Nitrogen Removal from Landfill Leachate by Microalgae
Abstract
:1. Introduction
2. Results
2.1. Biomass Production
2.2. Nutrient Uptake
3. Discussion
3.1. Biomass Production
3.2. Nutrient Uptake
4. Materials and Methods
4.1. Landfill Leachates
4.2. Microalgal Cultivation
4.3. Analytical Methods
4.4. Kinetic Models and Parameters
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y. Perspectives on technology for landfill leachate treatment. Arab. J. Chem. 2013. [Google Scholar] [CrossRef]
- Bakare, A.A.; Pandey, A.K.; Bajpayee, M.; Bhargav, D.; Chowdhuri, D.K.; Singh, K.P.; Murthy, R.C.; Dhawan, A. DNA damage induced in human peripheral blood lymphocytes by industrial solid waste and municipal sludge leachates. Environ. Mol. Mutagen. 2007, 48, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Toufexi, E.; Tsarpali, V.; Efthimiou, I.; Vidali, M.-S.; Vlastos, D.; Dailianis, S. Environmental and human risk assessment of landfill leachate: An integrated approach with the use of cytotoxic and genotoxic stress indices in mussel and human cells. J. Hazard. Mater. 2013, 260, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Chauhan, L.K.S.; Kumar, D.; Gupta, S.K. Municipal sludge leachate-induced genotoxicity in mice—A subacute study. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2005, 587, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tsarpali, V.; Dailianis, S. Investigation of landfill leachate toxic potency: An integrated approach with the use of stress indices in tissues of mussels. Aquat. Toxicol. 2012, 124, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tsarpali, V.; Kamilari, M.; Dailianis, S. Seasonal alterations of landfill leachate composition and toxic potency in semi-arid regions. J. Hazard. Mater. 2012, 233–234, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Li, G.; Xin, X. Municipal landfill leachate induces cytogenetic damage in root tips of hordeum vulgare. Ecotoxicol. Environ. Saf. 2006, 63, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.R.; Chiang, W. Sanitary Landfill Leachate—Generation, Control and Treatment; CRC Press LLC: Boca Raton, FL, USA, 1994. [Google Scholar]
- Lema, J.M.; Mendez, R.; Blazquez, R. Characteristics of landfill leachates and alternatives for their treatment: A review. Water Air Soil Pollut. 1988, 40, 223–250. [Google Scholar]
- Ozturk, I.; Altinbas, M.; Koyuncu, I.; Arikan, O.; Gomec-Yangin, C. Advanced physico-chemical treatment experiences on young municipal landfill leachates. Waste Manag. 2003, 23, 441–446. [Google Scholar] [CrossRef]
- Reinhart, D.R.; Basel Al-Yousfi, A. The impact of leachate recirculation on municipal solid waste landfill operating characteristics. Waste Manag. Res. 1996, 14, 337–346. [Google Scholar] [CrossRef]
- Ferraz, F.M.; Povinelli, J.; Pozzi, E.; Vieira, E.M.; Trofino, J.C. Co-treatment of landfill leachate and domestic wastewater using a submerged aerobic biofilter. J. Environ. Manag. 2014, 141, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, S.; Wang, W. Combined treatment of domestic wastewater with landfill leachate by using A2/O process. J. Hazard. Mater. 2010, 178, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- John, J. A Self-Sustainable Remediation System for Acidic Mine Voids. In Proceedings of the 4th International Conference of Diffuse Pollution, Bangkok, Thailand, 16–21 January 2000; pp. 506–511.
- Phang, S.-M.; Chu, W.-L.; Rabiei, R. Phycoremediation. In The Algae World; Sahoo, D., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 357–389. [Google Scholar]
- Cheung, K.C.; Chu, L.M.; Wong, M.H. Toxic effect of landfill leachate on microalgae. Water Air Soil Pollut. 1993, 69, 337–349. [Google Scholar] [CrossRef]
- Arunakumara, K.; Zhang, X. Heavy metal bioaccumulation and toxicity with special reference to microalgae. J. Ocean Univ. China 2008, 7, 60–64. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Ghosh, P.; Thakur, I.S. Landfill leachate treatment using bacto-algal co-culture: An integrated approach using chemical analyses and toxicological assessment. Ecotoxicol. Environ. Saf. 2016, 128, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, G.; Podola, B.; Melkonian, M. Continuous removal of zinc from wastewater and mine dump leachate by a microalgal biofilm PSBR. J. Hazard. Mater. 2015, 297, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chan, G.Y.S.; Jiang, B.L.; Lan, C.Y. Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag. 2007, 27, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Paskuliakova, A.; Tonry, S.; Touzet, N. Phycoremediation of landfill leachate with chlorophytes: Phosphate a limiting factor on ammonia nitrogen removal. Water Res. 2016, 99, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.G.; Mullins, B.J. Using microalgae for combined lipid production and heavy metal removal from leachate. Ecol. Model. 2013, 249, 59–67. [Google Scholar] [CrossRef]
- Sniffen, K.D.; Sales, C.M.; Olson, M.S. Nitrogen removal from raw landfill leachate by an algae-bacteria consortium. Water Sci. Technol. 2016, 73, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, Y.; Huang, S.; Qiu, D.; Schideman, L.; Chai, X.; Zhao, Y. Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour. Technol. 2014, 156, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.L.; Pires, J.C.M.; Simoes, M. Biotechnological potential of synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.L.; Rodrigues, C.M.; Pires, J.C.M.; Simoes, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Res. 2016, 14, 127–136. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Prentice Hall: Upper Saddle River, NJ, USA, 1997; Volume 514. [Google Scholar]
- Lee, Y.-K.; Shen, H. Basic culturing techniques. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Oxford, UK, 2003; pp. 40–56. [Google Scholar]
- Mustafa, E.M.; Phang, S.M.; Chu, W.L. Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J. Appl. Phycol. 2012, 24, 953–963. [Google Scholar] [CrossRef]
- Capasso, L.; Pinto, G. Evaluation of toxic effects of heavy metals on unicellular algae. V-influence of extracellular products on toxicity and on type of inhibition. G. Bot. Ital. 1983, 117, 121–128. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.P.; Goncalves, A.L.; Moreira, F.C.; Silva, T.F.C.V.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Boaventura, R.A.R.; Vilar, V.J.P.; Pires, J.C.M. Towards sustainable microalgal biomass production by phycoremediation of a synthetic wastewater: A kinetic study. Algal Res. 2015, 11, 350–358. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; Ji, M.-K.; Kim, H.-C.; Paeng, K.-J.; Jeon, B.-H. Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. J. Environ. Manag. 2013, 115, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sydney, E.B.; da Silva, T.E.; Tokarski, A.; Novak, A.C.; de Carvalho, J.C.; Woiciecohwski, A.L.; Larroche, C.; Soccol, C.R. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Maestrini, S.Y.; Robert, J.-M.; Leftley, J.W.; Collos, Y. Ammonium thresholds for simultaneous uptake of ammonium and nitrate by oyster-pond algae. J. Exp. Mar. Biol. Ecol. 1986, 102, 75–98. [Google Scholar] [CrossRef]
- Ji, M.-K.; Abou-Shanab, R.A.I.; Kim, S.-H.; Salama, E.-S.; Lee, S.-H.; Kabra, A.N.; Lee, Y.-S.; Hong, S.; Jeon, B.-H. Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng. 2013, 58, 142–148. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Thomas, P.B. Nutrient removal from membrane bioreactor permeate using microalgae and in a microalgae membrane photoreactor. Bioresour. Technol. 2012, 117, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.L.; Pires, J.C.M.; Simoes, M. Wastewater polishing by consortia of Chlorella vulgaris and activated sludge native bacteria. J. Clean. Prod. 2016, 133, 348–357. [Google Scholar] [CrossRef]
- Powell, N.; Shilton, A.; Chisti, Y.; Pratt, S. Towards a luxury uptake process via microalgae—Defining the polyphosphate dynamics. Water Res. 2009, 43, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. And Micractinium sp., in wastewater treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Directive 1991/271/EEC. Directive of the European Council of 21 May 1991 concerning urban waste-water treatment. Off. J. Eur. Union 1991, L135, 40–52.
- Directive 1998/15/EC. Directive of the European Commission of 27 February 1998 amending Council Directive 91/271/EEC with respect to certain requirements established in Annex I thereof. Off. J. Eur. Union 1998, L67, 29–30.
- Giordano, M.; Prioretti, L. Sulphur and algae: Metabolism, ecology and evolution. In The Physiology of Microalgae; Borowitzka, A.M., Beardall, J., Raven, A.J., Eds.; Springer: Cham, Switzerland, 2016; pp. 185–209. [Google Scholar]
- Giordano, M.; Raven, J.A. Nitrogen and sulfur assimilation in plants and algae. Aquat. Bot. 2014, 118, 45–61. [Google Scholar] [CrossRef]
- Iyer, G.; Gupte, Y.; Vaval, P.; Nagle, V. Uptake of potassium by algae and potential use as biofertilizer. Indian J. Plant Physiol. 2015, 20, 285–288. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Rittmann, B.E.; Parra-Saldivar, R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third generation biofuels. J. Clean. Prod. 2015, 98, 53–65. [Google Scholar] [CrossRef]
- Ogawa, T.; Aiba, S. Bioenergetic analysis of mixotrophic growth in Chlorella vulgaris and Scenedesmus acutus. Biotechnol. Bioeng. 1981, 23, 1121–1132. [Google Scholar] [CrossRef]
- Saraiva, I.M.A.; da, F.M.A.F.; Vilar, V.J.P.; Silva, T.F.C.V.; da Rocha Boaventura, R.A. Method of Treating Leachate, Phototreatment Reactors and Respective Use. Patent EP2784031 A1, 1 October 2014. [Google Scholar]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 230A, 205–221. [Google Scholar]
- Wett, B.; Rauch, W. The role of inorganic carbon limitation in biological nitrogen removal of extremely ammonia concentrated wastewater. Water Res. 2003, 37, 1100–1110. [Google Scholar] [CrossRef]
- Belianin, V.N.; Spirov, V.V.; Furiaev, E.A. Spectrophotometry of individual chlorella cells. Biofizika 1976, 20, 848–852. (In Russian) [Google Scholar]
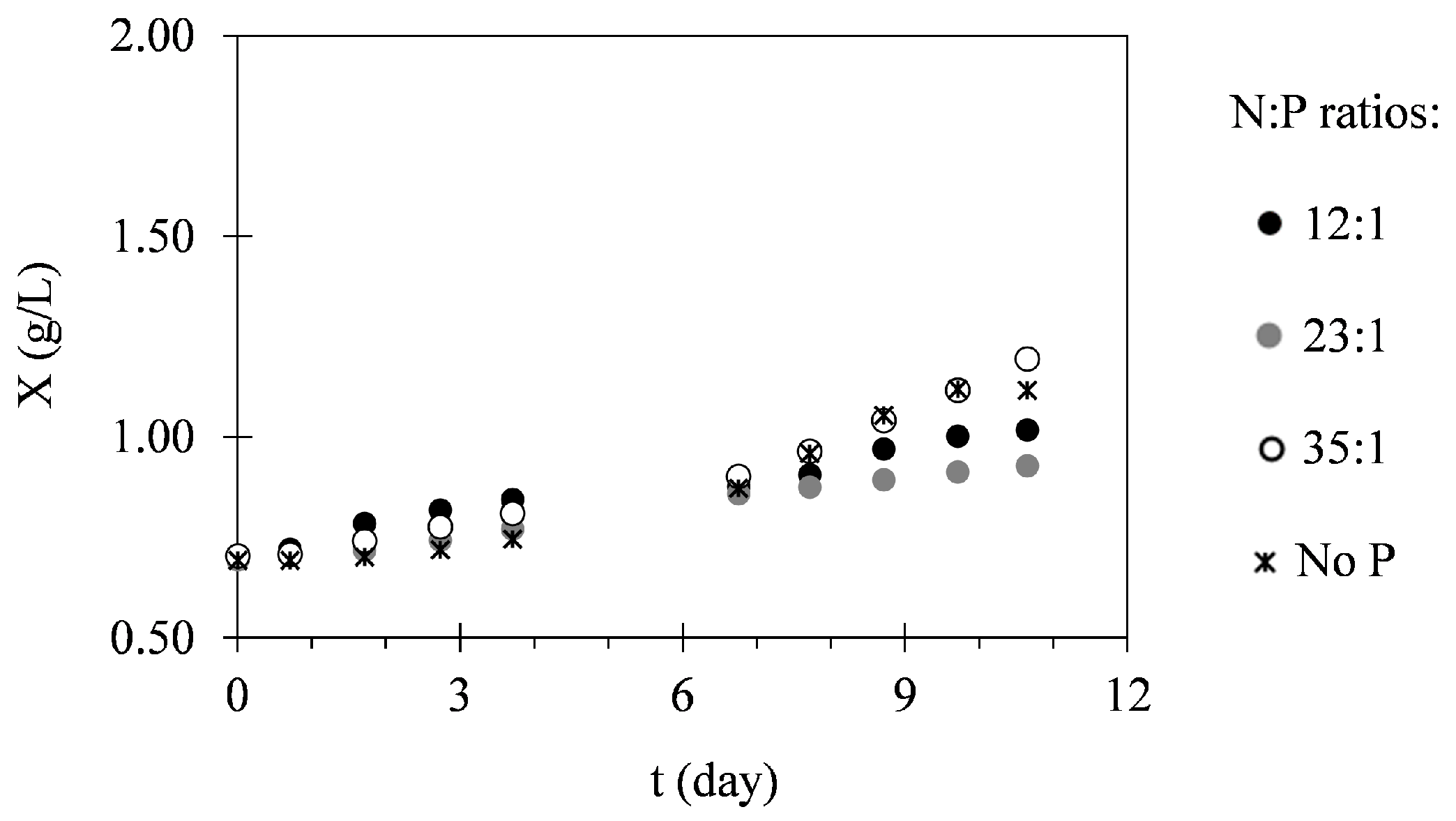


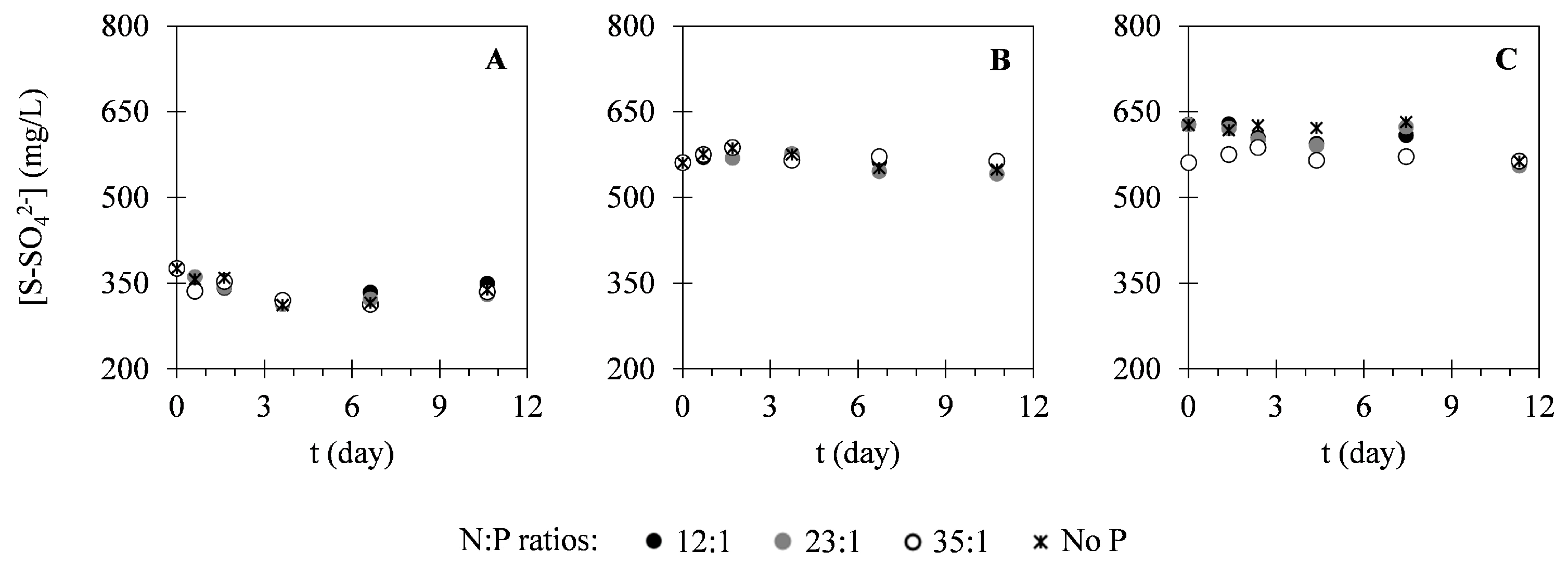
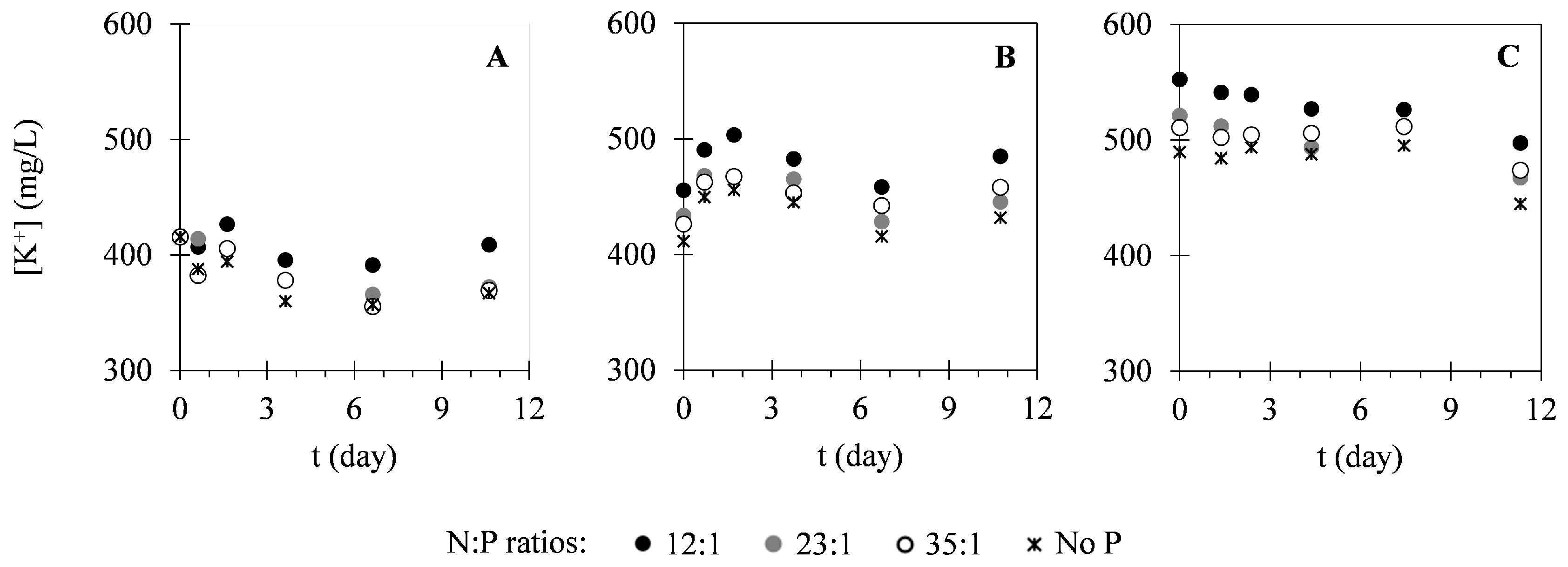
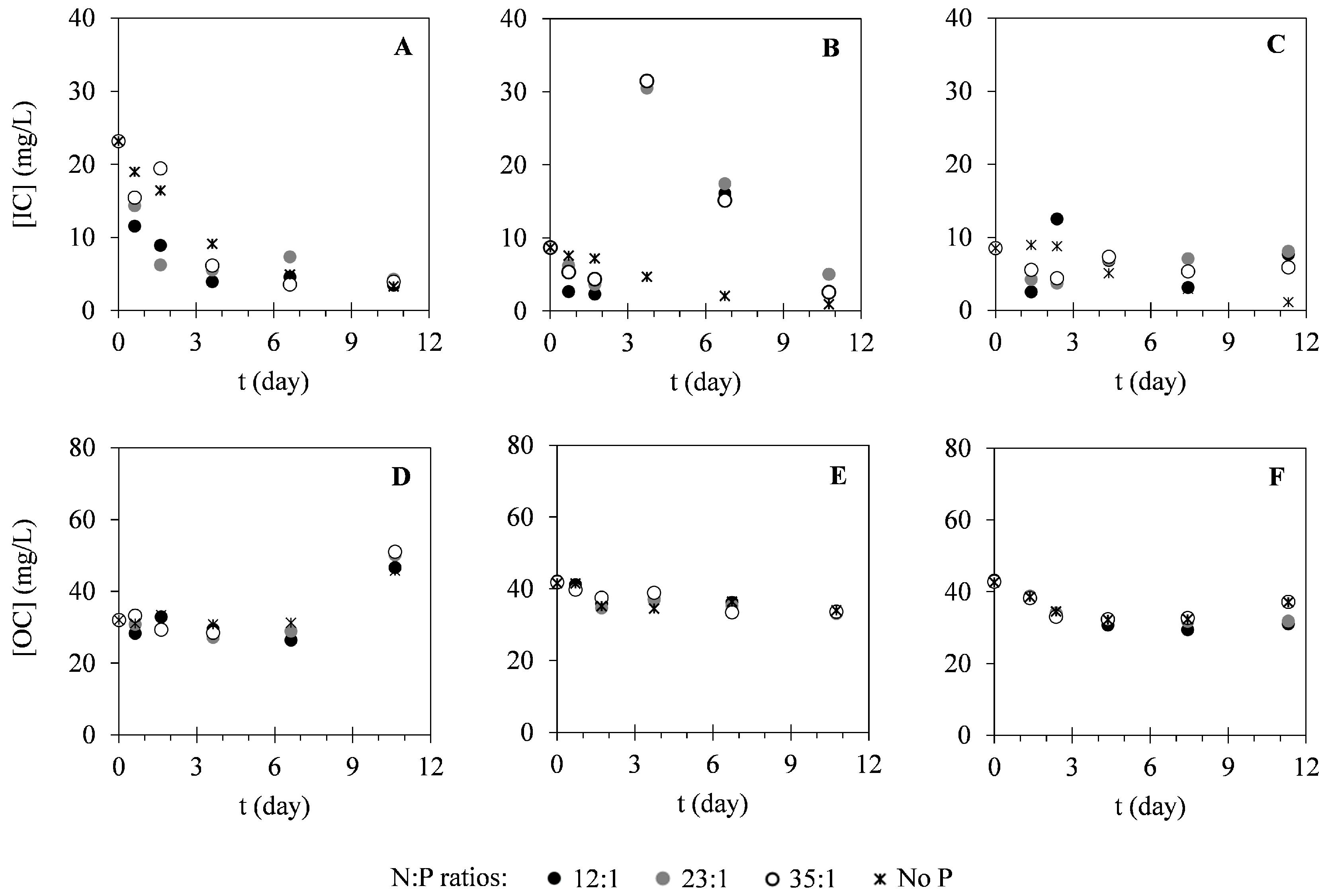
| Assay | N:P Ratio | Xi (g·L−1) | Xmax (g·L−1) | PX (g·L−1·Day−1) | μ (Day−1) |
|---|---|---|---|---|---|
| I | 12:1 | 0.194 | 0.91 ± 0.03 a | 0.107 ± 0.004 | – b |
| 23:1 | 0.194 | 0.83 ± 0.03 a | 0.095 ± 0.004 | – b | |
| 35:1 | 0.194 | 0.86 ± 0.03 a | 0.101 ± 0.005 | – b | |
| No P | 0.194 | 0.81 ± 0.09 a | 0.09 ± 0.02 | – b | |
| II | 12:1 | 0.607 | 1.52 ± 0.05 | 0.0988 ± 0.0004 | 0.13 ± 0.02 |
| 23:1 | 0.606 | 1.71 ± 0.06 | 0.11 ± 0.09 | 0.099 ± 0.005 | |
| 35:1 | 0.607 | 1.70 ± 0.05 | 0.11 ± 0.02 | 0.109 ± 0.003 | |
| No P | 0.608 | 1.44 ± 0.08 | 0.057 ± 0.002 | 0.068 ± 0.002 | |
| III | 12:1 | 0.701 | 0.970 ± 0.004 | 0.034 ± 0.003 | 0.060 ± 0.007 |
| 23:1 | 0.695 | 0.894 ± 0.007 | 0.020 ± 0.006 | 0.028 ± 0.003 | |
| 35:1 | 0.704 | 1.04 ± 0.02 | 0.049 ± 0.009 | 0.0724 ± 0.0007 | |
| No P | 0.693 | 1.06 ± 0.01 | 0.038 ± 0.002 | 0.085 ± 0.007 |
| Assay | N:P Ratio | N–NH4+ k (Day−1) | N–NH4+ RE (%) | N–NO3− RE (%) | Total-N RR (mg·L−1·Day−1) | YX/N (gX·gN−1) | P–PO43− k (Day−1) | P–PO43− RE (%) | P–PO43− RR (mg·L−1·Day−1) | YX/P (gX·gP−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 12:1 | 0.51 ± 0.08 | 100% | 22% | 4.4 | 24 a | 0.16 ± 0.08 | 54% | 1.4 | 76 a |
| 23:1 | 0.7 ± 0.2 | 100% | 27% | 5.1 | 19 a | 0.20 ± 0.03 | 92% | 1.2 | 80 a | |
| 35:1 | 0.7 ± 0.2 | 100% | 25% | 4.8 | 21 a | 0.6 ± 0.2 | 100% | 0.87 | 116 a | |
| No P | 0.41 ± 0.06 | 100% | 21% | 4.3 | 22 a | – | – | – | – a | |
| II | 12:1 | 0.135 ± 0.005 | 77% | <0% | 4.1 | 24 | 0.089 ± 0.006 | 38% | 1.3 | 74 |
| 23:1 | 0.120 ± 0.003 | 73% | 1% | 4.7 | 23 | 0.093 ± 0.007 | 65% | 1.2 | 95 | |
| 35:1 | 0.128 ± 0.006 | 75% | <0% | 4.2 | 27 | 0.09 ± 0.02 | 63% | 0.77 | 150 | |
| No P | 0.034 ± 0.007 | 22% | <0% | 1.2 | 46 | – | – | – | – | |
| III | 12:1 | 0.091 ± 0.007 | 63% | 6% | 5.0 | 6.8 | 0.045 ± 0.005 | 41% | 1.7 | 20 |
| 23:1 | 0.080 ± 0.002 | 57% | 9% | 5.0 | 4.1 | 0.11 ± 0.02 | 48% | 1.0 | 20 | |
| 35:1 | 0.090 ± 0.006 | 64% | 7% | 5.1 | 9.5 | 0.043 ± 0.008 | 54% | 0.76 | 64 | |
| No P | 0.040 ± 0.002 | 36% | 10% | 3.7 | 10 | – | – | – | – |
| Assay | N:P Ratio | S–SO42− RE (%) | S–SO42− RR (mg·L−1·Day−1) | K+ RE (%) | K+ RR (mg·L−1·Day−1) |
|---|---|---|---|---|---|
| I | 12:1 | 7% | 2.5 | 2% | 0.63 |
| 23:1 | 12% | 4.3 | 10% | 4.1 | |
| 35:1 | 11% | 3.9 | 11% | 4.3 | |
| No P | 10% | 3.5 | 12% | 4.6 | |
| II | 12:1 | 0% | <0 | <0% | <0 |
| 23:1 | 4% | 1.9 | <0% | <0 | |
| 35:1 | 0% | <0 | <0% | <0 | |
| No P | 2% | 1.1 | <0% | <0 | |
| III | 12:1 | 11% | 6.0 | 10% | 4.9 |
| 23:1 | 11% | 6.4 | 10% | 4.7 | |
| 35:1 | 8% | 4.4 | 7% | 3.3 | |
| No P | 10% | 5.6 | 9% | 4.0 |
| Assay | [N–NH4+] (mg·L−1) | [N–NO3−] (mg·L−1) | [P–PO43−] (mg·L−1) | [S–SO42−] (mg·L−1) | [K+] (mg·L−1) |
|---|---|---|---|---|---|
| I | 15 | 144 | <0.1 | 377 | 416 |
| II | 67 | 136 | 1 | 561 | 412 |
| III | 75 | 153 | 1 | 627 | 490 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.F.L.; Gonçalves, A.L.; Moreira, F.C.; Silva, T.F.C.V.; Vilar, V.J.P.; Pires, J.C.M. Nitrogen Removal from Landfill Leachate by Microalgae. Int. J. Mol. Sci. 2016, 17, 1926. https://doi.org/10.3390/ijms17111926
Pereira SFL, Gonçalves AL, Moreira FC, Silva TFCV, Vilar VJP, Pires JCM. Nitrogen Removal from Landfill Leachate by Microalgae. International Journal of Molecular Sciences. 2016; 17(11):1926. https://doi.org/10.3390/ijms17111926
Chicago/Turabian StylePereira, Sérgio F. L., Ana L. Gonçalves, Francisca C. Moreira, Tânia F. C. V. Silva, Vítor J. P. Vilar, and José C. M. Pires. 2016. "Nitrogen Removal from Landfill Leachate by Microalgae" International Journal of Molecular Sciences 17, no. 11: 1926. https://doi.org/10.3390/ijms17111926







