.; Chang, J.-H.; Chien, M.-H.; Tsao, S.-M.; Yu, M.-C.; Bai, K.-J.; Tsao, T.C.-Y.; Yang, S.-F. Plasma Monocyte Chemoattractant Protein-1 Level as a Predictor of the Severity of Community-Acquired Pneumonia. Int. J. Mol. Sci. 2016, 17, 179. https://doi.org/10.3390/ijms17020179
Yong K-K, Chang J-H, Chien M-H, Tsao S-M, Yu M-C, Bai K-J, Tsao TC-Y, Yang S-F. Plasma Monocyte Chemoattractant Protein-1 Level as a Predictor of the Severity of Community-Acquired Pneumonia. International Journal of Molecular Sciences. 2016; 17(2):179. https://doi.org/10.3390/ijms17020179
Chicago/Turabian StyleYong, Kok-Khun, Jer-Hwa Chang, Ming-Hsien Chien, Shih-Ming Tsao, Ming-Chih Yu, Kuan-Jen Bai, Thomas Chang-Yao Tsao, and Shun-Fa Yang. 2016. "Plasma Monocyte Chemoattractant Protein-1 Level as a Predictor of the Severity of Community-Acquired Pneumonia" International Journal of Molecular Sciences 17, no. 2: 179. https://doi.org/10.3390/ijms17020179







