The Control of Calcium Metabolism in Zebrafish (Danio rerio)
Abstract
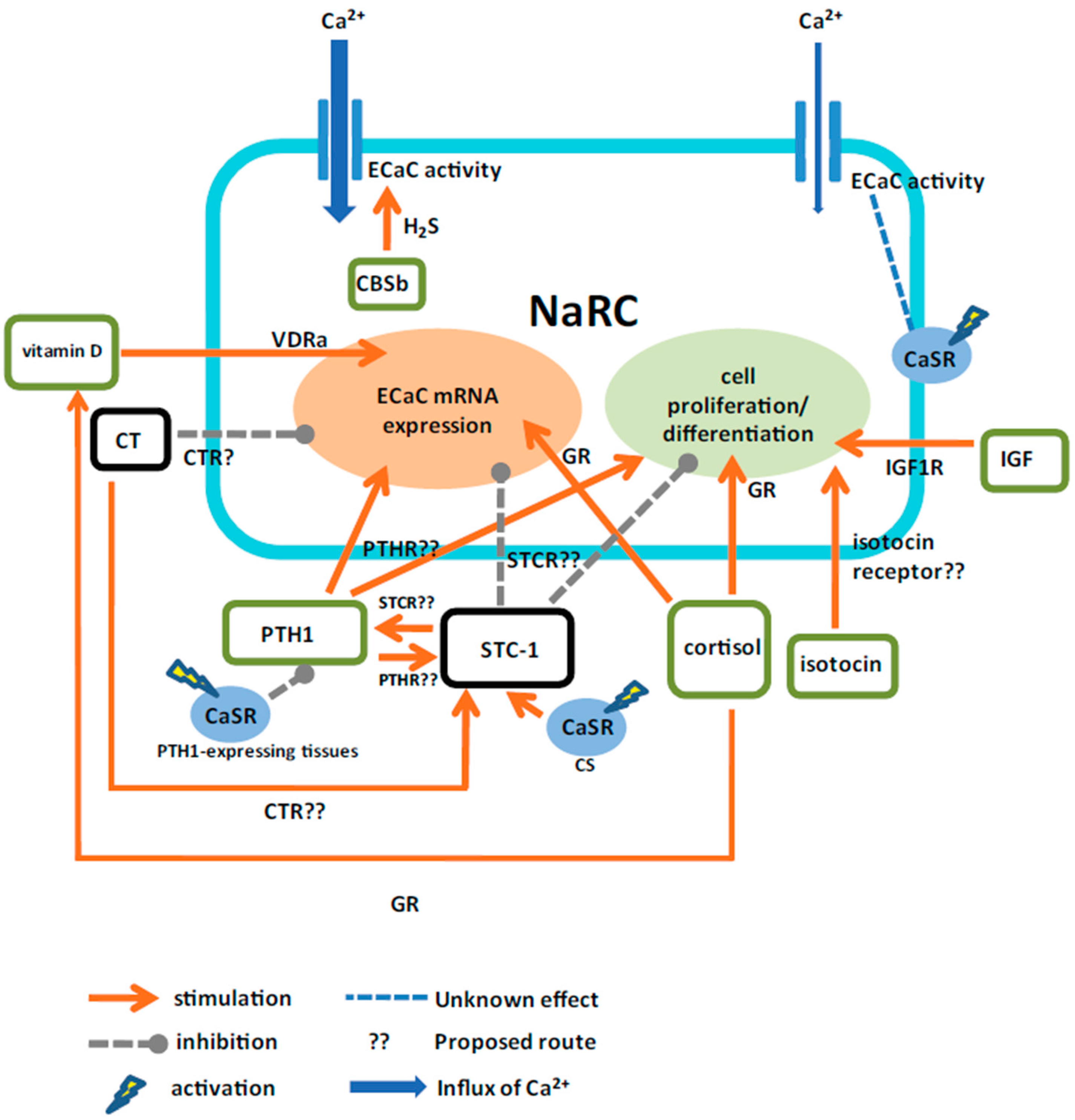
:1. Introduction
2. Ca2+ Regulation in Zebrafish
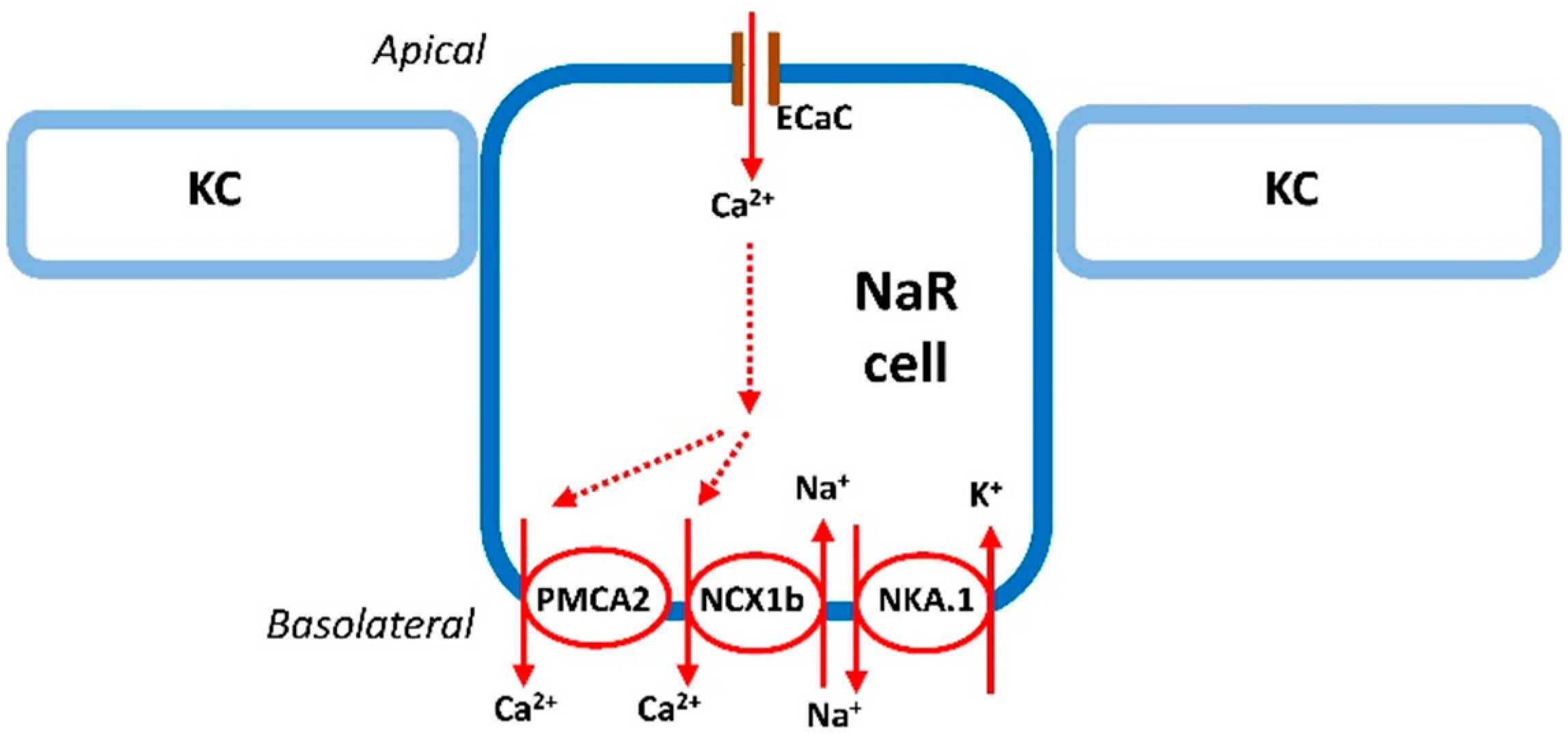
2.1. Ca2+ Uptake in Zebrafish
2.2. Ca2+ Excretion in Zebrafish
3. The Regulation of Ca2+ Uptake in Zebrafish
3.1. The Hormonal Control of Ca2+ Uptake
3.1.1. Cortisol
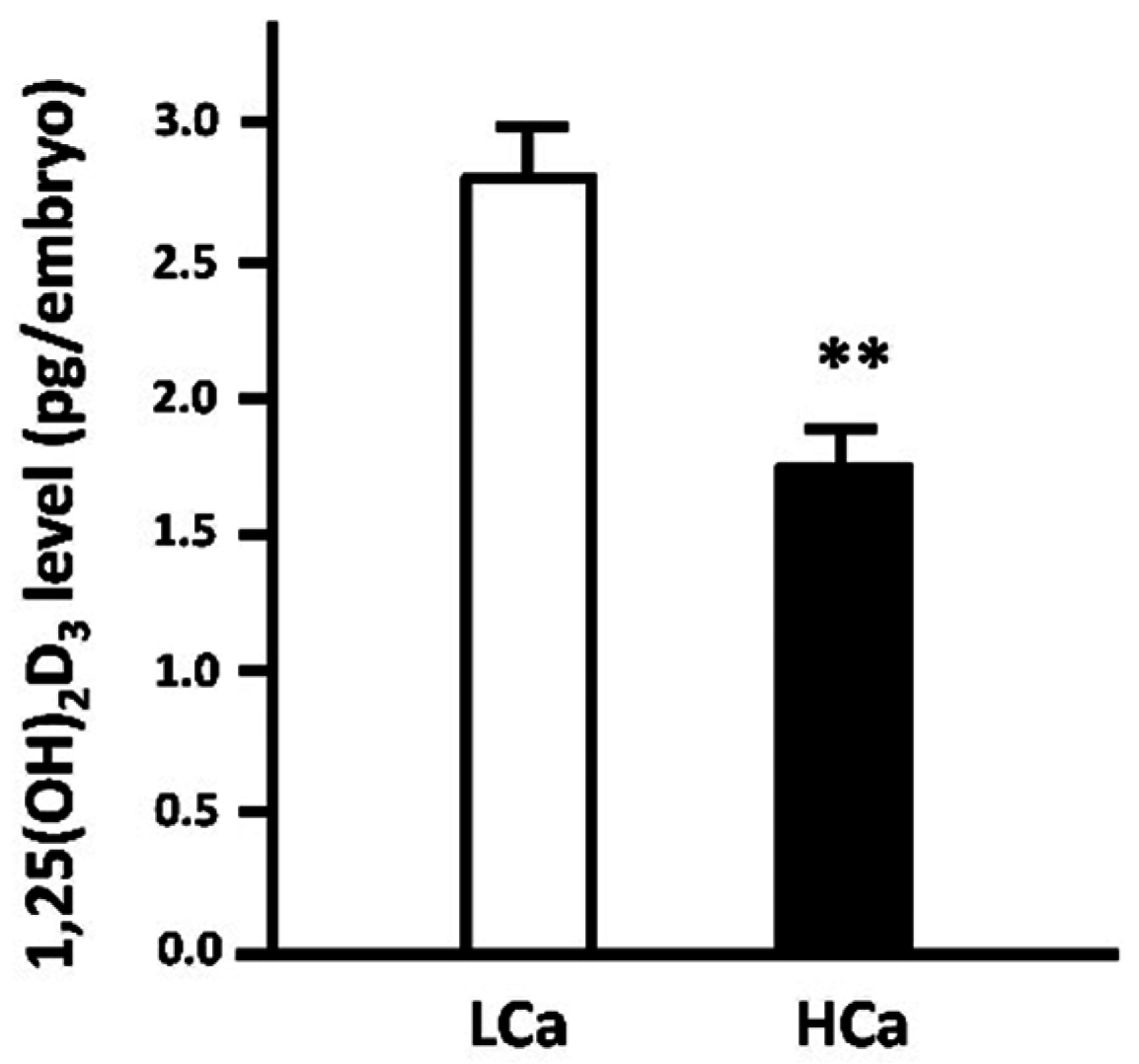
3.1.2. Vitamin D
3.1.3. Parathyroid Hormone (PTH)
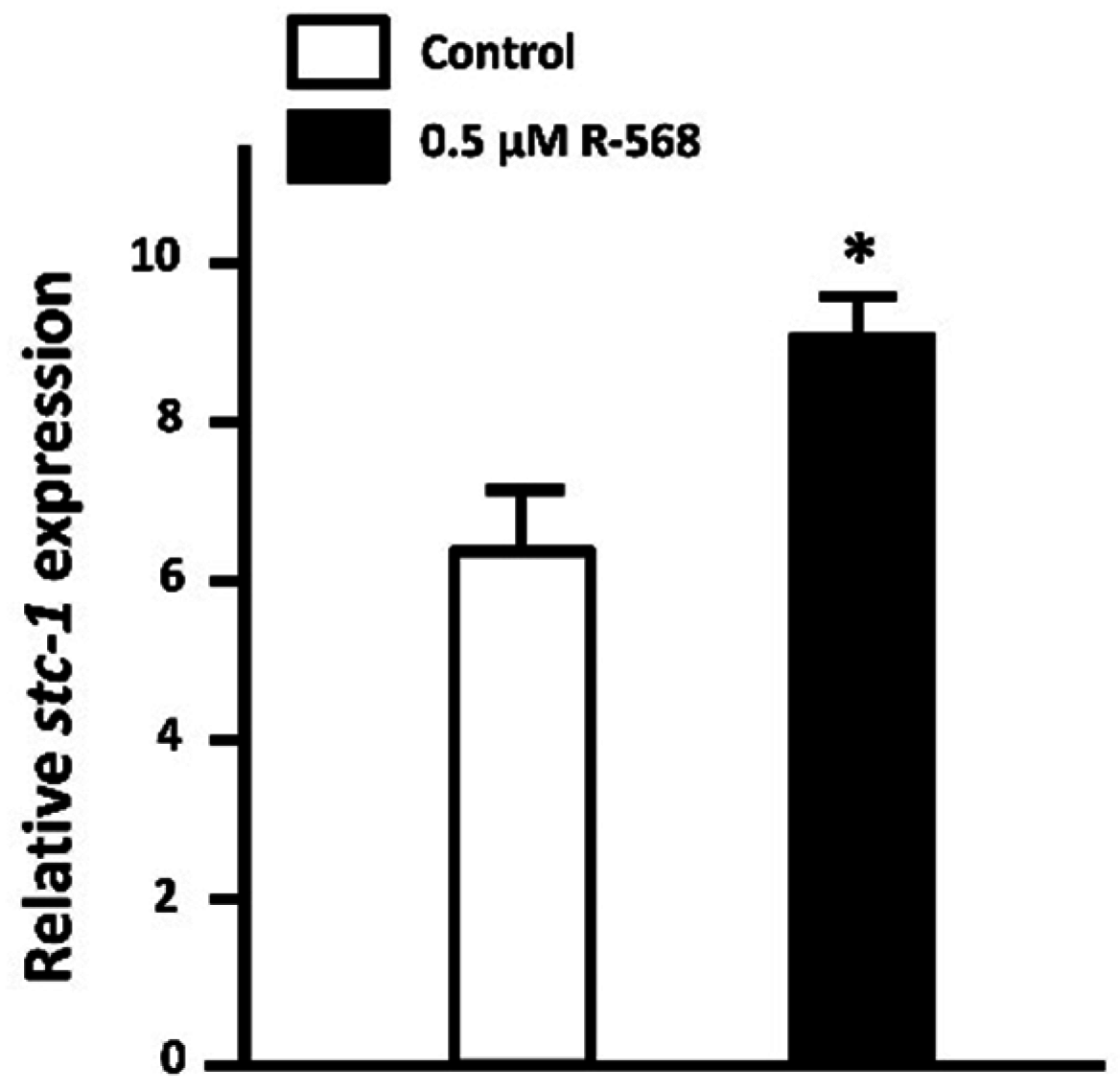
3.1.4. Stanniocalcin-1 (STC-1)
3.1.5. Calcitonin
3.1.6. Isotocin
3.1.7. Hydrogen Sulfide (H2S)
3.1.8. Insulin-Like Growth Factor 1 (IGF-1)
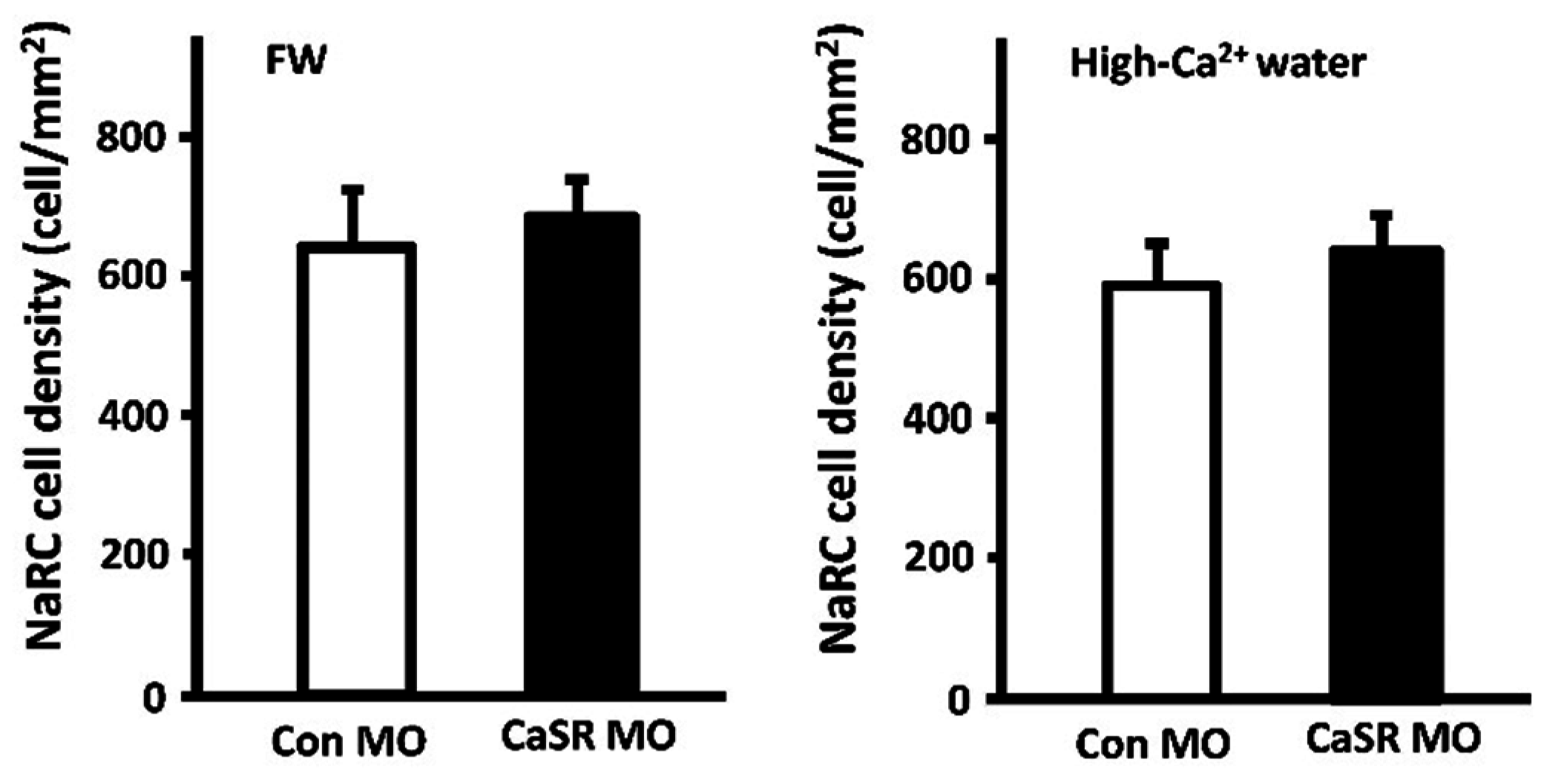
3.2. Calcium-Sensing Receptor (CaSR)
3.3. Mutual Counterbalance of Calciotropic Hormones
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoenderop, J.G.; Nilius, B.; Bindels, R.J. Calcium absorption across epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Perry, S.F. Ionic and acid-base regulation. In Fish Physiology, Zebrafish; Perry, S.F., Ekker, M., Farrell, A.P., Brauner, C.J., Eds.; Academic: San Diego, CA, USA, 2010; Volume 29, pp. 311–343. [Google Scholar]
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef] [PubMed]
- Bagnat, M.; Cheung, I.D.; Mostov, K.E.; Stainier, D.Y.R. Genetic control of single lumen formation in the zebrafish gut. Nat. Cell Biol. 2007, 9, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Kolosov, D.; Bui, P.; Chasiotis, H.; Kelly, S.P. Claudins in teleost fishes. Tissue Barriers 2013, 1, e25391. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.C.; Liao, B.K.; Huang, C.J.; Lin, L.Y.; Hwang, P.P. Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1202–R1211. [Google Scholar] [CrossRef] [PubMed]
- Shahsavarani, A.; McNeill, B.; Galvez, F.; Wood, C.M.; Goss, G.G.; Hwang, P.P.; Perry, S.F. Characterization of a branchial epithelial calcium channel (ECaC) in freshwater rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2006, 209, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.K.; Deng, A.N.; Chen, S.C.; Chou, M.Y.; Hwang, P.P. Expression and water calcium dependence of calcium transporter isoforms in zebrafish gill mitochondrion-rich cells. BMC Genom. 2007, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.K.; Chen, R.D.; Hwang, P.P. Expression regulation of Na+-K+-ATPase α1-subunit subtypes in zebrafish gill ionocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1897–R1906. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Lin, L.Y.; Tseng, Y.C.; Horng, J.L.; Hwang, P.P. A new model for fish ion regulation: Identification of ionocytes in freshwater- and seawater-acclimated medaka (Oryzias latipes). Cell Tissue Res. 2014, 357, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Kuan, W.C.; Liao, B.K.; Deng, A.N.; Tseng, D.Y.; Hwang, P.P. Environmental and cortisol-mediated control of Ca2+ uptake in tilapia (Oreochromis mossambicus). J. Comp. Physiol. B 2016, 186, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Löhr, H.; Hammerschmidt, M. Zebrafish in endocrine systems: Recent advances and implications for human disease. Annu. Rev. Physiol. 2011, 73, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Chou, M.Y. Zebrafish as an animal model to study ion homeostasis. Pflug. Arch. 2013, 465, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Guh, Y.J.; Lin, C.H.; Hwang, P.P. Osmoregulation in Zebrafish: Ion transport mechanisms and functional regulation. EXCLI J. 2015, 14, 627–659. [Google Scholar] [PubMed]
- Kwong, R.W.; Kumai, Y.; Perry, S.F. Neuroendocrine control of ionic balance in zebrafish. Gen. Comp. Endocrinol. 2016, 234, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.Y.; Chou, M.Y.; Tseng, Y.C.; Hsiao, C.D.; Huang, C.J.; Kaneko, T.; Hwang, P.P. Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo. Am. J. Physiol. 2009, 296, R549–R557. [Google Scholar]
- Lafont, A.G.; Wang, Y.F.; Chen, G.D.; Liao, B.K.; Tseng, Y.C.; Huang, C.J.; Hwang, P.P. Involvement of calcitonin and its receptor in the control of calcium-regulating genes and calcium homeostasis in zebrafish (Danio rerio). J. Bone Miner. Res. 2011, 26, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Y.; Hung, J.C.; Wu, L.C.; Hwang, S.P.; Hwang, P.P. Isotocin controls ion regulation through regulating ionocyte progenitor differentiation and proliferation. Cell. Mol. Life Sci. 2011, 68, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Su, C.H.; Hwang, P.P. Calcium-sensing receptor mediates Ca2+ homeostasis by modulating expression of PTH and stanniocalcin. Endocrinology 2014, 155, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Bai, Y.; Hebda, L.; Zhong, X.; Liu, J.; Kao, J.; Duan, C. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelialcell proliferation. Cell Death Differ. 2014, 21, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Perry, S.F. Hydrogen sulfide promotes calcium uptake in larval zebrafish. Am. J. Physiol. Cell Physiol. 2015, 309, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Y.; Lin, C.H.; Chao, P.L.; Hung, J.C.; Cruz, S.A.; Hwang, P.P. Stanniocalcin-1 controls ion regulation functions of ion-transporting epithelium other than calcium balance. Int. J. Biol. Sci. 2015, 11, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Auprix, D.; Perry, S.F. Involvement of the calcium-sensing receptor in calcium homeostasis in larval zebrafish exposed to low environmental calcium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R211–R221. [Google Scholar] [CrossRef] [PubMed]
- Flik, G.; Verbost, P.M.; Wendelaar Bonga, S.E. Calcium transport processes in fishes. Fish Physiol. 1995, 14, 317–342. [Google Scholar]
- Hwang, P.P. Ion uptake and acid secretion in zebrafish (Danio rerio). J. Exp. Biol. 2009, 212, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.D.; You, M.S.; Guh, Y.J.; Ma, M.; Jiang, Y.J.; Hwang, P.P. A positive regulatory loop between foxi3a and foxi3b is essential for specification and differentiation of zebrafish epidermal ionocytes. PLoS ONE 2007, 2, e302. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Lee, T.H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 479–97. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Lin, L.Y. Gill ion transport, acid-base regulation and nitrogen excretion. In The Physiology of Fishes, 4th ed.; Evans, D., Claiborne, J.B., Currie, S., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 205–233. [Google Scholar]
- Mobasheri, A.; Avila, J.; Cozar-Castellano, I.; Brownleader, M.D.; Trevan, M.; Francis, M.J.; Lamb, J.F.; Martin-Vasallo, P. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci. Rep. 2000, 20, 51–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Tsai, I.L.; Su, C.H.; Tseng, D.Y.; Hwang, P.P. Reverse effect of mammalian hypocalcemic cortisol in fish: Cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLoS ONE 2011, 6, e23689. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Su, C.H.; Tseng, D.Y.; Ding, F.C.; Hwang, P.P. Action of vitamin D and the receptor, VDRa, in calcium handling in zebrafish (Danio rerio). PLoS ONE 2012, 7, e45650. [Google Scholar]
- Vanoevelen, J.; Janssens, A.; Huitema, L.F.; Hammond, C.L.; Metz, J.R.; Flik, G.; Voets, T.; Schulte-Merker, S. Trpv5/6 is vital for epithelial calcium uptake and bone formation. FASEB J. 2011, 9, 3197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenderop, J.G.; van Leeuwen, J.P.; van der Eerden, B.C.; Kersten, F.F.; van der Kemp, A.W.; Mérillat, A.M.; Waarsing, J.H.; Rossier, B.C.; Vallon, V.; Hummler, E.; et al. Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Investig. 2003, 112, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, N.; Albrecht, N.; Harteneck, C.; Schultz, G.; Schaefer, M. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 2005, 118, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.D.; Peng, J.B.; Takanaga, H.; Suzuki, Y.; Crescenzi, A.; Kos, C.H.; Zhuang, L.; Freeman, M.R.; Gouveia, C.H.; Wu, J.; et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 2007, 22, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Quednau, B.D.; Nicoll, D.A.; Philipson, K.D. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997, 272, C1250–1261. [Google Scholar] [PubMed]
- Langenbacher, A.D.; Dong, Y.; Shu, X.; Choi, J.; Nicoll, D.A.; Goldhaber, J.I.; Philipson, K.D.; Chen, J.N. Mutation in sodium-calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc. Natl. Acad. Sci. USA 2005, 102, 17699–17704. [Google Scholar] [CrossRef] [PubMed]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.P.; Ronzaud, C.; Lagnaz, D.; Staub, O.; Gamba, G. Aldosterone paradox: Differential regulation of ion transport in distal nephron. Physiology 2011, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Comparison of P450s from human and fugu: 420 million years of vertebrate P450 evolution. Arch. Biochem. Biophys. 2003, 409, 18–24. [Google Scholar] [CrossRef]
- Hazon, N.; Balment, R.J. Endocrinology. In The Physiology of Fishes; Evans, D.H., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 441–463. [Google Scholar]
- Colombe, L.; Fostier, A.; Bury, N.; Pakdel, F.; Guigen, Y. A mineralocorticoid-like receptor in the rainbow trout, Oncorhynchus mykiss: Cloning and characterization of its steroid binding domain. Steroids 2000, 65, 319–328. [Google Scholar] [CrossRef]
- Bury, N.R.; Sturm, A.; le Rouzic, P.; Lethimonier, C.; Ducouret, B.; Cuiguen, Y.; Robinson-Rechavi, M.; Laudet, V.; Rafestin-Oblin, M.E.; Prunet, P. Evidence for two distinct functional glucocorticoid receptors in teleost fish. J. Mol. Endocrinol. 2003, 31, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, A.K.; Butler, P.C.; White, R.B.; DeMarco, U.; Pearce, D.; Fernald, R.D. Multiple corticosteroid receptors in a teleost fish: Distinct sequences, expressionpatterns, and transcriptional activities. Endocrinology 2003, 144, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Bury, N.R.; Dengreville, L.; Fagart, J.; Flouriot, G.; Rafestin-Oblin, M.E.; Prunet, P. 11-Deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology 2005, 146, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Nesan, D.; Vijayan, M.M.; Perry, S.F. Cortisol regulates Na+ uptake in zebrafish, Danio rerio, larvae via the glucocorticoid receptor. Mol. Cell. Endocrinol. 2012, 364, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.A.; Lin, C.H.; Chao, P.L.; Hwang, P.P. Glucocorticoid receptor, but not mineralocorticoid receptor, mediates cortisol regulation of epidermal ionocyte development and ion transport in zebrafish (Danio rerio). PLoS ONE 2013, 8, e77997. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Shih, T.H.; Liu, S.T.; Hsu, H.H.; Hwang, P.P. Cortisol regulates acid secretion of H(+)-ATPase-rich ionocytes in zebrafish (Danio rerio) embryos. Front. Physiol. 2015, 6, 328. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hu, H.J.; Hwang, P.P. Cortisol regulates sodium homeostasis by stimulating the transcription of sodium-chloride transporter (NCC) in zebrafish (Danio rerio). Mol. Cell. Endocrinol. 2016, 422, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Huybers, S.; Naber, T.H.; Bindels, R.J.; Hoenderop, J.G. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G92–G97. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, G.S.; Jung, E.M.; Choi, K.C.; Oh, G.T.; Jeung, E.B. Dexamethasone differentially regulates renal and duodenal calcium-processing genes in calbindin-D9k and -D28k knockout mice. Exp. Physiol. 2009, 94, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Shahsavarani, A.; Perry, S.F. Hormonal and environmental regulation ofepithelial calcium channel in gill of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1490–R1498. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; Wood, C.M. Kinetics of branchial calcium uptake in the rainbow trout: Effects of acclimation to various external calcium levels. J. Exp. Biol. 1985, 116, 411–433. [Google Scholar]
- Cruz, S.A.; Chao, P.L.; Hwang, P.P. Cortisol promotes differentiation of epidermal ionocytes through Foxi3 transcription factors in zebrafish (Danio rerio). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochem. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Prosser, D.E.; Jones, G. Enzymes involved in the activation and in activation of vitamin D. Trends Biochem. Sci. 2004, 29, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Howarth, D.L.; Law, S.H.; Barnes, B.; Hall, J.M.; Hinton, D.E.; Moore, L.; Maglich, J.M.; Moore, J.T.; Kullman, S.W. Paralogous vitamin D receptors in teleosts: Transition of nuclear receptor function. Endocrinology 2008, 149, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.L. The 25-hydroxyvitamin D3 1α-hydroxylase. In Vitamin D; Feldman, D., Glorieux, F.H., Pike, J.W., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 57–68. [Google Scholar]
- Murayama, A.; Takeyama, K.; Kitanaka, S.; Kodera, Y.; Kawaguchi, Y.; Hosoya, T.; Kato, S. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α hydroxylase gene by parathyroid hormone, calcitonin, and 1α, 25(OH)2D3 in intact animals. Endocrinology 1999, 140, 2224–2231. [Google Scholar] [PubMed]
- Lechner, D.; Ka’llay, E.; Cross, HS. 1α,25-dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol. Cell. Endocrinol. 2007, 263, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.E.; Guilland-Cumming, D.F.; Russell, R.G.; Henderson, I.W. Metabolism of 25-hydroxycholecalciferol in a teleost fish, the rainbow trout (Salmo gairdneri). Gen. Comp. Endocrinol. 1986, 64, 143–150. [Google Scholar] [CrossRef]
- Bailly du Bois, M.; Milet, C.; Garabedian, M.; Guillozo, H.; Martelly, E.; Lopez, E.; Balsan, S. Calcium-dependent metabolism of 25-hydroxycholecalciferol in silver eel tissues. Gen. Comp. Endocrinol. 1988, 71, 1–9. [Google Scholar] [CrossRef]
- Takeuchi, A.; Okano, T.; Kobayashi, T. The existence of 25-hydroxyvitaminD3–1α-hydroxylase in the liver of carp and bastard halibut. Life Sci. 1991, 48, 275–282. [Google Scholar] [CrossRef]
- Hogan, B.M.; Danks, J.A.; Layton, J.E.; Hall, N.E.; Heath, J.K.; Lieschke, G.J. Duplicate zebrafish pth genes are expressed along the lateral line and in the central nervous system during embryogenesis. Endocrinology 2005, 146, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.; Perry, S.F. An essential role for parathyroid hormone in gill formation and differentiation of ion-transporting cells in developing zebrafish. Endocrinology 2015, 156, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, K.; Hirose, S. Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J. Endocrinol. 2007, 193, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Gensure, R.C.; Ponugoti, B.; Gunes, Y.; Papasani, M.R.; Lanske, B.; Bastepe, M.; Rubin, D.A.; Jüppner, H. Identification and characterization of two parathyroid hormone-like molecules in zebrafish. Endocrinology 2004, 145, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Yeung, B.H.; Law, A.Y.; Wong, C.K. Evolution and roles of stanniocalcin. Mol. Cell. Endocrinol. 2012, 349, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.F.; Vozzolo, B.L.; Jaworski, E.; Haddad, M.; Kline, R.L.; Olsen, H.S.; Rosen, C.A.; Davidson, M.B.; Renfro, J.L. Human stanniocalcin inhibits renal phosphate excretion in the rat. J. Bone Miner. Res. 1997, 12, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, R.G.; Lafeber, F.P.; Flik, G.; Wendelaar Bonga, S.E. Ionic and total calcium levels in the blood of the European eel (Anguilla anguilla): Effects of stanniectomy and hypocalcin replacement therapy. J. Exp. Biol. 1989, 141, 177–186. [Google Scholar] [PubMed]
- Lafeber, F.P.; Flik, G.; Wendelaar Bonga, S.E.; Perry, S.F. Hypocalcin from Stannius corpuscles inhibits gill calcium uptake in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988, 254, R891–R896. [Google Scholar]
- Khanal, R.C.; Nemere, I. Endocrine regulation of calcium transport in epithelia. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Claveau, D.; Hilal, G.; Leclerc, M.; Brunette, M.G. Effect of calcitonin on calcium transport by the luminal and basolateral membranes of the rabbit nephron. Kidney Int. 1997, 51, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Carney, S.L. Calcitonin and human renal calcium and electrolyte transport. Miner. Electrol. Metab. 1997, 23, 43–47. [Google Scholar]
- Chakrabarti, P.; Mukherjee, D. Studies on the hypocalcemic actions of salmon calcitonin and ultimobranchial gland extracts in the freshwater teleost Cyprinus carpio. Gen. Comp. Endocrinol. 1993, 90, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, Y.; Suzuki, N.; Oguro, C.; Takei, Y.; Takahashi, A.; Watanabe, T.X.; Nakajima, K.; Sakakibara, S. Calcitonin of the stingray: Comparison of the hypocalcemic activity with other calcitonins. Gen. Comp. Endocrinol. 1992, 86, 269–274. [Google Scholar] [CrossRef]
- Sasayama, Y.; Ukawa, K.; Kai-Ya, H.; Oguro, C.; Takei, Y.; Watanabe, T.X.; Nakajima, K.; Sakakibara, S. Goldfish calcitonin: Purification, characterization, and hypocalcemic potency. Gen. Comp. Endocrinol. 1993, 89, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.L. Nonapeptides and the evolutionary patterning of sociality. Prog. Brain Res. 2008, 170, 3–15. [Google Scholar] [PubMed]
- Venkatesh, B.; Brenner, S. Structure and organization of the isotocin and vasotocin genes from teleosts. Adv. Exp. Med. Biol. 1995, 395, 629–638. [Google Scholar] [PubMed]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [PubMed]
- Eaton, J.L.; Glasgow, E. Zebrafish orthopedia (otp) is required for isotocin cell development. Dev. Genes Evol. 2007, 217, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.L.; Glasgow, E. Expression of isotocin-neurophysin mRNA in developing zebrafish. Gene Expr. Patterns 2003, 3, 105–108. [Google Scholar] [CrossRef]
- Motohashi, E.; Hamabata, T.; Ando, H. Structure of neurohypophysialhormone genes and changes in the levels of expression during spawning season in grass puffer (Takifugu niphobles). Gen. Comp. Endocrinol. 2008, 155, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, E.; Hasegawa, S.; Mishiro, K.; Ando, H. Osmoregulatory responses of expression of vasotocin, isotocin, prolactin and growth hormone genes following hypoosmotic challenge in a stenohaline marine teleost, tiger puffer (Takifugu rubripes). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Hu, Y.S.; Hu, L.F.; Lu, Q.; Dawe, G.S.; Moore, P.K.; Wong, P.T.; Bian, J.S. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 2006, 54, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Markova, J.; Hudecova, S.; Soltysova, A.; Sirova, M.; Csaderova, L.; Lencesova, L.; Ondrias, K.; Krizanova, O. Sodium/calcium exchanger is upregulated by sulfide signaling, forms complex with the β1 and β3 but not β2 adrenergic receptors, and induces apoptosis. Pflug. Arch. 2014, 466, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Bertoni, G.; Pla, A.F.; Dragoni, S.; Pupo, E.; Merlino, A.; Mancardi, D.; Munaron, L.; Tanzi, F. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Curr. Pharm. Biotechnol. 2011, 12, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.K.; Wu, T.; Huang, C.L. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am. J. Physiol. Ren. Physiol. 2008, 294, F1212–F1221. [Google Scholar] [CrossRef] [PubMed]
- De Groot, T.; Lee, K.; Langeslag, M.; Xi, Q.; Jalink, K.; Bindels, R.J.; Hoenderop, J.G. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J. Am. Soc. Nephrol. 2009, 20, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Menaa, C.; Vrtovsnik, F.; Friedlander, G.; Corvol, M.; Garabédian, M. Insulin-like growth factor I, a unique calcium dependent stimulator of 1,25-dihydroxyvitamin D3 production: Studies in cultured mouse kidney cells. J. Biol. Chem. 1995, 270, 25461–25467. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Landowski, C.P.; Hediger, M.A. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu. Rev. Physiol. 2008, 70, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.; Zhou, J.; Bondy, C.A. Renal growth hormone receptor gene expression: Relationship to renal insulin-like growth factor system. Endocrinology 1992, 131, 3061–3066. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.; Zhou, J.; Bondy, C.A. Anatomical relationships in the patterns of insulin-like growth factor (IGF)-I, IGF binding protein-1, and IGF-I receptor gene expression in the rat kidney. Endocrinology 1992, 130, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.; Bondy, C.A. Insulin-like growth factor system gene expression in the human kidney. J. Clin. Endocrinol. Metab. 1992, 75, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.; Michels, K.; Bondy, C.A. Partition of insulin-like growth factor(IGF)-binding sites between the IGF-I and IGF-II receptors and IGF-binding proteins in the human kidney. J. Clin. Endocrinol. Metab. 1994, 78, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, R.; Brody, M.; Lu, L.H.; Chan, C.; Shaheen, A.M.; Gillett, N. Expression of the genes encoding the rat renal insulin like growth factor-I system. J. Am. Soc. Nephrol. 1995, 6, 1511–1518. [Google Scholar] [PubMed]
- Reinecke, M.; Schmid, A.; Ermatinger, R.; Loffing-Cueni, D. Insulin-like growth factor I in the teleost Oreochromis mossambicus, the tilapia: Gene sequence, tissue expression, and cellular localization. Endocrinology 1997, 138, 3613–3619. [Google Scholar] [PubMed]
- Sakamoto, T.; Hirano, T.; Madsen, S.S.; Nishioka, R.S.; Bern, H.A. Insulin-like growth factor I gene expression during parr-smolt transformation of coho salmon. Zool. Sci. 1995, 12, 249–252. [Google Scholar] [CrossRef]
- Seidelin, M.; Madsen, S.S. Endocrine control of Na+, K+-ATPase and chloride cell development in brown trout (Salmo trutta): Interaction of insulin-like growth factor-I with prolactin and growth hormone. J. Endocrinol. 1999, 162, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pozios, K.C.; Ding, J.; Degger, B.; Upton, Z.; Duan, C. IGFs stimulate zebrafish cell proliferation by activating MAP kinase and PI3-kinase-signaling pathways. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1230–R1239. [Google Scholar] [PubMed]
- Loretz, C.A.; Pollina, C.; Hyodo, S.; Takei, Y.; Chang, W.; Shoback, D. cDNA cloning and functional expression of a Ca2+-sensing receptor with truncated C-terminal tail from the Mozambique tilapia (Oreochromis mossambicus). J. Biol. Chem. 2004, 279, 53288–53297. [Google Scholar] [CrossRef] [PubMed]
- Loretz, C.A. Extracellular calcium-sensing receptors in fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Radman, D.P.; McCudden, C.; James, K.; Nemeth, E.M.; Wagner, G.F. Evidence for calcium-sensing receptor mediated stanniocalcin secretion in fish. Mol. Cell. Endocrinol. 2002, 186, 111–119. [Google Scholar] [CrossRef]
- Loretz, C.A.; Pollina, C.; Hyodo, S.; Takei, Y. Extracellular calcium sensing receptor distribution in osmoregulatory and endocrine tissues of the tilapia. Gen. Comp. Endocrinol. 2009, 161, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Herberger, A.L.; Loretz, C.A. Morpholino oligonucleotide knockdown of the extracellular calcium-sensing receptor impairs early skeletal development in zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Kwong, R.W.M.; Perry, S.F. A role for transcription factor glial cell missing 2 in Ca2+ homeostasis in zebrafish, Danio rerio. Pflug. Arch. 2014, 467, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, N.; Ye, C.P.; Yamaguchi, T.; Kerner, R.; Vassilev, P.M.; Brown, E.M. Extracellular calcium-sensing receptor induces cellular proliferation and activation of a nonselective cation channel in U373 human astrocytoma cells. Brain Res. 1999, 851, 116–124. [Google Scholar] [CrossRef]
- Chen, R.A.; Goodman, W.G. Role of the calcium-sensing receptor in parathyroid gland physiology. Am. J. Physiol. Ren. Physiol. 2004, 286, F1005–F1011. [Google Scholar] [CrossRef] [PubMed]
- Rey, O.; Young, S.H.; Jacamo, R.; Moyer, M.P.; Rozengurt, E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J. Cell. Physiol. 2010, 225, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Topala, C.N.; Schoeber, J.P.; Searchfield, L.E.; Riccardi, D.; Hoenderop, J.G.; Bindels, R.J. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium 2009, 45, 331–339. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Hwang, P.-P. The Control of Calcium Metabolism in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2016, 17, 1783. https://doi.org/10.3390/ijms17111783
Lin C-H, Hwang P-P. The Control of Calcium Metabolism in Zebrafish (Danio rerio). International Journal of Molecular Sciences. 2016; 17(11):1783. https://doi.org/10.3390/ijms17111783
Chicago/Turabian StyleLin, Chia-Hao, and Pung-Pung Hwang. 2016. "The Control of Calcium Metabolism in Zebrafish (Danio rerio)" International Journal of Molecular Sciences 17, no. 11: 1783. https://doi.org/10.3390/ijms17111783




