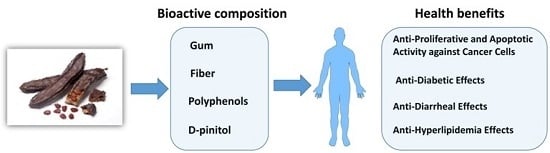
Functional Components of Carob Fruit: Linking the Chemical and Biological Space
Abstract
:1. Introduction
2. Functional Chemical Components of Carob Fruit
2.1. Sugars
2.2. Cyclitols
2.3. Fibers
2.4. Gum
2.5. Polyphenols
2.6. Amino Acids
2.7. Minerals
3. Health Benefits of Carob
3.1. Anti-Proliferative and Apoptotic Activity against Cancer Cells
3.2. Anti-Diabetic Effects
3.3. Anti-Diarrheal Effects
3.4. Anti-Hyperlipidemia Effects
3.5. Carob and Clinical Trials
3.5.1. Infant Regurgitation
3.5.2. Hypercholesterolemia
3.5.3. Diarrhea
4. Carob’s Polyphenols and Bioavailability
5. LBG as Carrier Agent for Controlled Release of Drugs
6. Economic Impact
7. Data Collection
8. Future Directions—Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LBG | locust bean gum |
| CBG | carob bean gum |
| d.m. | dry matter |
| IC50 | half maximal inhibitory concentration |
| BrdU | bromodeoxyuridine |
| AGE | advanced glycation end product |
| ARF | anti-regurgitation formulae |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| CBJ | carob bean juice |
| WHO | World Health Organization |
| ORS | oral rehydration solution |
| IPN | interpenetrating polymer network |
| MLBG | modified locust bean gum |
| PVA | poly (vinyl alcohol) |
| Tmax | time that drug is present at the maximum concentration in serum |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
References
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional characterisation and bioactive components of commercial carobs flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations, Final 2012 Data. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx#ancor (accessed on 12 July 2016).
- Battle, I.; Tous, J. Carob Tree: Ceratonia Siliqua L.—Promoting the Conservation Anduse of Underutilized and Neglected Crops. 17; Bioversity International: Rome, Italy, 1997; p. 91. [Google Scholar]
- Wursch, P.; Delvedovo, S.; Rosset, J.; Smiley, M. The tannin granules from ripe carob pod. Lebensm. Wiss. Technol. 1984, 17, 351–354. [Google Scholar]
- Miyazawa, R.; Tomomasa, T.; Kaneko, H.; Arakawa, H.; Morikawa, A. Effect of formula thickened with reduced concentration of locust bean gum on gastroesophageal reflux. Acta Paediatr. 2007, 96, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, R.; Tomomasa, T.; Kaneko, H.; Morikawa, A. Effect of formula thickened with locust bean gum on gastric emptying in infants. J. Paediatr. Child. Health 2006, 42, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Leluyer, B.; Cazaubiel, M.; Housez, B.; Bocquet, A. Double-blind comparative trial with 2 antiregurgitation formulae. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Vivatvakin, B.; Buachum, V. Effect of carob bean on gastric emptying time in thai infants. Asia Pac. J. Clin. Nutr. 2003, 12, 193–197. [Google Scholar] [PubMed]
- Maiti, S.; Dey, P.; Banik, A.; Sa, B.; Ray, S.; Kaity, S. Tailoring of locust bean gum and development of hydrogel beads for controlled oral delivery of glipizide. Drug. Deliv. 2010, 17, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Aj, R.; Hn, Y.; Sb, S. Natural gums as sustained release carriers: Development of gastroretentive drug delivery system of ziprasidone hcl. DARU J. 2012, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Panghal, D.; Nagpal, M.; Thakur, G.S.; Arora, S. Dissolution improvement of atorvastatin calcium using modified locust bean gum by the solid dispersion technique. Sci. Pharm. 2014, 82, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Kaity, S.; Isaac, J.; Ghosh, A. Interpenetrating polymer network of locust bean gum-poly (vinyl alcohol) for controlled release drug delivery. Carbohydr. Polym. 2013, 94, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Kaity, S.; Ghosh, A. Comparative bio-safety and in vivo evaluation of native or modified locust bean gum-PVA IPN microspheres. Int. J. Biol. Macromol. 2015, 72, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, C.; Vasanthakumar, S.; Ramakrishnan, A. In vitro and in vivo evaluation of locust bean gum and chitosan combination as a carrier for buccal drug delivery. Pharmazie 2008, 63, 342–347. [Google Scholar] [PubMed]
- Jana, S.; Gandhi, A.; Sheet, S.; Sen, K.K. Metal ion-induced alginate-locust bean gum ipn microspheres for sustained oral delivery of aceclofenac. Int. J. Biol. Macromol. 2015, 72, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Trotta, A.M.; Marianecci, C.; Carafa, M.; di Marzio, L.; Rinaldi, F.; di Meo, C.; Alhaique, F.; Matricardi, P. Gel-embedded niosomes: Preparation, characterization and release studies of a new system for topical drug delivery. Colloids Surf. B 2015, 125, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tsatsaragkou, K.; Yiannopoulos, S.; Kontogiorgi, A.; Poulli, E.; Krokida, M.; Mandala, I. Effect of carob flour addition on the rheological properties of gluten-free breads. Food Bioprocess. Technol. 2014, 7, 868–876. [Google Scholar] [CrossRef]
- Šebečić, B.; Dragojević, I.V.; Vitali, D.; Hečimović, M.; Dragičević, I. Raw materials in fibre enriched biscuits production as source of total phenols. Poljopr. Znanst. Smotra 2007, 72, 265–270. [Google Scholar]
- Seczyk, L.; Swieca, M.; Gawlik-Dziki, U. Effect of carob (Ceratonia siliqua L.) flour on the antioxidant potential, nutritional quality, and sensory characteristics of fortified durum wheat pasta. Food Chem. 2016, 194, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Lar, A.C.; Erol, N.; Elgun, M.S. Effect of carob flour substitution on chemical and functional properties of tarhana. J. Food Process. Preserv. 2013, 37, 670–675. [Google Scholar]
- Martin-Diana, A.B.; Izquierdo, N.; Albertos, I.; Sanchez, M.S.; Herrero, A.; Sanz, M.A.; Rico, D. Valorization of carob’s germ and seed peel as natural antioxidant ingredients in gluten-free crackers. J. Food Process. Preserv. 2016. [Google Scholar] [CrossRef]
- Srour, N.; Daroub, H.; Toufeili, I.; Olabi, A. Developing a carob-based milk beverage using different varieties of carob pods and two roasting treatments and assessing their effect on quality characteristics. J. Sci. Food Agric. 2016, 96, 3047–3057. [Google Scholar] [CrossRef] [PubMed]
- Custodio, L.; Patarra, J.; Albericio, F.; Neng, N.R.; Nogueira, J.M.; Romano, A. In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase. Nat. Prod. Res. 2015, 29, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Pramod, T.; Lingappa, K. Immobilization of aspergillus niger in polyurethane foam for citric acid production from carob pod extract. Am. J. Food Technol. 2008, 3, 252–256. [Google Scholar]
- Turhan, I.; Bialka, K.L.; Demirci, A.; Karhan, M. Enhanced lactic acid production from carob extract by lactobacillus casei using invertase pretreatment. Food Biotechnol. 2010, 24, 364–374. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Moniz, P.; Duarte, L.C.; Esteves, M.P.; Gírio, F.M. Mannitol production by lactic acid bacteria grown in supplemented carob syrup. J. Ind. Microbiol. Biotechnol. 2011, 38, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Roca, C.; Reis, M.A. Improving succinic acid production by actinobacillus succinogenes from raw industrial carob pods. Bioresour. Technol. 2016, 218, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Ercan, Y.; Irfan, T.; Mustafa, K. Optimization of ethanol production from carob pod extract using immobilized saccharomyces cerevisiae cells in a stirred tank bioreactor. Bioresour. Technol. 2013, 135, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.H.; Jones, R.B.; Bailey, C.J. Insulin-like effect of pinitol. Br. J. Pharmacol. 2000, 130, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Gruendel, S.; Otto, B.; Garcia, A.L.; Wagner, K.; Mueller, C.; Weickert, M.O.; Heldwein, W.; Koebnick, C. Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. Br. J. Nutr. 2007, 98, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Zunft, H.J.; Luder, W.; Harde, A.; Haber, B.; Graubaum, H.J.; Koebnick, C.; Grunwald, J. Carob pulp preparation rich in insoluble fibre lowers total and LDL cholesterol in hypercholesterolemic patients. Eur. J. Nutr. 2003, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Klenow, S.; Glei, M.; Haber, B.; Owen, R.; Pool-Zobel, B.L. Carob fibre compounds modulate parameters of cell growth differently in human ht29 colon adenocarcinoma cells than in LT97 colon adenoma cells. Food Chem. Toxicol. 2008, 46, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Perez-Olleros, L.; Garcia-Cuevas, M.; Ruiz-Roso, B.; Requejo, A. Comparative study of natural carob fibre and psyllium husk in rats. Influence on some aspects of nutritional utilisation and lipidaemia. J. Sci. Food Agric. 1999, 79, 173–178. [Google Scholar] [CrossRef]
- Guggenbichler, J.P. Adherence of enterobacteria in infantile diarrhea and its prevention. Infection 1983, 11, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Turhan, I. Relationship between sugar profile and d-pinitol content of pods of wild and cultivated types of carob bean (Ceratonia siliqua L.). Int. J. Food Prop. 2013, 17, 363–370. [Google Scholar] [CrossRef]
- Sigge, G.O.; Lipumbua, L.; Britza, T.J. Proximate composition of carob cultivars growing in south africa. S. Afr. J. Plant Soil 2011, 28, 17–22. [Google Scholar] [CrossRef]
- Diaz, C.S. Syrup of Natural Carob Sugars and a Process for Its Production. U.S. Patent 5,624,500 A, 29 April 1997. [Google Scholar]
- Livesey, G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Gennerritzmann, R.; Haas, J.; Amado, R.; Neukom, H. Isolation and identification of cyclitols in carob pods (Ceratonia-siliqua L). J. Agric. Food Chem. 1986, 34, 827–829. [Google Scholar] [CrossRef]
- Camero, B.M.; Merino, C.S. Method of Obtaining Pinitol from Carob Extracts. U.S. Patent 6699511 B2, 2 March 2004. [Google Scholar]
- Haber, B. Carob fiber benefits and applications. Cereal Foods World 2002, 47, 365–369. [Google Scholar]
- Marco, A.M.R.; De Mora, B.R.C.; Diaz, C.S. Method of Making Natural Carob Fiber. U.S. Patent 5,609,905 A, 11 March 1997. [Google Scholar]
- Nasar-Abbas, S.M.; E-Huma, Z.; Vu, T.-H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob kibble: A bioactive-rich food ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef]
- Nawrocka, A.; Miś, A.; Szymańska-Chargot, M. Characteristics of relationships between structure of gluten proteins and dough rheology—Influence of dietary fibres studied by FT-Raman spectroscopy. Food Biophys. 2016, 11, 81–90. [Google Scholar] [CrossRef]
- Miś, A.; Dziki, D. Extensograph curve profile model used for characterising the impact of dietary fibre on wheat dough. J. Cereal Sci. 2013, 57, 471–479. [Google Scholar] [CrossRef]
- Rizzo, V.; Tomaselli, F.; Gentile, A.; La Malfa, S.; Maccarone, E. Rheological properties and sugar composition of locust bean gum from different carob varieties (Ceratonia siliqua L.). J. Agric. Food Chem. 2004, 52, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W.; Reichel, M. Producing a Locust Bean Gum Comprising Food Product, Preferably a Powdered Baby Food, Comprises Heating a Liquid Foodstuff Mixture and Spray Drying the Mixture. German Patent DE102,011,106,409A, 26 July 2012. [Google Scholar]
- Aoki, K.K.; Sasaki, J.; Shiotani, T. Jelly Foods Containing Agar, Xanthan and Locust Bean Gum. Europe Patent EP1,074,183 A2, 7 Febraury 2001. [Google Scholar]
- Yan, C.; Given, P.S.; Huvard, G.; Mallepally, R.R.; McHugh, M.A. Method of Loading Flavor into an Aerogel and Flavor Impregnated Aerogel Based on Food Grade Materials. U.S. Patent 20,160,058,045 A1, 3 March 2016. [Google Scholar]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSN. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Comp. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Cavdarova, M.; Makris, D.P. Extraction kinetics of phenolics from carob (Ceratonia siliqua L.) kibbles using environmentally benign solvents. Waste Biomass Valoriz. 2014, 5, 773–779. [Google Scholar] [CrossRef]
- Novotni, D.; Curic, D.; Bituh, M.; Colic Baric, I.; Skevin, D.; Cukelj, N. Glycemic index and phenolics of partially-baked frozen bread with sourdough. Int. J. Food Sci. Nutr. 2011, 62, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepulveda, C.; Almeida, J.; Meireles, M.; Girio, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crops. Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Almanasrah, M.; Roseiro, L.B.; Bogel-Lukasik, R.; Carvalheiro, F.; Brazinha, C.; Crespo, J.; Kallioinen, M.; Mänttäri, M.; Duarte, L.C. Selective recovery of phenolic compounds and carbohydrates from carob kibbles using water-based extraction. Ind. Crop. Prod. 2015, 70, 443–450. [Google Scholar] [CrossRef]
- Baraldi, M. Extract of Ceratonia Siliqua Leaves and Pods Containing Polyphenols with Antioxidant and Antitumor Activities. U.S. Patent 20,040,265,404 A1, 30 December 2004. [Google Scholar]
- Custodio, L.; Escapa, A.L.; Fernandes, E.; Fajardo, A.; Aligue, R.; Albericio, F.; Neng, N.; Nogueira, J.M.F.; Romano, A. Phytochemical profile, antioxidant and cytotoxic activities of the carob tree (Ceratonia siliqua L.) germ flour extracts. Plant. Food. Hum. Nutr. 2011, 66, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Haubner, R.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem. Toxicol. 2003, 41, 1727–1738. [Google Scholar] [CrossRef]
- Custodio, L.; Fernandes, E.; Escapa, A.L.; Fajardo, A.; Aligue, R.; Albericio, F.; Neng, N.R.; Nogueira, J.M.; Romano, A. Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J. Agric. Food Chem. 2011, 59, 7005–7012. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Gruz, J.; Strnad, M. Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): Sugars, amino and organic acids, minerals and phenolic compounds. J. Food Qual. 2007, 30, 1040–1055. [Google Scholar] [CrossRef]
- Ortega, N.; Macia, A.; Romero, M.P.; Trullols, E.; Morello, J.R.; Angles, N.; Motilva, M.J. Rapid determination of phenolic compounds and alkaloids of carob flour by improved liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 7239–7244. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Ereifej, K.I.; Esoh, R.B.; Al-u'datt, M.H.; Alrababah, M.A.; Yang, W. Antioxidant activities, total phenolics and hplc analyses of the phenolic compounds of extracts from common mediterranean plants. Nat. Prod. Rep. 2011, 25, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [PubMed]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of chemical composition of carob (Ceratonia siliqua): Protein, fat, carbohydrates, and tannins. J. Food Comp. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Glew, R.H.; Bak, Z.D.; Chuang, L.T.; Presley, J.M.; Andrews, R. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. Plant Foods Hum. Nutr. 2009, 64, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Oziyci, H.R.; Tetik, N.; Turhan, I.; Yatmaz, E.; Ucgun, K.; Akgul, H.; Gubbuk, H.; Karhan, M. Mineral composition of pods and seeds of wild and grafted carob (Ceratonia siliqua L.) fruits. Sci. Hortic. 2014, 167, 149–152. [Google Scholar] [CrossRef]
- Singh, G.; Arora, S.; Sharma, G.S.; Sangwan, R.B. Heat stability and calcium bioavailability of calcium-fortified milk. Lebensm. Wiss. Technol. 2007, 40, 625–631. [Google Scholar] [CrossRef]
- Tetik, N.; Yuksel, E. Ultrasound-assisted extraction of d-pinitol from carob pods using response surface methodology. Ultrason. Sonochem. 2014, 21, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Wursch, P. Influence of tannin-rich carob pod fiber on the cholesterol metabolism in the rat. J. Nutr. 1979, 109, 685–692. [Google Scholar] [PubMed]
- Gruendel, S.; Garcia, A.L.; Otto, B.; Mueller, C.; Steiniger, J.; Weickert, M.O.; Speth, M.; Katz, N.; Koebnick, C. Carob pulp preparation rich in insoluble dietary fiber and polyphenols enhances lipid oxidation and lowers postprandial acylated ghrelin in humans. J. Nutr. 2006, 136, 1533–1538. [Google Scholar] [PubMed]
- Ruiz-Roso, B.; Quintela, J.C.; de la Fuente, E.; Haya, J.; Perez-Olleros, L. Insoluble carob fiber rich in polyphenols lowers total and LDL cholesterol in hypercholesterolemic subjects. Plant Food Hum. Nutr. 2010, 65, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Aksit, S.; Caglayan, S.; Cukan, R.; Yaprak, I. Carob bean juice: A powerful adjunct to oral rehydration solution treatment in diarrhoea. Paediatr. Perinat. Epidemiol. 1998, 12, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Primikyri, A.; Chatziathanasiadou, M.V.; Karali, E.; Kostaras, E.; Mantzaris, M.D.; Hatzimichael, E.; Shin, J.S.; Chi, S.W.; Briasoulis, E.; Kolettas, E.; et al. Direct binding of Bcl-2 family proteins by quercetin triggers its pro-apoptotic activity. ACS Chem. Biol. 2014, 9, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Angst, E.; Park, J.L.; Moro, A.; Lu, Q.Y.; Lu, X.; Li, G.; King, J.; Chen, M.; Reber, H.A.; Go, V.L.; et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas 2013, 42, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Q.; Yang, S.; Chen, C.; Li, X.; Liu, J.; Zou, Z.; Cai, D. Quercetin inhibits angiogenesis by targeting calcineurin in the xenograft model of human breast cancer. Eur. J. Pharmacol. 2016, 781, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Z.; Zhang, X.; Li, H.; Tao, Y.Q.; Tao, L.J.; Yang, Z.R.; Zhou, X.P.; Shi, Z.L.; Tao, H.M. Gallic acid induces the apoptosis of human osteosarcoma cells in vitro and in vivo via the regulation of mitogen-activated protein kinase pathways. Cancer Biother. Radiopharm. 2012, 27, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer pc3 cells in vitro and in vivo: Involvement of nuclear factor-κB signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef] [PubMed]
- Haber, B.D. Carob Product Based Antiinflammatory or Chemopreventative Agent. Europe Patent EP1,366,673 A3, 10 December 2003. [Google Scholar]
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Antiproliferative effects of Ceratonia siliqua L. On mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684. [Google Scholar] [CrossRef]
- Klenow, S.; Jahns, F.; Pool-Zobel, B.L.; Glei, M. Does an extract of carob (Ceratonia siliqua L.) have chemopreventive potential related to oxidative stress and drug metabolism in human colon cells? J. Agric. Food Chem. 2009, 57, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Klenow, S.; Glei, M. New insight into the influence of carob extract and gallic acid on hemin induced modulation of HT29 cell growth parameters. Toxicol. In Vitro 2009, 23, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Lee, J.W.; Lee, P.; Bae, K.H. Glycemic index of insu 100® herbal preparation containing koreanred ginseng, carob, mulberry, and banaba. J. Ginseng Res. 2010, 34, 89–92. [Google Scholar] [CrossRef]
- Forestieri, A.M.; Galati, E.M.; Trovato, A.; Tumino, G. Effects of guar and carob gums on glucose, insulin and cholesterol plasma levels in the rat. Phytother. Res. 1989, 3, 1–4. [Google Scholar] [CrossRef]
- Milek Dos Santos, L.; Tomzack Tulio, L.; Fuganti Campos, L.; Ramos Dorneles, M.; Carneiro Hecke Kruger, C. Glycemic response to carob (Ceratonia siliqua L.) in healthy subjects and with the in vitro hydrolysis index. Nutr. Hosp. 2015, 31, 482–487. [Google Scholar]
- Loeb, H.; Vandenplas, Y.; Wursch, P.; Guesry, P. Tannin-rich carob pod for the treatment of acute-onset diarrhea. J. Pediatr. Gastroenterol. Nutr. 1989, 8, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, Y.; Xu, W.; Wang, H.; Lin, N. Rhubarb tannins extract inhibits the expression of aquaporins 2 and 3 in magnesium sulphate-induced diarrhoea model. BioMed Res. Int. 2014, 2014, 619465. [Google Scholar] [CrossRef] [PubMed]
- Wursch, P. Treatment of Diarrhoea with Compositions Derived from Carob Pod. U.S. Patent 5,043,160 A, 27 August 1991. [Google Scholar]
- Haber, B.; Ter, M.H.U.; Hausmanns, S. Cholesterol-Reducing Agent Made of Dietary Fibre and Cholesterol-Reducing Substances. WO2,004,009,093 A1, 29 January 2004. [Google Scholar]
- Haber, B.; Kiy, T.; Hausmanns, S.; Ruesing, M. Dietary Foodstuff for Positively Influencing Cardiovascular Health. U.S. Patent 20,060,110,476 A1, 25 May 2006. [Google Scholar]
- Ershoff, B.H.; Wells, A.F. Effects of gum guar, locust bean gum and carrageenan on liver cholesterol of cholesterolfed rats. Proc. Soc. Exp. Biol. Med. 1962, 110, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sogawa, I.; Nishina, A.; Saeki, S.; Ichikawa, N.; Iibata, S. Improved hypolipidemic effects of xanthan gum-galactomannan mixtures in rats. Biosci. Biotechnol. Biochem. 2000, 64, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, K.M.A.; Youssef, M.K.E.; Ali, H.M.; El-Manfaloty, M.M. The influence of carob powder on lipid profile and histopathologyof some organs in rats. Comp. Clin. Pathol. 2015, 24, 1509–1513. [Google Scholar] [CrossRef]
- Valero-Munoz, M.; Martin-Fernandez, B.; Ballesteros, S.; Lahera, V.; de las Heras, N. Carob pod insoluble fiber exerts anti-atherosclerotic effects in rabbits through sirtuin-1 and peroxisome proliferator-activated receptor-gamma coactivator-1α. J. Nutr. 2014, 144, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.D.; Spiller, G.A.; Gates, J.E.; Miller, A.F.; Whittam, J.H. The effect of acacia gum and a water-soluble dietary fiber mixture on blood lipids in humans. J. Am. Coll. Nutr. 1993, 12, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Greally, P.; Hampton, F.J.; MacFadyen, U.M.; Simpson, H. Gaviscon and carobel compared with cisapride in gastro-oesophageal reflux. Arch. Dis. Child. 1992, 67, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Haskell, W.L.; Spiller, G.A.; Jensen, C.D.; Ellis, B.K.; Gates, J.E. Role of water-soluble dietary fiber in the management of elevated plasma cholesterol in healthy subjects. Am. J. Cardiol. 1992, 69, 433–439. [Google Scholar] [CrossRef]
- Jensen, C.D.; Haskell, W.; Whittam, J.H. Long-term effects of water-soluble dietary fiber in the management of hypercholesterolemia in healthy men and women. Am. J. Cardiol. 1997, 79, 34–37. [Google Scholar] [CrossRef]
- Zavoral, J.H.; Hannan, P.; Fields, D.J.; Hanson, M.N.; Frantz, I.D.; Kuba, K.; Elmer, P.; Jacobs, D.R., Jr. The hypolipidemic effect of locust bean gum food products in familial hypercholesterolemic adults and children. Am. J. Clin. Nutr. 1983, 38, 285–294. [Google Scholar] [PubMed]
- Hostettler, M.; Steffen, R.; Tschopp, A. Efficacy and tolerance of insoluble carob fraction in the treatment of travellers’ diarrhoea. J. Diarrhoeal Dis. Res. 1995, 13, 155–158. [Google Scholar] [PubMed]
- Benchikh, Y.; Louaileche, H.; George, B.; Merlin, A. Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Ind. Crops Prod. 2014, 60, 298–303. [Google Scholar] [CrossRef]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Hu, M. Commentary: Bioavailability of flavonoids and polyphenols: Call to arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Urso, R.; Blardi, P.; Giorgi, G. A short introduction to pharmacokinetics. Eur. Rev. Med. Pharmacol. Sci. 2002, 6, 33–44. [Google Scholar] [PubMed]
- Lu, C.M.; Lin, L.C.; Tsai, T.H. Determination and pharmacokinetic study of gentiopicroside, geniposide, baicalin, and swertiamarin in chinese herbal formulae after oral administration in rats by LC-MS/MS. Molecules 2014, 19, 21560–21578. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Ni, B.; Feng, L.; Yin, X.; Dou, H.; Fu, J.; Lin, L.; Ni, J. Simultaneous determination of typhaneoside and Isorhamnetin-3-O-Neohesperidoside in rats after oral administration of pollen typhae extract by UPLC-MS/MS. J. Chromatogr. Sci. 2015, 53, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Law, M.C.; Wong, M.S.; Chan, T.H. Development of a UPLC-MS/MS bioanalytical method for the pharmacokinetic study of (−)-epiafzelechin, a flavan-3-ol with osteoprotective activity, in C57BL/6J mice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 967, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Li, R.F.; Sun, M.; Hu, D.M.; Qiu, J.F.; Yan, Y.H. UPLC-MS/MS method for determination of avicularin in rat plasma and its application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 965, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Guo, J.; Chen, S.L.; Liu, X.; Zhou, Y.; Zhang, X.P.; Xu, X.D. Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography-mass spectrometry. J. Pharm. Biomed. 2013, 72, 267–291. [Google Scholar] [CrossRef] [PubMed]
- Kaity, S.; Isaac, J.; Kumar, P.M.; Bose, A.; Wong, T.W.; Ghosh, A. Microwave assisted synthesis of acrylamide grafted locust bean gum and its application in drug delivery. Carbohydr. Polym. 2013, 98, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J. Locust bean gum: A versatile biopolymer. Carbohydr. Polym. 2013, 94, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A.; Rathbone, M.J. Overview of controlled release mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmannl, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: St. Paul, MN, USA, 2011; pp. 19–43. [Google Scholar]
- Dionisio, M.; Grenha, A. Locust bean gum: Exploring its potential for biopharmaceutical applications. J. Pharm. Bioallied Sci. 2012, 4, 175–185. [Google Scholar] [PubMed]
- Tamarkin, D.; Friedman, D.; Eini, M. Foam Carrier Containing Amphiphilic Copolymeric Gelling Agent. Patent WO2005,011,567 A3, 27 October 2005. [Google Scholar]
- Baichwal, A.R.; Staniforth, J.N. Controlled Release Insufflation Carrier for Medicaments Comprising Xanthan Gum and Locust Bean Gum. Patent EP 0818991 B1, 4 January 2006. [Google Scholar]
- Biasutto, L.; Zoratti, M. Prodrugs of quercetin and resveratrol: A strategy under development. Curr. Drug Metab. 2014, 15, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Cavalli, R.; Trotta, F. Cyclodextrin-based nanosponges: A versatile platform for cancer nanotherapeutics development. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gulec, K.; Demirel, M. Characterization and antioxidant activity of quercetin/methyl-B-cyclodextrin complexes. Curr. Drug Deliv. 2015, 13, 444–451. [Google Scholar] [CrossRef]


| Polyphenol | Carob Part/Fraction | Reference |
|---|---|---|
| Phenolic acids | ||
| 4-hydroxybenzoic acid | Pulp | [57] |
| Caffeic acid | Pulp | [57] |
| Chlorogenic acid | Seed | [60] |
| Cinnamic acid | Fiber, pulp | [57,61] |
| Coumaric acid | Fiber, pulp | [57,61] |
| Ferulic acid | Fiber, pulp, seed | [57,60,61] |
| Gallic acid | Pulp, fiber, seed | [53,60,62] |
| Gentisic acid | Seed | [60] |
| Syringic acid | Pulp, seed | [57,60] |
| Flavonoids | ||
| (epi)gallocatechin | Fiber | [53] |
| (epi)gallocatechingallate | Fiber | [53] |
| Apigenin | Fiber, pulp | [57,61] |
| Catechin | Pulp, seed | [53,60,62] |
| Chrysoeriol | Fiber, pulp | [57,61] |
| Eridictyol | Pulp | [57] |
| Genistein | Pulp | [57] |
| Isorhamnetin | Fiber, pulp | [57,61] |
| Kaempferol | Fiber, pulp | [53,57,61] |
| Kaempferol rhamnoside | Fiber | [61] |
| Kaempferol-desoxyhexoside and -dihexoside | Pulp | [53] |
| Luteolin | Fiber, pulp | [57,61] |
| Myricetin | Seed | [60] |
| Myricetin rhamnoside and -desoxyhexoside | Fiber | [61] |
| Myricetin-hexoside | Fiber, pulp | [53,57,61] |
| Naringenin | Fiber, pulp | [57,61] |
| Quercetin | Fiber, seed | [60,61] |
| Quercetin-arabinoside | Fiber | [61] |
| Quercetin-desoxyhexoside and -hexoside | Fiber, pulp | [53] |
| Quercetin rhamnoside | Pulp | [57] |
| Tricetin 3′, 5′ dimethyl ether | Fiber, pulp | [57,61] |
| Tannins | ||
| (epi)gallocatechin + 4 gallic acid units | Fiber | [53] |
| Hexose + 2 or 3 or 4 or 5 Gallic acid Units | Fiber | [53,61] |
| Pentoses + 2 gallic acid units | Fiber | [53] |
| prodelphinidin dimer and trimer | Fiber | [53] |
| Group of Chemical Constituents/Individual Substances | Biological Evaluation of Constituents/Disease | Carob Parts/Fraction | Reference |
|---|---|---|---|
| LBG/galactomannan | Gastrointestinal effects | Seed endosperm | [5,6,7,8] |
| d-Pinitol | Anti-diabetic activity | Carob pulp | [29,71] |
| Soluble and Insoluble Dietary Fiber Polyphenols/Gallic acid, Gallotannins, Flavonol Glycosides | Glycemic control, Enhanced lipid metabolism, Lowers total and LDL cholesterol | Carob Pulp | [30,31] |
| Insoluble Dietary Fiber Polyphenols/Tannins, Cellulose, Semicellulose, Lignin, Pectin | Cholesterol metabolism, Enhances lipid oxidation, Lowers postprandial acylated ghrelin | Carob Fiber | [72,73] |
| Polyphenols/ Gallic acid, Catechin, Myricetin rhamnoside, Eriodictyol glucoside, Quercetin glucoside, Quercetin rhamnoside | Anticancer effects | Carob Fiber | [32] |
| Polyphenols—Alkaloids/(+)-Catechin; Gentisic acid; Chlorogenic acid; Catechol; Ferulic acid; Gallic acid; Myricetin; Methyl gallate; Quercetin; Rutin; Syringic acid; Theophylline; Vanillin | Cytotoxic activities | Germ Flour Extracts (seed) | [31] |
| Fiber | Nutritional utilization, Induction of lipodemia | Carob Fiber | [33] |
| Fiber | Hyperlipidemia effects | Carob fiber | [31,74] |
| Tannins—Polyphenols | Anti-diarrheal effects | Carob pod | [34] |
| Tannins—Pectin | Anti-diarrheal effects | Carob bean juice | [75] |
| Carrier Agent | Role of Carrier Agent | Active Substance | Reference |
|---|---|---|---|
| Carboxymethyl derivative of LBG | Controlled release | Glipizide | [9] |
| LBG | Controlled release, Floatability | Ziprasidone HCl | [10] |
| Modified LBC (heating method) | Increased solubility | Atorvastatin | [11] |
| Interpenetrating polymer network microspheres of LBG and poly (vinyl alcohol) | Oral controlled release | Buflomedil HCl | [13] |
| LBG and chitosan mixtures | Mucoadhesive component in tablets | Propranolol HCl | [14] |
| Ca2+ alginate–LBG microspheres | Prolonged release | Aceclofenac | [15] |
| LBG gel-embedded niosomes | Preparation of vesicular systems | Monoammonium glycyrrhizinate | [16] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. https://doi.org/10.3390/ijms17111875
Goulas V, Stylos E, Chatziathanasiadou MV, Mavromoustakos T, Tzakos AG. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. International Journal of Molecular Sciences. 2016; 17(11):1875. https://doi.org/10.3390/ijms17111875
Chicago/Turabian StyleGoulas, Vlasios, Evgenios Stylos, Maria V. Chatziathanasiadou, Thomas Mavromoustakos, and Andreas G. Tzakos. 2016. "Functional Components of Carob Fruit: Linking the Chemical and Biological Space" International Journal of Molecular Sciences 17, no. 11: 1875. https://doi.org/10.3390/ijms17111875