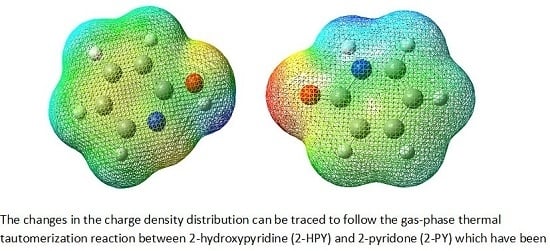
The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit
Abstract
:1. Introduction
2. Results and Discussion
2.1. Geometry
2.2. Activation Energies
2.3. Therodynamic Analysis
2.4. Natural Bond Orbital (NBO) Analysis
2.5. Nonlinear Optical (NLO) Properties
3. Computational Details
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rawson, J.M.; Winpenny, R.E.P. The coordination derivatives chemistry of 2-pyridone and its derivatives. Coord. Chem. Rev. 1995, 139, 313–374. [Google Scholar] [CrossRef]
- Yang, H.W.; Craven, B.M. Charge Density Study of 2-Pyridone. Acta Crystallogr. Sect. B Struct. Sci. 1998, 54, 912–920. [Google Scholar] [CrossRef]
- Smets, J.; Maes, G. Matrix-isolation FT-IR study on the protomeric tautomerism 2-hydroxypyridine⇌2-pyridone. Chem. Phys. Lett. 1991, 187, 532–536. [Google Scholar] [CrossRef]
- Takasuka, M.; Saito, T.; Nakai, H. Fourier transform infrared spectrometric study of tautomeric (enol-keto) and dimeric equilibria in 2-hydroxypyridines and 1,3-cyclohexanediones in CHCl3 and/or CCl4 solutions. Vib. Spectrosc. 1996, 13, 65–74. [Google Scholar] [CrossRef]
- Dkhissi, A.; Houben, L.; Smets, J.; Adamowicz, L.; Maes, G. Density functional theory and ab-initio computational study of the 2-hydroxypyridine/2-pyridone system: A comparison with FT-IR data from matrix isolation experiments. J. Mol. Struct. 1999, 484, 215–227. [Google Scholar] [CrossRef]
- Kim, S.K.; Ha, T.; Schermann, J.-P. Photoionization of 2-pyridone and 2-hydroxypyridine. Phys. Chem. Chem. Phys. 2010, 12, 3334–3335. [Google Scholar] [PubMed]
- Hatherley, L.D.; Brown, R.D.; Godfrey, P.D.; Pierlot, A.P.; Caminati, W.; Damiani, D.; Melandri, S.; Favero, L.B. Gas-phase tautomeric equilibrium of 2-pyridinone and 2-hydroxypyridine by microwave spectroscopy. J. Phys. Chem. 1993, 97, 46–51. [Google Scholar] [CrossRef]
- Schlegel, H.B.; Gund, P.; Fluder, E.M. Tautomerization of formamide, 2-pyridone, and 4-pyridone: An ab initio study. J. Am. Chem. Soc. 1982, 104, 5347–5351. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Szafran, M.; Stevens, J. AM1 study of the tautomerism of 2- and 4-pyridones and their thio-analogs. J. Mol. Struct. THEOCHEM 1989, 184, 179–192. [Google Scholar] [CrossRef]
- Parchment, O.G.; Burton, N.A.; Hiller, I.H. Tautomeric equilibrium of 2-hydroxypyridine/2-pyridone: Does a theoretical—Experimental discrepancy exist? Chem. Phys. Lett. 1993, 203, 46–48. [Google Scholar] [CrossRef]
- Beak, P.; Fry, F.S.; Lee, J.; Steele, F. Equilibration studies. Protomeric equilibria of 2- and 4-hydroxypyridines, 2- and 4-hydroxypyrimidines, 2- and 4-mercaptopyridines, and structurally related compounds in the gas phase. J. Am. Chem. Soc. 1976, 98, 171–179. [Google Scholar] [CrossRef]
- Cook, M.J.; El-Abbady, S.; Katritzky, A.R.; Guimon, C.; Pfister-Guillouzo, G. Photoelectron spectra of hydroxy- and mercapto-pyridines and models of fixed structure. J. Chem. Soc. Perkin Trans. 2 1977, 13, 1652–1656. [Google Scholar] [CrossRef]
- Aue, D.H.; Betowski, L.D.; Davidson, W.R.; Bowers, M.T.; Beak, P.; Lee, J. Gas-phase basicities of amides and imidates. Estimation of protomeric equilibrium constants by the basicity method in the gas phase. J. Am. Chem. Soc. 1979, 101, 1361–1368. [Google Scholar] [CrossRef]
- Frank, J.; Katritzky, A.R. Tautomeric pyridines. Part XV. Pyridone–hydroxypyridine equilibria in solvents of differing polarity. J. Chem. Soc. Perkin Trans. 2 1979, 12, 1428–1431. [Google Scholar] [CrossRef]
- Penfold, B.R. The Electron Distribution in Crystalline α Pyridone. Acta Crystallogr. 1953, 6, 591–600. [Google Scholar] [CrossRef]
- Ohms, U.; Guth, H.; Heller, E.; Dannöhl, H.; Schweig, A. Comparison of Observed and Calculated Electron-Density 2-Pyridone, C5H5NO, Crystal-Structure Refinements at 295 K and 120 K, Experimental and Theoretical Deformation Density Studies. Z. Kristallogr. 1984, 169, 185–200. [Google Scholar] [CrossRef]
- Beak, P.; Covington, J.B. Solvent effects on protomeric equilibriums: Quantitative correlation with an electrostatic hydrogen-bonding model. J. Am. Chem. Soc. 1978, 100, 3961–3963. [Google Scholar] [CrossRef]
- Forlani, L.; Cristoni, G.; Boga, C.; Todesco, P.E.; del Vecchio, E.; Selva, S.; Monari, M. Reinvestigation of the tautomerism of some substituted 2-hydroxypyridines. Arkivoc 2002, 11, 198–215. [Google Scholar]
- Mirek, J.; Syguła, A. MNDO study on relative stabilities of monosubstituted pyridine tautomers. J. Mol. Struct. THEOCHEM 1981, 86, 85–90. [Google Scholar] [CrossRef]
- Wong, M.W.; Wiberg, K.B.; Frisch, M.J. Solvent effects. 3. Tautomeric equilibria of formamide and 2-pyridone in the gas phase and solution: An ab initio SCRF study. J. Am. Chem. Soc. 1992, 114, 1645–1652. [Google Scholar] [CrossRef]
- Parchment, O.G.; Burton, N.A.; Hillier, I.H.; Vincent, M.A. Predictions of tautomeric equilibria in 2-hydroxypyridine and pyridine-2-thiol: Correlation effects and possible discrepancies with experiment. J. Chem. Soc. Perkin Trans. 2 1993, 5, 861–863. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Hillier, I.H.; Davies, R.H. Prediction of tautomeric equilibria of hydroxypyridines by ab initio molecular orbital methods. J. Chem. Soc. Chem. Commun. 1982, 12, 685–686. [Google Scholar] [CrossRef]
- Hall, R.J.; Burton, N.A.; Ian, H.; Hillier, I.H.; Phillip, E.; Young, P.E. Tautomeric equilibria in 2-hydroxypyridine and in cytosine: An assessment of density functional methods, including gradient corrections. Chem. Phys. Lett. 1994, 220, 129–132. [Google Scholar] [CrossRef]
- Lledos, A.; Bertran, J. Lactam/lactim tautomeric interconversion mechanism of 2-pyridone in aqueous solution. Tetrahedron Lett. 1981, 22, 775–778. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Hillier, I.H. On the mechanism of proton transfer in the 2-hydroxypyridine α 2-pyridone tautomeric equilibrium. Chem. Phys. Lett. 1984, 107, 330–332. [Google Scholar] [CrossRef]
- Moreno, M.; Miller, W.H. On the tautomerization reaction 2-pyridone*2-hydroxypyridine: An ab initio study. Chem. Phys. Lett. 1990, 171, 475–479. [Google Scholar] [CrossRef]
- Abdulla, H.I.; El-Bermani, M.F. Infrared studies of tautomerism in 2-hydroxypyridine 2-thiopyridine and 2-aminopyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2659–2671. [Google Scholar] [CrossRef]
- Leś, A.; Adamowicz, L.; Nowak, M.J.; Lapinski, L. Concerted biprotonic tautomerism of 2-hydroxypyridine. J. Mol. Struct. THEOCHEM 1994, 312, 157–166. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C.A. New hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Wiberg, K.B. Basis Set Effects on Calculated Geometries: 6-311-G** vs. aug-cc-pvdz. J. Comput. Chem. 2004, 25, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Scuseria, G.E.; Janssen, C.L.; Schaefer, H.F., III. An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J. Chem. Phys. 1988, 89, 7382–7387. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis-sets for anion calculations. 3. The 3-21+G basis set for 1st-row elements, Li-F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Wollrab, J.E.; Laurie, V.W. Microwave Spectrum of Dimethylamine. J. Chem. Phys. 1968, 48, 5058–5066. [Google Scholar] [CrossRef]
- Lifshitz, A.; Frenklach, M.; Burcat, A. Structural isomerization allene↔propyne. Studies with a single pulse shock tube. J. Phys. Chem. 1975, 79, 1148–1152. [Google Scholar] [CrossRef]
- Jimenez, V.; Alderete, J.B. Complete basis set calculations on the tautomerism and protonation of triazoles and tetrazoles. J. Mol. Struct. THEOCHEM 2006, 775, 1–7. [Google Scholar] [CrossRef]
- Tautermann, C.S.; Voegele, A.F.; Liedl, K.R. The ground state tunneling splitting of the 2-pyridone 2-hydroxypyridine dimer. Chem. Phys. 2003, 292, 47–52. [Google Scholar] [CrossRef]
- Lia, G.-S.; Ruiz-Lbpez, M.F.; Zhang, M.-S.; Maigret, B. Ab initio calculations of tautomer equilibrium and protonation enthalpy of 4- and 5-methylimidazole in the gas phase: Basis set and correlation effects. J. Mol. Struct. THEOCHEM 1998, 422, 197–204. [Google Scholar] [CrossRef]
- Rendell, A.P.; Guest, M.F.; Kendall, R.A. Distributed data parallel coupled-cluster algorithm: Application to the 2-hydroxypyridine/2-pyridone tautomerism. J. Comput. Chem. 1993, 14, 1429–1439. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near Hartree–Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Wang, K.; Shan, X.; Chen, X. Electron propagator theory study of 2-aminoethanol conformers. J. Mol. Struct. THEOCHEM 2009, 909, 91–95. [Google Scholar] [CrossRef]
- Song, L.; Lin, Y.; Wu, W.; Zhang, Q.; Mo, Y. Steric Strain versus Hyperconjugative Stabilization in Ethane Congeners. J. Phys. Chem. A 2005, 109, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kaneno, D.; Tomoda, D. The Origin of Cis Effect in 1,2-Dihaloethenes: The Quantitative Comparison of Electron Delocalizations and Steric Exchange Repulsions. Bull. Chem. Soc. Jpn. 2008, 81, 1415–1422. [Google Scholar] [CrossRef]
- Pophristic, V.; Goodman, L. Hyperconjugation not steric repulsion leads to the staggered structure of ethane. Nature 2001, 411, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Glendenning, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Version 3.1, Gaussian Inc.: Pittsburg, PA, USA, 2001.
- Gaussian, Inc. Gaussian 09, Revision A.02, Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., et al., Eds.; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Garza, A.J.; Osman, O.I.; Scuseria, G.E.; Wazan, N.A.; Khan, S.B.; Asiri, A.M. Nonlinear optical properties of DPO and DMPO: A theoretical and computational study. Theor. Chem. Acc. 2013, 132, 1384–1390. [Google Scholar] [CrossRef]
- Kaatz, P.; Donley, E.A.; Shelton, D.P. A comparison of molecular hyperpolarizabilities from gas and liquid phase measurements. J. Chem. Phys. 1998, 108, 849–856. [Google Scholar] [CrossRef]
- Cramer, C.J. (Ed.) Essentials of Computational Chemistry, 2nd ed.; Theories and Models, Chapter 8.5 Advantage and Disadvantages of DFT Compared to MO Theory; John Wily & Sons Ltd.: New York, NY, USA, 2002.
- Feller, D.; Peterson, K.A. Probing the limits of accuracy in electronic structure calculations: Is theory capable of results uniformly better than “chemical accuracy”? J. Chem. Phys. 2007, 126, 114105. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.J.; Scuseria, G.E.; Khan, S.B.; Asiri, A.M. Assessment of long-range corrected functionals for the prediction of non-linear optical properties of organic materials. Chem. Phys. Lett. 2013, 575, 122–125. [Google Scholar] [CrossRef]
- Champagne, B.; Perpete, E.A.; Jacquemin, D.; van Gisbergen, S.J.A.; Baerends, E.J.; Soubra-Ghaou, C.; Robins, K.A.; Kirtman, B. Assessment of conventional density functional schemes for computing the dipole moment and (hyper)polarizabilities of push–pull π-conjugated systems. J. Phys. Chem. A 2000, 104, 4755–4763. [Google Scholar] [CrossRef]
- Solomon, R.V.; Veerapandian, P.; Vedha, S.A.; Venuvanalingam, P. Tuning nonlinear optical and optoelectronic properties of vinyl coupled triazene chromophores: A density functional theory and time-dependent density functional theory investigation. J. Phys. Chem. A 2012, 116, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Loboda, O.; Zalesny, R.; Avramopoulos, A.; Luis, J.M.; Kirtman, B.; Tagmatarchis, N.; Reis, H.; Papadopoulos, M.G. Linear and nonlinear optical properties of [60]fullerene derivatives. J. Phys. Chem. A 2009, 113, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Thanthiriwatte, K.S.; Nalin de Silva, K.M. Non-linear optical properties of novel fluorenyl derivatives—Ab initio quantum chemical calculations. J. Mol. Struct. THEOCHEM 2002, 617, 169–175. [Google Scholar] [CrossRef]
- Sriyanka Mendis, B.A.; Nalin de Silva, K.M. A comprehensive study of non-linear optical properties of novel charge transfer molecular systems. J. Mol. Struct. THEOCHEM 2004, 678, 31–38. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A.; Al-Amoudi, M.S.; Alamry, K.A. Synthesis, characterization, absorbance, fluorescence and non-linear optical properties of some donor acceptor chromophores. Bull. Korean Chem. Soc. 2012, 33, 1900–1906. [Google Scholar] [CrossRef]
- Tillekaratne, A.D.; de Silva, R.M.; Nalin de Silva, K.M. Push–pull porphyrins as non-linear optical materials: Ab initio quantum chemical calculations. J. Mol. Struct. THEOCHEM 2003, 638, 169–176. [Google Scholar] [CrossRef]
- Marder, S.R.; Cheng, L.-T.; Tiemann, B.G.; Friedli, A.C.; Blanchard-Desce, M.; Perry, J.W.; Skindhøj, J. Large First Hyperpolarizabilities in Push–Pull Polyenes by Tuning of the Bond Length Alternation and Aromaticity. Science 1994, 263, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.; Millam, J. GaussView, version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009.
- Geomodeling in GeoGraphix. Available online: http://www.chemcraftprog.com (accessed on 8 April 2016).
- Berry, R.S.; Davidovits, P.; McFadden, D.L. Alkali Halide Vapors; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.V.; Ori, O.; Cataldo, F.; Putz, A. Parabolic Reactivity “Coloring” Molecular Topology: Application to Carcinogenic PAHs. Curr. Org. Chem. 2016, 20, 2816–2830. [Google Scholar] [CrossRef]
- Matito, E.; Putz, M.V. New Link between Conceptual Density Functional Theory and Electron Delocalization. J. Phys. Chem. A 2011, 115, 12459–12462. [Google Scholar] [CrossRef] [PubMed]


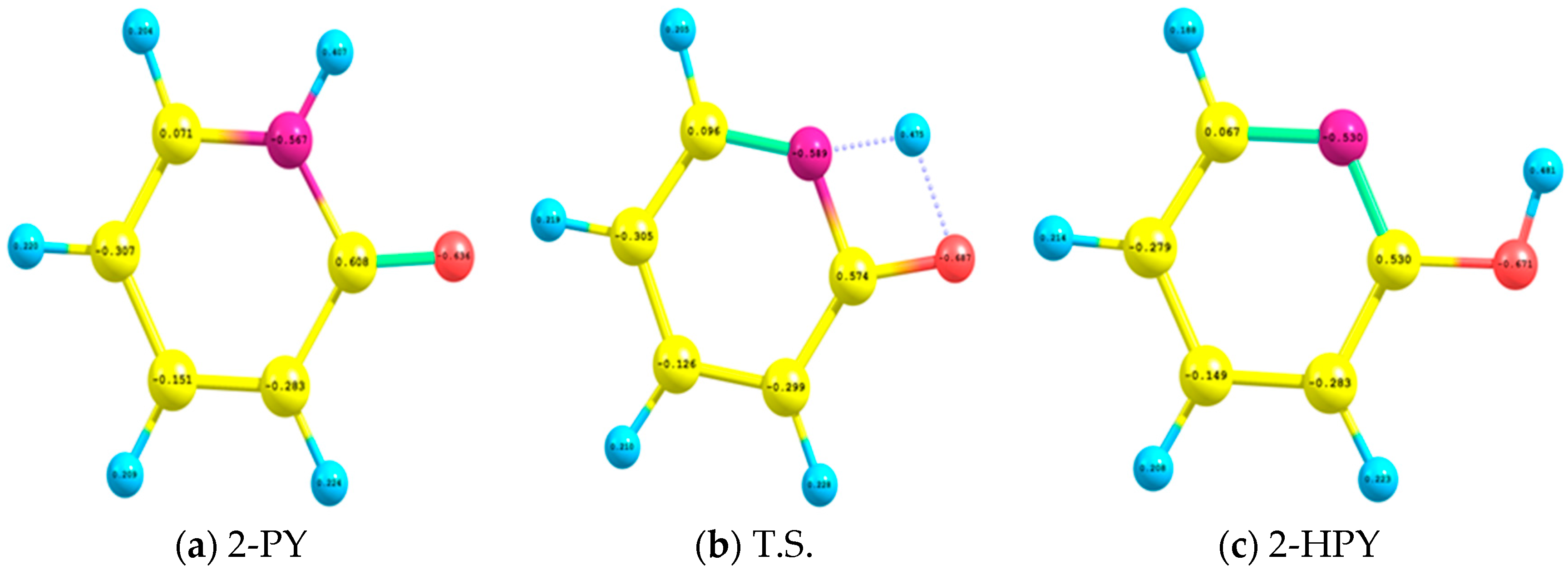

| Parameter | 2-HPY | T.S. | 2-PY |
|---|---|---|---|
| C2O11 | 1.351 | 1.289 | 1.225 |
| C2N10 | 1.323 | 1.355 | 1.399 |
| C2C3 | 1.399 | 1.407 | 1.450 |
| C1N10 | 1.339 | 1.336 | 1.363 |
| O11H5 | 0.968 | 1.366 | - |
| N11H5 | - | 1.290 | 1.013 |
| O11C2C3 | 118.5 | 134.3 | 126.6 |
| N10C2C3 | 124.1 | 120.5 | 113.5 |
| C2C3C4 | 117.4 | 116.4 | 121.4 |
| Functional | Basis Set | 2-HPY | T.S. | 2-PY |
|---|---|---|---|---|
| B3LYP | 6-311++G** | −323.5205 | −323.4665 | −323.5218 |
| Activ. Energy | 135.544 | 138.080 | ||
| aug-cc-pvdz | 323.4725 | −323.4195 | −323.4727 | |
| Activ. Energy | 133.032 | 133.536 | ||
| CAM-B3LYP | 6-311++G** | −323.3592 | −323.3034 | −323.3595 |
| Activ. Energy | 140.06 | 140.812 | ||
| aug-cc-pvdz | −323.3102 | −323.2555 | −323.3094 | |
| Activ. Energy | 137.3 | 135.292 | ||
| ωB97XD | 6-311++G** | −323.4021 | −323.3469 | −323.4031 |
| Activ. Energy | 138.556 | 141.064 | ||
| aug-cc-pvdz | −323.3591 | −323.3049 | −323.3590 | |
| Activ. Energy | 136.044 | 135.792 | ||
| M062X | 6-311++G** | −323.3834 | −323.3258 | −323.3810 |
| Activ. Energy | 144.58 | 138.556 | ||
| aug-cc-pvdz | −323.3480 | −323.2916 | −323.3446 | |
| Activ. Energy | 141.568 | 133.032 |
| Functional | Basis Set | 2-HPY–2-PY | T.S. | Activ. Energy |
|---|---|---|---|---|
| B3LYP | 6-311++G** | −647.2530 | −647.2398 | 33.532 |
| aug-cc-pvdz | −647.1573 | −647.1452 | 30.372 | |
| CAM-B3LYP | 6-311++G** | −646.9344 | −646.9214 | 32.632 |
| aug-cc-pvdz | −646.8367 | −646.8249 | 29.62 | |
| ωB97XD | 6-311++G** | −647.0232 | −647.0091 | 35.392 |
| aug-cc-pvdz | −646.9371 | −646.9239 | 33.532 | |
| M062X | 6-311++G** | −646.9798 | −646.9688 | 27.612 |
| aug-cc-pvdz | −646.9088 | −646.8989 | 24.848 |
| Level of Theory | ΔE | ΔH° | ΔG° | ΔS° | K |
|---|---|---|---|---|---|
| B3LYP/6-311++G** | 52.72 | 50.32 | 58.56 | −27.632 | 3.648 |
| B3LYP/aug-cc-pvdz | 10.416 | 8.576 | 15.632 | −23.664 | 10.784 |
| CAM-B3LYP/6-311++G** | 12.096 | 9.536 | 18.192 | −29.04 | 10.112 |
| CAM-B3LYP/aug-cc-pvdz | −31.808 | −33.648 | −26.464 | −24.096 | 31.168 |
| M062X/6-311++G** | −102.496 | −105.312 | −95.952 | −31.392 | 179.76 |
| M062X/aug-cc-pvdz | −143.92 | −145.936 | −138.368 | −25.376 | 524.048 |
| ωB97XD/6-311++G** | 42.928 | 40.288 | 49.152 | −29.728 | 4.64 |
| ωB97XD/aug-cc-pvdz | −4.16 | −6.224 | 1.344 | −25.376 | 15.472 |
| CCSD/6-311++G** | −77.296 | −92.288 | −50.16 | −141.296 | 56.688 |
| CCSD/aug-cc-pvdz | −81.2 | −83.808 | −74.352 | −31.712 | 104.336 |
| Parameter | 2-HPY | T.S. | 2-PY |
|---|---|---|---|
| πC1–C5→π*C2–N10 | 20.42 | 0.974 | 1.45 |
| πC1–C5→π*C3–C4 | 36.25 | 18.04 | 26.07 |
| πC2–N10→π*C1–C5 | 38.71 | 0.504 | 0.50 |
| πC2–N10→π*C3–C4 | 15.33 | 0.894 | 5.07 |
| πC3–C4→π*C1–C5 | 21.85 | 9.10 | 16.61 |
| πC3–C4→π*C2–N10 | 40.94 | 0.50 | 32.18 |
| σ*C1–N10→σ*C2–O11 | 4.99 | 0.56 | 2.24 |
| σ*O11–H12→σ*C2–C3 | 6.33 | 0.5 | 0.5 |
| n1N10→σ*C1–C5 | 9.98 | 36.17 | (55.80) # |
| n1N10→σ*C2–C3 | 12.49 | 0.45 | 0.5 |
| n1N10→π*C2–O11 | 0.5 | 8.42 | 66.97 |
| n1N10→σ*C2–O11 | 8.36 | 0.5 | 2.50 |
| n1O11→σ*C2–N10 | 7.52 | 5.61 | 1.57 |
| n2O11→π*C2–N10 | 43.08 | 0.50 | 35.24 |
| n2O11→σ*C2–C3 | 0.5 | 4.51 | 20.57 |
| n2O11→n*H12 | - | 70.11 | - |
| n2N10→n*H12 | - | 100.36 | - |
| Parameter | 2-PY | 2-HPY | ΔE a |
|---|---|---|---|
| Total SCF Energy (Full) | −323.402843 | −323.403258 | −1.090 |
| Energy of Deletion (L) | −322.535752 | −322.566444 | −80.582 |
| Hyperconjugative Energy (NL) | −0.867091 | −0.836814 | +79.492 |
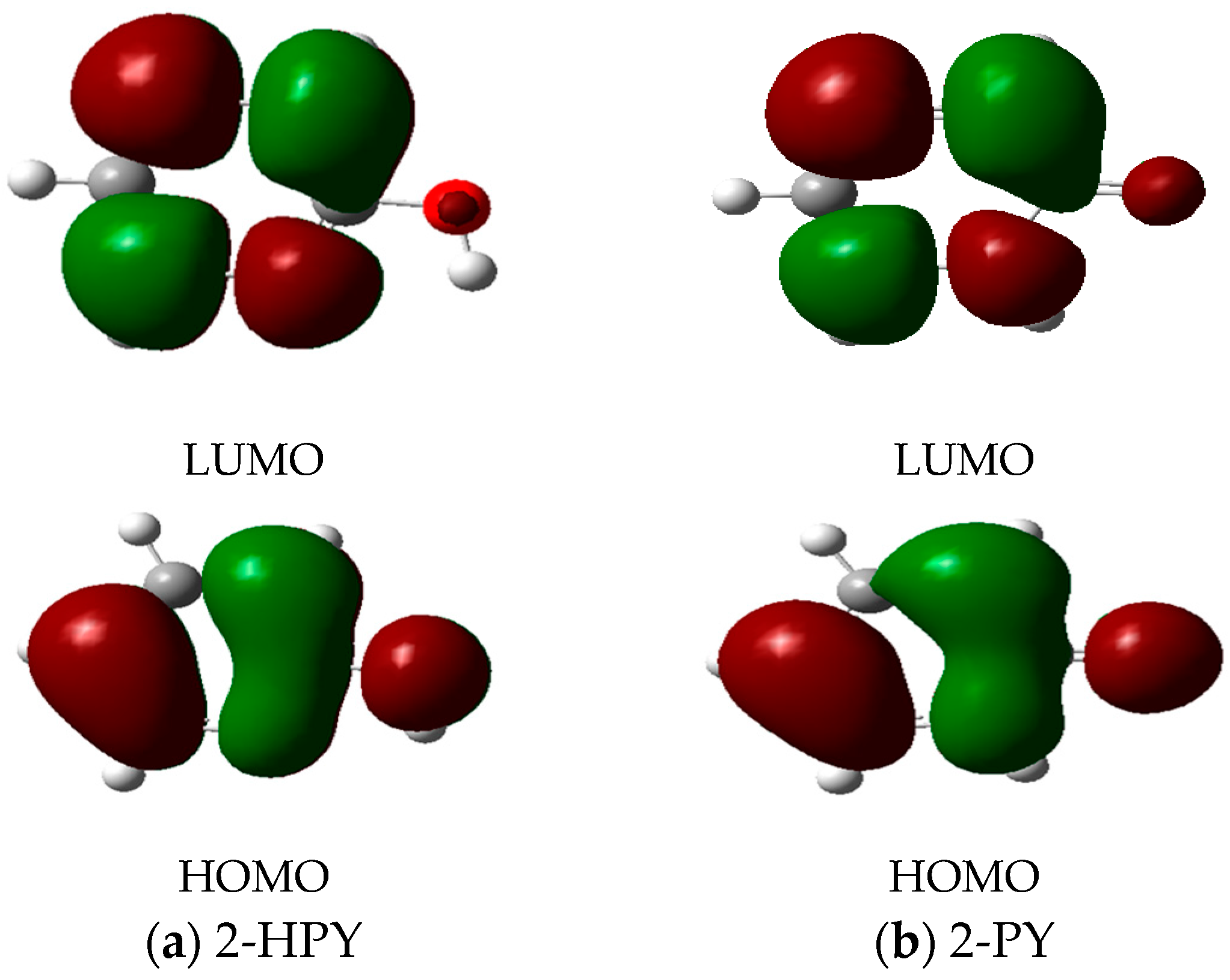
| Level of Theory | Parameter | 2-HPY | 2-PY | p-NA a |
|---|---|---|---|---|
| B3LYP/6-311++G** | Μ | 1.464 | 4.506 | 7.17 |
| HOMO | −6.817 | −6.349 | ||
| LUMO | −1.135 | −1.598 | ||
| E.G. | 5.682 | 4.751 | 4.290 | |
| βtot | 209.27 | 177.85 | 1327 | |
| B3LYP/aug-cc-pvdz | Μ | 1.360 | 4.428 | |
| HOMO | −6.771 | −6.312 | ||
| LUMO | −1.133 | −1.608 | ||
| E.G. | 5.638 | 4.704 | ||
| βtot | 203.55 | 195.01 | ||
| CAM-B3LYP/6-311++G** | Μ | 1.523 | 4.556 | 7.23 |
| HOMO | −8.254 | −7.756 | - | |
| LUMO | 0.172 | −0.275 | - | |
| E.G. | 8.426 | 7.481 | 6.78 | |
| βtot | 197.44 | 149.25 | 1350 | |
| CAM-B3LYP/aug-cc-pvdz | Μ | 1.415 | 4.427 | |
| HOMO | −8.198 | −7.742 | ||
| LUMO | 0.173 | −0.253 | ||
| E.G. | 8.371 | 7.489 | ||
| βtot | 192.10 | 162.10 | ||
| M062X/6-311++G** | Μ | 1.480 | 4.456 | |
| HOMO | −8.155 | −7.626 | ||
| LUMO | −0.101 | −0.541 | ||
| E.G. | 8.054 | 7.085 | ||
| βtot | 194.19 | 158.64 | ||
| M062X/aug-cc-pvdz | Μ | 1.357 | 4.365 | |
| HOMO | −8.069 | −7.645 | ||
| LUMO | −0.111 | −0.528 | ||
| E.G. | 7.958 | 7.117 | ||
| βtot | 204.14 | 181.59 | ||
| ωB97XD/6-311++G** | Μ | 1.460 | 4.516 | 7.160 |
| HOMO | −8.773 | −8.278 | - | |
| LUMO | 0.838 | 0.389 | - | |
| E.G. | 9.611 | 8.667 | 7.96 | |
| βtot | 200.14 | 150.31 | 1350 | |
| ωB97XD/aug-cc-pvdz | Μ | 1.371 | 4.444 | |
| HOMO | −8.724 | −8.241 | ||
| LUMO | 0.839 | 0.383 | ||
| E.G. | 9.563 | 8.624 | ||
| βtot | 198.65 | 167.58 | ||
| Expermintal b | μ | 1.39 | 4.26 | |
| Experimental c | βП(−2ω;ω;ω) | - | - | 1072 ± 44 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejazi, S.A.; Osman, O.I.; Alyoubi, A.O.; Aziz, S.G.; Hilal, R.H. The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit. Int. J. Mol. Sci. 2016, 17, 1893. https://doi.org/10.3390/ijms17111893
Hejazi SA, Osman OI, Alyoubi AO, Aziz SG, Hilal RH. The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit. International Journal of Molecular Sciences. 2016; 17(11):1893. https://doi.org/10.3390/ijms17111893
Chicago/Turabian StyleHejazi, Safiyah A., Osman I. Osman, Abdulrahman O. Alyoubi, Saadullah G. Aziz, and Rifaat H. Hilal. 2016. "The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit" International Journal of Molecular Sciences 17, no. 11: 1893. https://doi.org/10.3390/ijms17111893