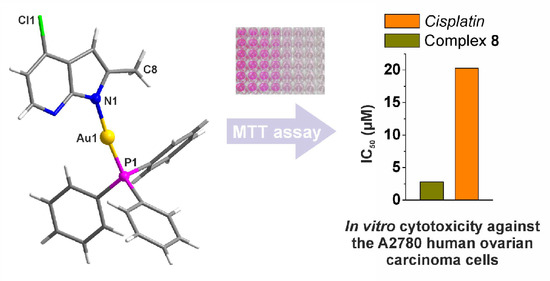
In Vitro Antitumor Active Gold(I) Triphenylphosphane Complexes Containing 7-Azaindoles
Abstract
:1. Introduction
2. Results
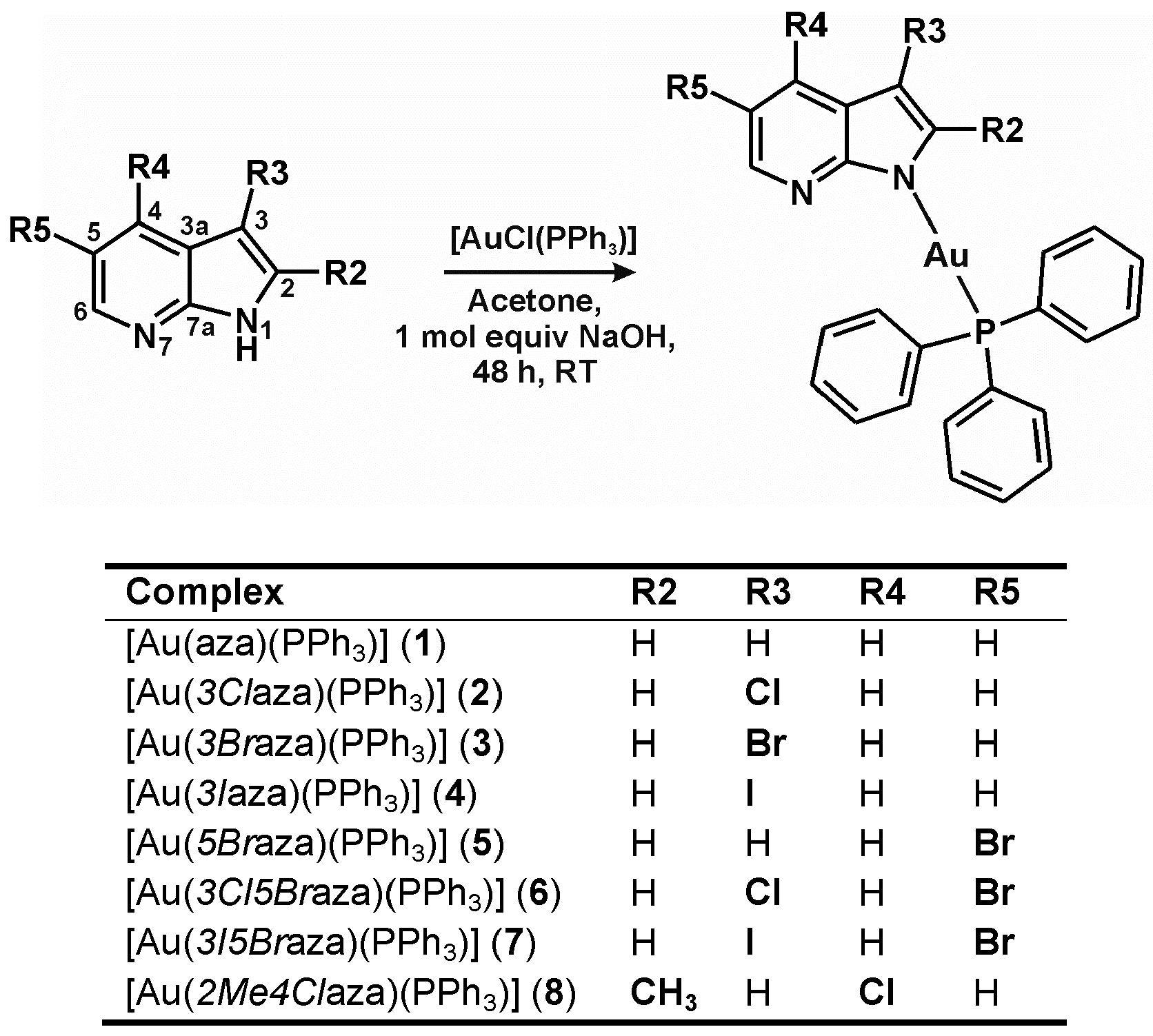
2.1. Chemistry
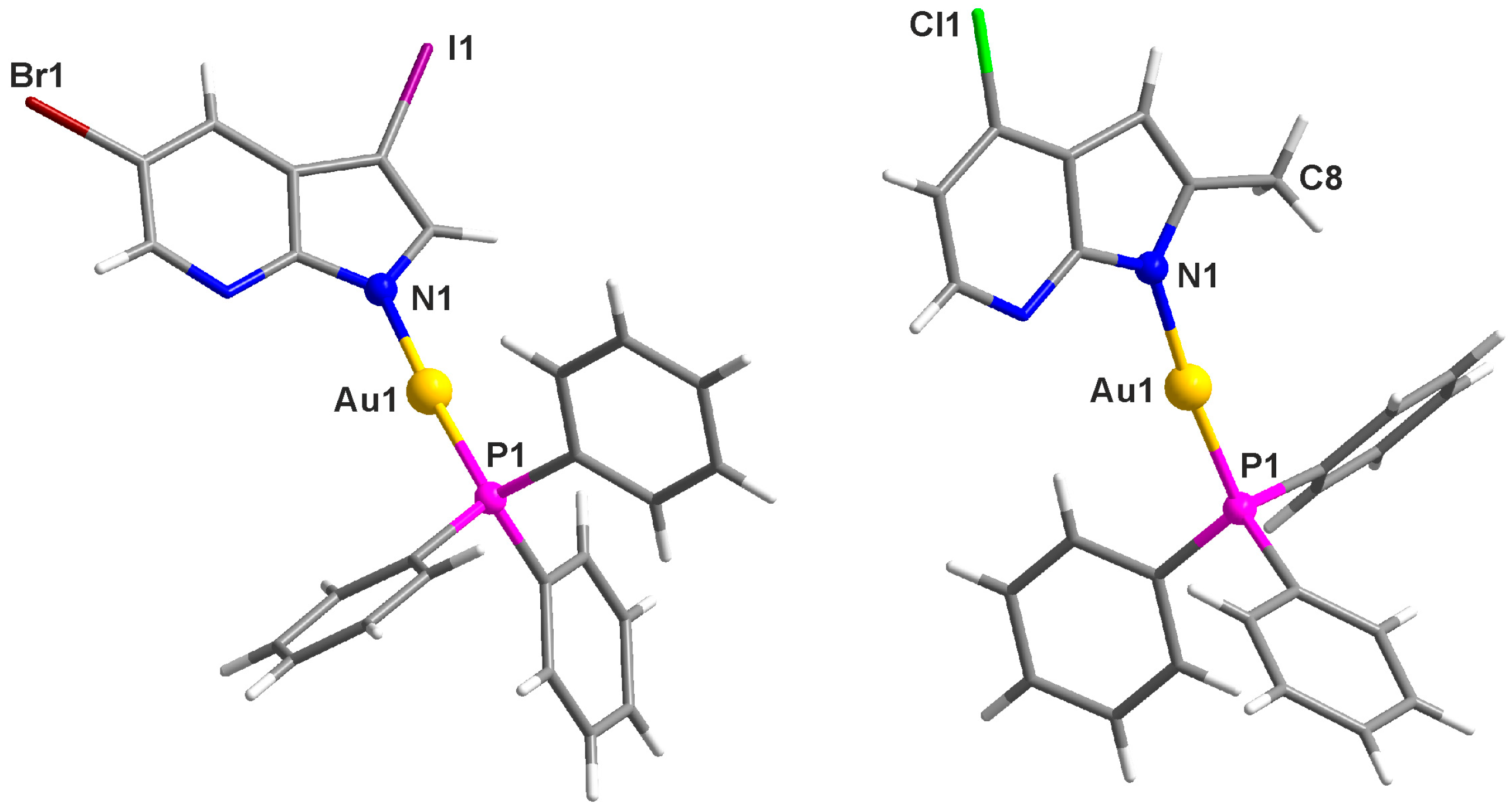
2.2. Single Crystal X-ray Analysis
2.3. Aqueous Chemistry and Interaction Studies
2.3.1. Solution Behaviour in a Water/DMF Mixture
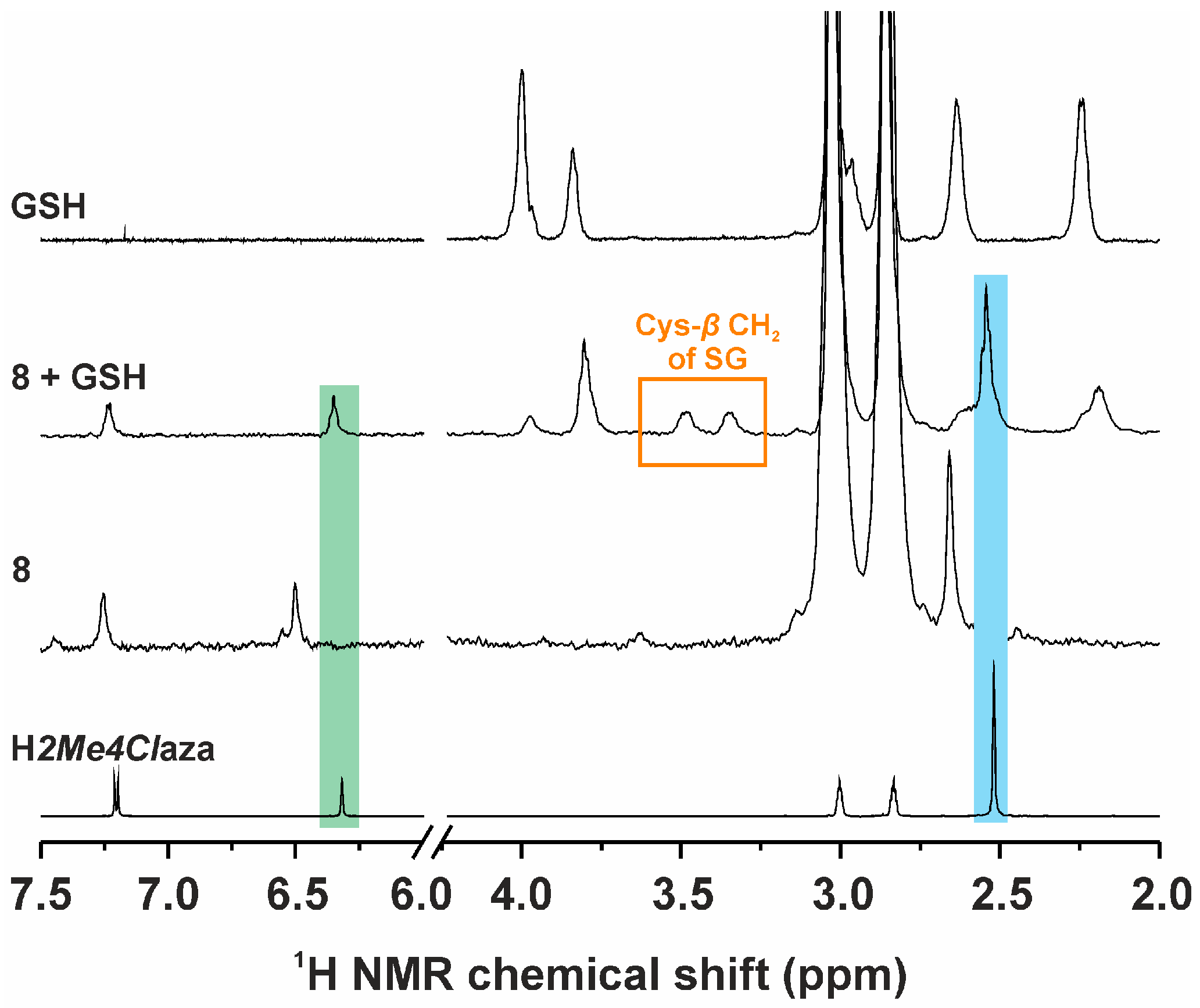
2.3.2. Interaction Studies with Biomolecules
2.4. In Vitro Antitumor Activity
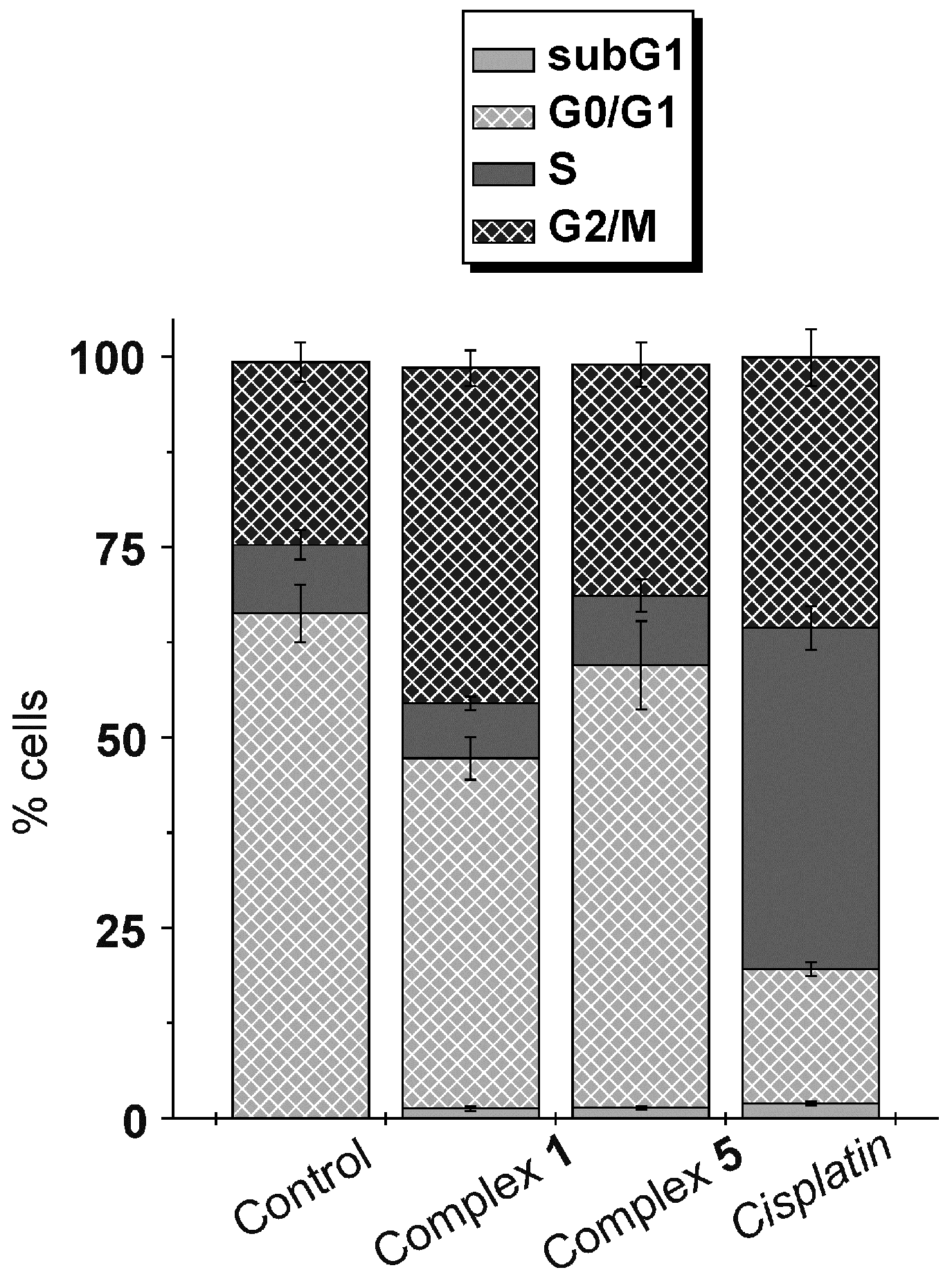
2.5. Cell Cycle Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.3. General Methods
4.4. Single Crystal X-ray Analysis
4.5. Studies of Aqueous Chemistry and Interaction with Biomolecules
4.6. In Vitro Cytotoxicity Testing
4.7. Cell Cycle Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gielen, M.; Tiekink, E.R.T. Metallotherapeutic Drugs and Metal Based Diagnostic Agents; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.F., III. Gold-Based therapeutic agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Nardon, C.; Pettenuzzo, N.; Fregona, D. Gold complexes for therapeutic purposes: An updated patent review (2010–2015). Curr. Med. Chem. 2016, 23, 3374–3403. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Zou, T.; Tung Lum, C.; Lok, C.N.; Zhang, J.J.; Che, C.M. Chemical biology of anticancer gold(III) and gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- University of Kansas. Phase I and II Study of Auranofin in Chronic Lymphocytic Leukemia (CLL). US National Institutes of Health. Available online: https://clinicaltrials.gov/ct2/show/NCT01419691 (accessed on 23 September 2016).
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an old drug for a golden new age. Drugs R. D. 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic Cancer Center. Auranofin in Treating Patients with Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. US National Institutes of Health. Available online: https://clinicaltrials.gov/ct2/show/NCT01747798 (accessed on 23 September 2016).
- Gromer, S.; Arscott, L.D.; Williams, C.H.; Schirmer, R.H.; Becker, K. Human placenta thioredoxin reductase. J. Biol. Chem. 1998, 273, 20096–20101. [Google Scholar] [CrossRef] [PubMed]
- Coffer, M.T.; Shaw, C.F., III; Eidsness, M.K.; Watkins, J.W., II; Elder, R.C. Reactions of auranofin and chloro(triethylphosphine)gold with bovine serum albumin. Inorg. Chem. 1986, 25, 333–339. [Google Scholar]
- Coffer, M.T.; Shaw, C.F., III; Hormann, A.L.; Mirabelli, C.K.; Crooke, S.T. Thiol competition for Et3PAuS-albumin: A nonenzymatic mechanism for Et3PO formation. J. Inorg. Biochem. 1987, 30, 177–185. [Google Scholar] [CrossRef]
- Rubbiani, R.; Kitanovic, I.; Alborzinia, H.; Can, S.; Kitanovic, A.; Onambele, L.A.; Stefanopoulou, M.; Geldmacher, Y.; Sheldrick, W.S.; Wolber, G.; et al. Benzimidazol-2-ylidene gold(I) complexes are thioredoxin reductase inhibitors with multiple antitumor properties. J. Med. Chem. 2010, 53, 8608–8618. [Google Scholar] [CrossRef] [PubMed]
- Rubbiani, R.; Can, S.; Kitanovic, I.; Alborzinia, H.; Stefanopoulou, M.; Kokoschka, M.; Mönchgesang, S.; Sheldrick, W.S.; Wölfl, S.; Ott, I. Comparative in vitro evaluation of N-heterocyclic carbene gold(I) complexes of the benzimidazolylidene type. J. Med. Chem. 2011, 54, 8646–8657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Cao, R.; Zhang, W.; Yin, M.; Xiao, X.; Liu, Q.; Huang, N. A soluble bis-chelated gold(I) diphosphine compound with strong anticancer activity and low toxicity. J. Med. Chem. 2013, 56, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Marchetti, L.; Massai, L.; Scaletti, F.; Guerri, A.; Landini, I.; Nobili, S.; Perrone, G.; Mini, E.; Leoni, P.; et al. Chemistry and biology of two novel gold(I) carbene complexes as prospective anticancer agents. Inorg. Chem. 2014, 53, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Trávníček, Z.; Štarha, P.; Vančo, J.; Šilha, T.; Hošek, J.; Suchý, P.; Pražanová, G. Antiinflammatory active gold(I) complexes involving 6-substituted purine derivatives. J. Med. Chem. 2012, 55, 4568–4579. [Google Scholar] [CrossRef] [PubMed]
- Hošek, J.; Vančo, J.; Štarha, P.; Paráková, L.; Trávníček, Z. Effect of 2-chloro-substitution of adenine moiety in mixed-ligand gold(I) triphenylphosphine complexes on anti-inflammatory activity: The discrepancy between the in vivo and in vitro models. PLoS ONE 2013, 8, e82441. [Google Scholar] [CrossRef] [PubMed]
- Křikavová, R.; Hošek, J.; Vančo, J.; Hutyra, J.; Dvořák, Z.; Trávníček, Z. Gold(I)-triphenylphosphine complexes with hypoxanthine-derived ligands: In vitro evaluations of anticancer and anti-inflammatory activities. PLoS ONE 2014, 9, e107373. [Google Scholar] [CrossRef] [PubMed]
- Vančo, J.; Gáliková, J.; Hošek, J.; Dvořák, Z.; Paráková, L.; Trávníček, Z. Gold(I) complexes of 9-deazahypoxanthine as selective antitumor and antiinflammatory agents. PLoS ONE 2014, 9, e109901. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Martín, A.; del Pilar Brandi-Blanco, M.; Matilla-Hernández, A.; El Bakkali, H.; Nurchi, V.M.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. Unravelling the versatile metal binding modes of adenine: Looking at the molecular recognition patterns of deaza- and aza-adenines in mixed ligand metal complexes. Coord. Chem. Rev. 2013, 257, 2814–2838. [Google Scholar] [CrossRef]
- Zhao, S.B.; Wang, S. Luminescence and reactivity of 7-azaindole derivatives and complexes. Chem. Soc. Rev. 2010, 39, 3142–3156. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P.; Marek, J.; Trávníček, Z. Cisplatin and oxaliplatin derivatives involving 7-azaindole: Structural characterisations. Polyhedron 2012, 33, 404–409. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z.; Popa, A.; Popa, I.; Muchová, T.; Brabec, V. How to modify 7-azaindole to form cytotoxic Pt(II) complexes: Highly in vitro anticancer effective cisplatin derivatives involving halogeno-substituted 7-azaindole. J. Inorg. Biochem. 2012, 115, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P.; Hanousková, L.; Trávníček, Z. Organometallic half-sandwich, dichloridoruthenium(II) complexes with 7-azaindoles: Synthesis, characterization and elucidation of their anticancer inactivity against A2780 cell line. PLoS ONE 2015, 10, e0143871. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Guo, C.X.; Cheung, K.K.; Li, D.; Che, C.M. A luminescent heterometallic AuI∙∙∙CuI complex. apectroscopic properties and crystal structures of [Au(PPh3)(C7H5N2)] and [{Au(PPh3)(µ-C7H5N2)Cu(µ-C7H5N2)}2] (C7H5N2 = 7-azaindo1ate). J. Chem. Soc. Dalton Trans. 1994, 3677–3682. [Google Scholar] [CrossRef]
- Peng, S.M.; Lin, Y.N. Structure of CuII and CoII clusters of 7-azaindolate: [Cu2(C7H5N2)4(dmf)2] (I), [Cu4(OCH3)4(C7H5N2)4(dmf)2] (II) and [Co4O(C7H5N2)6]·CHCl3 (III). Acta Crystallogr. 1986, C42, 1725–1731. [Google Scholar] [CrossRef]
- Nomiya, K.; Noguchi, R.; Ohsawa, K.; Tsuda, K.; Oda, M. Synthesis, crystal structure and antimicrobial activities of two isomeric gold(I) complexes with nitrogen-containing heterocycle and triphenylphosphine ligands, [Au(L)(PPh3)] (HL = pyrazole and imidazole). J. Inorg. Biochem. 2000, 78, 363–370. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic, and Bioinorganic Chemistry; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Faggianhi, R.; Howard-Locck, H.E.; Lock, C.J.L.; Turner, M.A. The reaction of chloro(triphenylphosphine)gold(I) with 1-methylthymine. Can. J. Chem. 1987, 65, 1568–1575. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Brammer, L.; Bruton, E.A.; Sherwood, P. Understanding the behavior of halogens as hydrogen bond acceptors. Cryst. Growth Des. 2001, 1, 277–290. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, T.; Wang, Y.; Yang, H.; Yan, X.; Luo, X.; Jiang, H.; Zhu, W. Halogen bonding—A novel interaction for rational drug design? J. Med. Chem. 2009, 52, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Shaw, C.F., III. Inhibition of erythrocyte selenium-glutathione peroxidase by auranofin analogues and metabolites. Biochem. Pharmacol. 1998, 55, 1291–1299. [Google Scholar] [CrossRef]
- Graham, G.G.; Champion, G.D.; Ziegler, J.B. The cellular metabolism and effects of gold complexes. Metal-Based Drugs 1994, 1, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Bhuyan, B.J.; Mugesh, G. Bioinorganic and medicinal chemistry: Aspects of gold(I)-protein complexes. Dalton Trans. 2011, 40, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.E.; Reglinski, J.; Hoey, S.; Brown, D.H.; Sturrock, R.D. Action of sodium gold(I) thiomalate on erythrocyte membrane. Inorg. Chem. 1990, 29, 5190–5196. [Google Scholar] [CrossRef]
- Salemi, G.; Gueli, M.C.; D’Amelio, M.; Saia, V.; Mangiapane, P.; Aridon, P.; Ragonese, P.; Lupo, I. Blood levels of homocysteine, cysteine, glutathione, folic acid, and vitamin B12 in the acute phase of atherothrombotic stroke. Neurol. Sci. 2009, 30, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Abbehausen, C.; Peterson, E.J.; de Paiva, R.E.F.; Corbi, P.P.; Formiga, A.L.B.; Qu, Y.; Farrell, N.P. Gold(I)-phosphine-N-heterocycles: Biological activity and specific (ligand) interactions on the C-Terminal HIVNCp7 zinc finger. Inorg. Chem. 2013, 52, 11280–11287. [Google Scholar] [CrossRef] [PubMed]
- Guidi, F.; Landini, I.; Puglia, M.; Magherini, F.; Gabbiani, C.; Cinellu, M.A.; Nobili, S.; Fiaschi, T.; Bini, L.; Mini, E.; et al. Proteomic analysis of ovarian cancer cell responses to cytotoxic gold compounds. Metallomics 2012, 4, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Casas, J.S.; Couce, M.D.; Sánchez, A.; Sánchez-Gonzalez, A.; Sordo, J.; Varela, J.M.; Vázquez López, E.M. Synthesis, structure and cytotoxicity of triphenylphosphinegold(I) sulfanylpropenoates. J. Inorg. Biochem. 2008, 102, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.S.; Castellano, E.E.; Couce, M.D.; Crespo, O.; Ellena, J.; Laguna, A.; Sánchez, A.; Sordo, J.; Taboada, C. Novel gold(I) 7-azacoumarin complex: Synthesis, structure, optical properties, and cytotoxic effects. Inorg. Chem. 2007, 46, 6236–6238. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.S.; Castellano, E.E.; Couce, M.D.; Ellena, J.; Sánchez, A.; Sordo, J.; Taboada, C. A gold(I) complex with a vitamin K3 derivative: Characterization and antitumoral activity. J. Inorg. Biochem. 2006, 100, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Machado, R.C.; Grazul, R.M.; Paz Lopes, M.T.; Corrêa, C.C.; Dos Santos, H.F.; de Almeida, M.V.; Silva, H. Novel antitumor adamantane–azole gold(I) complexes as potential inhibitors of thioredoxin reductase. J. Biol. Inorg. Chem. 2016, 21, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Muchová, T.; Prachařová, J.; Štarha, P.; Olivová, R.; Vrána, O.; Benešová, B.; Kašpárková, J.; Trávníček, Z.; Brabec, V. Insight into the toxic effects of cis-dichloridoplatinum(II) complexes containing 7-azaindole halogeno derivatives in tumor cells. J. Biol. Inorg. Chem. 2013, 18, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zheng, W.; Fu, X.; Li, X.; Wong, Y.S.; Chen, T. Enhancement of auranofin-induced lung cancer cell apoptosis by selenocystine, a natural inhibitor of TrxR1 in vitro and in vivo. Cell Death Dis. 2014, 5, e1191. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Fernandes, A.P.; Rigobello, M.P.; Dani, B.; Sorrentino, F.; Tisato, F.; Björnstedt, M.; Bindoli, A.; Sturaro, A.; Rella, R.; et al. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem. Pharmacol. 2010, 79, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, A.; Sagawa, M.; Muto, A.; Uchida, H.; Ikeda, Y.; Kizaki, M. The gold compound auranofin induces apoptosis of human multiple myeloma cells through both down-regulation of STAT3 and inhibition of NF-κB activity. Leukemia Res. 2011, 35, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Mann, F.G.; Wells, A.F.; Purdie, D. The constitution of complex metallic salts: Part IV. The constitution of the phosphine and arsine derivatives of silver and aurous halides. The coordination of the coordinated argentous and aurous complex. J. Chem. Soc. 1937, 1828–1836. [Google Scholar] [CrossRef]
- Bruker. Apex3; Bruker AXS Inc.: Madison, WI, USA, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Diamond Version 4.0.3; Crystal Impact GbR: Bonn, Germany, 2015. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
| 7 | 8′ | |
|---|---|---|
| Empirical Formula | C25H18AuBrIN2P | C52H44Au2Cl2N4OP2 |
| Formula weight | 781.16 | 1267.68 |
| Temperature (K) | 120(2) | 120(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, C2/c |
| Unit cell dimensions | ||
| a (Å) | 6.8563(3) | 24.2844(14) |
| b (Å) | 24.2482(12) | 15.1186(14) |
| c (Å) | 14.7081(8) | 16.3330(14) |
| α (°) | 90 | 90 |
| β (°) | 101.313(2) | 128.203(4) |
| γ (°) | 90 | 90 |
| V (Å3) | 2397.8(2) | 4712.3(7) |
| Z, Dcalc (g·cm−3) | 4, 2.164 | 4, 1.787 |
| Absorption coefficient (mm−1) | 9.172 | 6.444 |
| Crystal size (mm) | 0.170 × 0.140 × 0.120 | 0.160 × 0.120 × 0.120 |
| F (000) | 1456 | 2456 |
| θ range for data collection (°) | 2.825 to 27.513 | 2.500 to 27.940 |
| Index ranges (h, k, l) | −7 ≤ h ≤ 8 | −31 ≤ h ≤ 31 |
| −31 ≤ k ≤ 31 | −19 ≤ k ≤ 19 | |
| −19 ≤ l ≤ 19 | −21 ≤ l ≤ 21 | |
| Reflections collected/unique | 56041 | 104819 |
| Data/restraints/parameters | 5508/0/280 | 5648/0/289 |
| Goodness–of–fit on F2 | 1.071 | 1.012 |
| Final R indices [I > 2σ(I)] | R1 = 0.0279, wR2 = 0.0453 | R1 = 0.0193, wR2 = 0.0340 |
| R indices (all data) | R1 = 0.0427, wR2 = 0.0485 | R1 = 0.0301, wR2 = 0.0366 |
| Largest peak and hole (e Å−3) | 0.900 and −1.237 | 0.550 and −0.702 |
| Cambridge Crystallographic Data Centre nos. | 1507789 | 1507790 |
| Parameter | 7 | 8′ |
|---|---|---|
| Au1–N1 | 2.047(3) | 2.030(2) |
| Au1–P1 | 2.2349(9) | 2.2277(6) |
| P1–Au1–N1 | 176.41(8) | 173.27(6) |
| Au1–P1–C10 | 112.34(12) | 107.47(8) |
| Au1–P1–C20 | 111.24(12) | 117.05(8) |
| Au1–P1–C30 | 115.36(11) | 114.19(8) |
| Au1–N1–C2 | 126.1(2) | 125.1(2) |
| Au1–N1–C7a | 125.9(2) | 127.7(2) |
| Complex | A2780 | A2780R | MRC5 | RF 1 | SI 2 |
|---|---|---|---|---|---|
| [Au(aza)(PPh3)] (1) | 3.8 ± 1.1 *** | 4.4 ± 0.8 | 7.7 ± 1.7 | 1.2 | 2.0 |
| [Au(3Claza)(PPh3)] (2) | 22.4 ± 5.7 | 21.7 ± 0.8 | 31.4 ± 4.9 | 1.0 | 1.4 |
| [Au(3Braza)(PPh3)] (3) | 23.3 ± 3.4 | 21.3 ± 0.8 | 27.3 ± 1.7 | 0.9 | 1.2 |
| [Au(3Iaza)(PPh3)] (4) | 3.5 ± 0.4 *** | 11.0 ± 4.7 | 29.2 ± 5.9 | 3.1 | 8.3 |
| [Au(5Braza)(PPh3)] (5) | 3.1 ± 0.5 *** | 14.4 ± 3.3 | 26.0 ± 4.2 | 4.6 | 8.4 |
| [Au(3Cl5Braza)(PPh3)] (6) | 22.9 ± 2.3 | 13.8 ± 2.1 | 26.5 ± 3.7 | 0.6 | 1.2 |
| [Au(3I5Braza)(PPh3)] (7) | 20.2 ± 2.5 | 22.1 ± 0.3 | 27.3 ± 2.6 | 1.1 | 1.4 |
| [Au(2Me4Claza)(PPh3)] (8) | 2.8 ± 0.7 *** | 8.9 ± 3.8 | 26.9 ± 3.6 | 3.2 | 9.6 |
| Cisplatin | 20.3 ± 2.3 | >50.0 | >50.0 | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štarha, P.; Trávníček, Z.; Drahoš, B.; Dvořák, Z. In Vitro Antitumor Active Gold(I) Triphenylphosphane Complexes Containing 7-Azaindoles. Int. J. Mol. Sci. 2016, 17, 2084. https://doi.org/10.3390/ijms17122084
Štarha P, Trávníček Z, Drahoš B, Dvořák Z. In Vitro Antitumor Active Gold(I) Triphenylphosphane Complexes Containing 7-Azaindoles. International Journal of Molecular Sciences. 2016; 17(12):2084. https://doi.org/10.3390/ijms17122084
Chicago/Turabian StyleŠtarha, Pavel, Zdeněk Trávníček, Bohuslav Drahoš, and Zdeněk Dvořák. 2016. "In Vitro Antitumor Active Gold(I) Triphenylphosphane Complexes Containing 7-Azaindoles" International Journal of Molecular Sciences 17, no. 12: 2084. https://doi.org/10.3390/ijms17122084
APA StyleŠtarha, P., Trávníček, Z., Drahoš, B., & Dvořák, Z. (2016). In Vitro Antitumor Active Gold(I) Triphenylphosphane Complexes Containing 7-Azaindoles. International Journal of Molecular Sciences, 17(12), 2084. https://doi.org/10.3390/ijms17122084