Next-Generation Sequencing of Two Mitochondrial Genomes from Family Pompilidae (Hymenoptera: Vespoidea) Reveal Novel Patterns of Gene Arrangement
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Features of Mitochondrial Genomes
2.2. Nucleotide Composition
2.3. Protein-Coding Genes
2.4. Transfer RNA Gene
2.5. Ribosomal RNA Genes
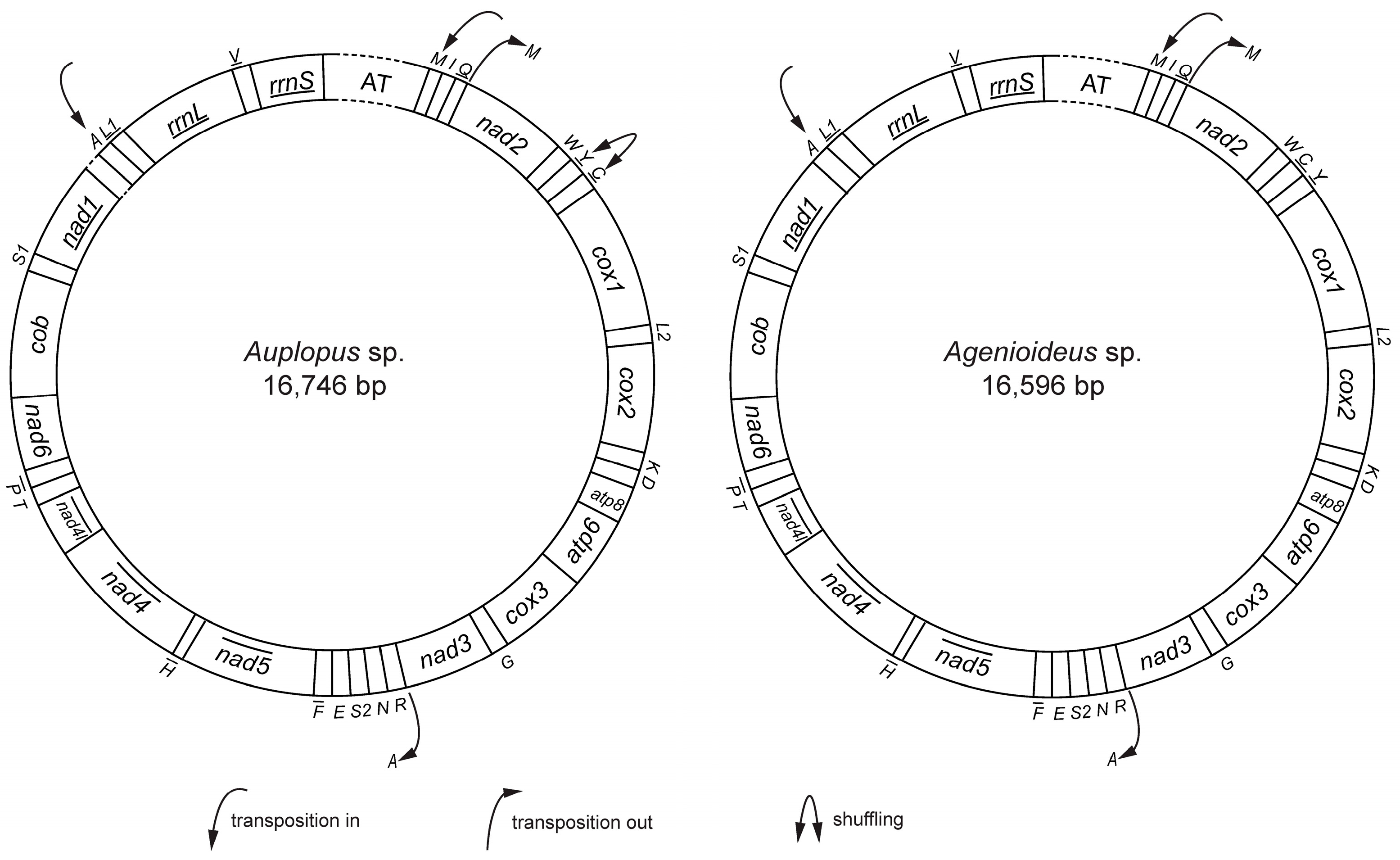
2.6. Gene Rearrangement
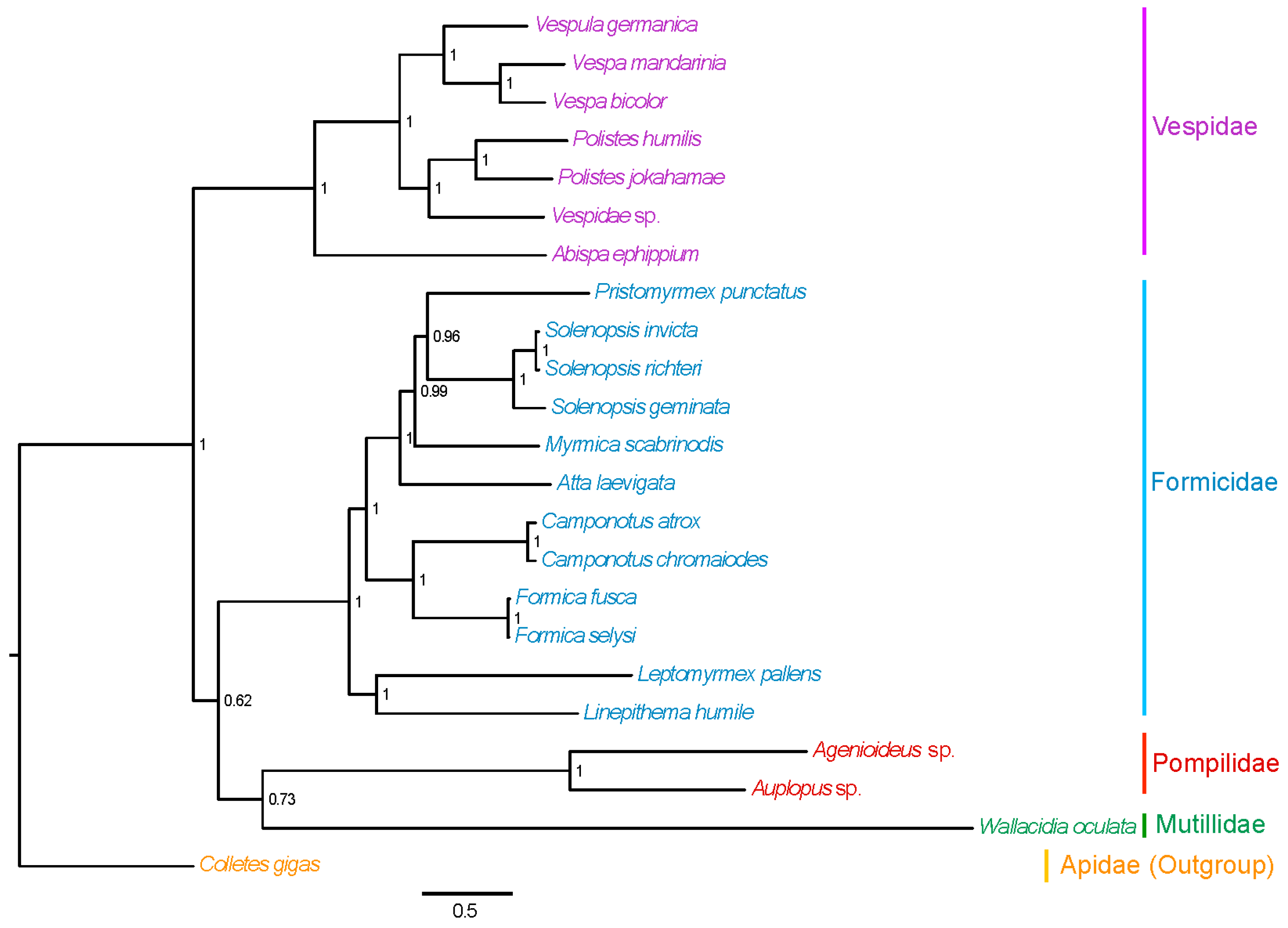
2.7. Phylogenetic Relationships
3. Materials and Methods
3.1. Sample Collection and DNA Extraction
3.2. Mitochondrial Genome Sequencing and Assembly
3.3. Mitochondrial Genome Annotation
3.4. Comparative Analysis of the Mitochondrial Genomes
3.5. Phylognetic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.X.; Hewitt, G.M. Insect mitochondrial control region: A review of its structure, evolution and usefulness in evolutionary studies. Biochem. Syst. Ecol. 1997, 25, 99–120. [Google Scholar] [CrossRef]
- Barr, C.M.; Neiman, M.; Taylor, D.R. Inheritance and recombination of mitochondrial genomes in plants, fungi and animals. New Phytol. 2005, 168, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.M.; Neiman, M.; Taylor, D.R. Inheritance and recombination of mitochondrial genomes in plants, fungi and animals. New Phytol. 2005, 168, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Shi, M.; He, J.H.; Sharkey, M.; Chen, X.X. The complete mitochondrial genome of Diadegma semiclausum (Hymenoptera: Ichneumonidae) indicates extensive independent evolutionary events. Genome 2009, 52, 308–319. [Google Scholar] [PubMed]
- Curole, J.P.; Kocher, T.D. Mitogenomics: Digging deeper with complete mitochondrial genomes. Trends Ecol. Evol. 1999, 14, 394–398. [Google Scholar] [CrossRef]
- Moritz, C.; And, T.E.D.; Brown, W.M. Evolution of animal mitochondrial DNA: Relevance for population biology and systematics. Annu. Rev. Ecol. Syst. 1987, 18, 269–292. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Raychoudhury, R.; Lavrov, D.V.; Werren, J.H. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera: Pteromalidae). Mol. Biol. Evol. 2008, 25, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Boore, J.L.; Brown, W.M. Big trees from little genomes: Mitochondrial gene order as a phylogenetic tool. Curr. Opin. Genet. Dev. 1998, 8, 668–674. [Google Scholar] [CrossRef]
- Dowton, M.; Castro, L.R.; Austin, A.D. Mitochondrial gene rearrangements as phylogenetic characters in the invertebrates: The examination of genome ‘morphology’. Invertebr. Syst. 2002, 16, 345–356. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.Y.; Liu, S.L.; Yang, Q.; Su, X.; Zhou, L.L.; Tang, M.; Fu, R.B.; Li, J.G.; Huang, Q.F. Ultra-deep sequencing enables high-fidelity recovery of biodiversity for bulk arthropod samples without PCR amplification. Gigascience 2013, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Hwang, U.W.; Chan, J.P.; Tai, S.Y.; Kim, W. One-step PCR amplification of complete arthropod mitochondrial genomes. Mol. Phylogenet. Evol. 2001, 19, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.M.; Miya, M.U.; Nishida, M. Use of a PCR-based approach for sequencing whole mitochondrial genomes of insects: Two examples (cockroach and dragonfly) based on the method developed for decapod crustaceans. Insect Mol. Biol. 2004, 13, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hardman, C.J.; Ji, Y.; Meng, G.; Liu, S.; Tan, M.; Yang, S.; Moss, E.D.; Wang, J.; Yang, C.; et al. High-throughput monitoring of wild bee diversity and abundance via mitogenomics. Methods Ecol. Evol. 2015, 6, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Gillett, C.P.; Crampton-Platt, A.; Timmermans, M.J.; Jordal, B.H.; Emerson, B.C.; Vogler, A.P. Bulk de novo mitogenome assembly from pooled total DNA elucidates the phylogeny of weevils (Coleoptera: Curculionoidea). Mol. Biol. Evol. 2014, 31, 2223–2237. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M.; Austin, A.D. Evolutionary dynamics of a mitochondrial rearrangement “hot spot” in the Hymenoptera. Mol. Biol. Evol. 1999, 16, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.R.; Dowton, M. Molecular analyses of the Apocrita (Insecta: Hymenoptera) suggest that the Chalcidoidea and sister to the diaprioid complex. Invertebr. Syst. 2006, 20, 603–614. [Google Scholar] [CrossRef]
- Cameron, S.L.; Whiting, M.F. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta, (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene 2008, 408, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, N.C.; Song, H.; Cameron, S.L.; Whiting, M.F. A comparative analysis of mitochondrial genomes in Coleoptera (Arthropoda: Insecta) and genome descriptions of six new beetles. Mol. Biol. Evol. 2008, 25, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M.; Cameron, S.L.; Dowavic, J.I.; Austin, A.D.; Whiting, M.F. Characterization of 67 mitochondrial tRNA gene rearrangements in the Hymenoptera suggests that mitochondrial tRNA gene position is selectively neutral. Mol. Biol. Evol. 2009, 26, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Li, Q.; van Achterberg, K.; Chen, X.X. Two mitochondrial genomes from the families Bethylidae and Mutillidae: Independent rearrangement of protein-coding genes and higher-level phylogeny of the Hymenoptera. Mol. Phylogenet. Evol. 2014, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Shi, M.; Sharkey, M.J.; van Achterberg, C.; Chen, X.X. Comparative mitogenomics of Braconidae (Insecta: Hymenoptera) and the phylogenetic utility of mitochondrial genomes with special reference to Holometabolous insects. BMC Genom. 2010, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Austin, A.D.; Johnson, N.F.; Dowton, M. Coexistence of minicircular and a highly rearranged mtDNA molecule suggests that recombination shapes mitochondrial genome organization. Mol. Biol. Evol. 2014, 31, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Gibson, T.; Dowton, M. Evolutionary dynamics of the mitochondrial genome in the Evaniomorpha (Hymenoptera)-a group with an intermediate rate of gene rearrangement. Genome Biol. Evol. 2014, 6, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M.; Cameron, S.L.; Austin, A.D.; Whiting, M.F. Phylogenetic approaches for the analysis of mitochondrial genome sequence data in the Hymenoptera – A lineage with both rapidly and slowly evolving mitochondrial genomes. Mol. Phylogenet. Evol. 2009, 52, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Song, S.N.; Tang, P.; Wei, S.J.; Chen, X.X. Comparative and phylogenetic analysis of the mitochondrial genomes in basal hymenopterans. Sci. Rep. 2016, 6, 20972. [Google Scholar] [CrossRef] [PubMed]
- Brothers, D.J.; Finnamore, A.T. Superfamily Vespoidea. In Hymenoptera of the World: An Identification Guide to Families; Goulet, H., Huber, J.T., Eds.; Canada Communication Group: Ottawa, ON, Canada, 1993; pp. 161–278. [Google Scholar]
- Pitts, J.P.; Wasbauer, M.S.; Dohlen, C.D.V. Preliminary morphological analysis of relationships between the spider wasp subfamilies (Hymenoptera: Pompilidae): Revisiting an old problem. Zool. Scr. 2006, 35, 63–84. [Google Scholar] [CrossRef]
- Costa, F.G.; Pérez-Miles, F.; Mignone, A. Pompilid wasp interactions with burrowing tarantulas: Pepsis cupripennis versus Eupalaestrus weijenberghi and Acanthoscurria suina (Araneae, Theraphosidae). Stud. Neotrop. Fauna Environ. 2004, 39, 37–43. [Google Scholar] [CrossRef]
- Punzo, F. The effect of encounter experience on hunting behavior in the spider wasp, Pepsis cerberus Lucas (Hymenoptera: Pompilidae). Texas J. Sci. 2005, 57, 165–174. [Google Scholar]
- Punzo, F. The biology of the spider wasp Pepsis thisbe (Hymenoptera: Pompilidae) from trans Pecos, Texas. I. adult morphometrics, larval development and the ontogeny of larval feeding patterns. Psyche J. Entomol. 1994, 101, 229–241. [Google Scholar] [CrossRef]
- Goulet, H.; Huber, J.T. Hymenoptera of the World: An Identification Guide to Families; Canada Communication Group: Ottawa, ON, Canada, 1993. [Google Scholar]
- Zhou, Y.; Hu, Y.L.; Xu, Z.F.; Wei, S.J. The mitochondrial genome of the German wasp Vespula germanica (Fabricius, 1793)(Hymenoptera: Vespoidea: Vespidae). Mitochondr. DNA 2016, 27, 2917–2918. [Google Scholar] [CrossRef] [PubMed]
- Song, S.N.; Chen, P.Y.; Wei, S.J.; Chen, X.X. The mitochondrial genome of Polistes jokahamae and a phylogenetic analysis of the Vespoidea (Insecta: Hymenoptera). Mitochondr. DNA 2016, 27, 2783–2784. [Google Scholar]
- Chen, P.Y.; Wei, S.J.; Liu, J.X. The mitochondrial genome of the Vespa mandarinia Smith (Hymenoptera: Vespidae: Vespinae) and a phylogenetic analysis of the Vespoidea. Mitochondr. DNA 2015. [Google Scholar] [CrossRef]
- Szafranski, P. The mitochondrial trn-cox1 locus: Rapid evolution in Pompilidae and evidence of bias in cox1 initiation and termination codon usage. Mitochondr. DNA 2009, 20, 15–25. [Google Scholar] [CrossRef]
- Wei, S.J.; Tang, P.; Zheng, L.H.; Shi, M.; Chen, X.X. The complete mitochondrial genome of Evania appendigaster (Hymenoptera: Evaniidae) has low A + T content and a long intergenic spacer between atp8 and atp6. Mol. Biol. Rep. 2010, 37, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Wu, Q.L.; Liu, W. Sequencing and characterization of the Monocellicampa pruni (Hymenoptera: Tenthredinidae) mitochondrial genome. Mitochondr. DNA 2015, 26, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L.; Dowton, M.; Castro, L.R.; Ruberu, K.; Whiting, M.F.; Austin, A.D.; Diement, K.; Stevens, J. Mitochondrial genome organization and phylogeny of two vespid wasps. Genome 2008, 51, 800–808. [Google Scholar] [PubMed]
- Crampton-Platt, A.; Timmermans, M.J.; Gimmel, M.L.; Kutty, S.N.; Cockerill, T.D.; Vun Khen, C.; Vogler, A.P. Soup to Tree: The phylogeny of beetles inferred by mitochondrial metagenomics of a bornean rainforest sample. Mol. Biol. Evol. 2015, 32, 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.; Léger, N.; Deutsch, J. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of metazoa, and consequences for phylogenetic inferences. Syst. Biol. 2015, 54, 277–298. [Google Scholar] [CrossRef]
- Wei, S.J.; Shi, M.; Chen, X.X.; Sharkey, M.J.; van Achterberg, C.; Ye, G.Y.; He, J.H. New views on strand asymmetry in insect mitochondrial genomes. PLoS ONE 2010, 5, e12708. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar] [PubMed]
- Crozier, R.H.; Crozier, Y.C. The mitochondrial genome of the honeybee Apis mellifera: Complete sequence and genome organization. Genetics 1993, 133, 97–117. [Google Scholar] [PubMed]
- Masta, S.E.; Boore, J.L. The complete mitochondrial genome sequence of the spider Habronattus oregonensis reveals rearranged and extremely truncated tRNAs. Mol. Biol. Evol. 2004, 21, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Valerio, A.; Austin, A.D.; Dowton, M.; Johnson, N.F. The first mitochondrial genome for the wasp superfamily Platygastroidea: The egg parasitoid Trissolcus basalis. Genome 2012, 55, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.R.; Ruberu, K.; Dowton, M. Mitochondrial genomes of Vanhornia eucnemidarum (Apocrita: Vanhorniidae) and Primeuchroeus spp. (Aculeata: Chrysididae): Evidence of rearranged mitochondrial genomes within the Apocrita (Insecta: Hymenoptera). Genome 2006, 49, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Hamm, D.M.; Collins, F.H. The mitochondrial genome of the mosquito Anopheles gambiae: DNA sequence, genome organization, and comparisons with mitochondrial sequences of other insects. Insect Mol. Biol. 1993, 2, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Barker, S.C. The highly rearranged mitochondrial genome of the plague thrips, Thrips imaginis (Insecta: Thysanoptera): Convergence of two novel gene boundaries and an extraordinary arrangement of rRNA genes. Mol. Biol. Evol. 2003, 20, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Li, Q.; Gu, Y.; Shi, B.C.; van Achterberg, C.; Wei, S.J.; Chen, X.X. The complete mitochondrial genome of Taeniogonalos taihorina (Bischoff) (Hymenoptera: Trigonalyidae) reveals a novel gene rearrangement pattern in the Hymenoptera. Gene 2014, 543, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.J.; Johnston, J.S.; Cannone, J.J.; Gutell, R.R. Characteristics of the nuclear (18S, 5.8S, 28S and 5S) and mitochondrial (12S and 16S) rRNA genes of Apis mellifera (Insecta: Hymenoptera): Structure, organization, and retrotransposable elements. Insect Mol. Biol. 2006, 15, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.R.; Simon, C.; Flook, P.K.; Misof, B. Secondary structure and conserved motifs of the frequently sequenced domains IV and V of the insect mitochondrial large subunit rRNA gene. Insect Mol. Biol. 2000, 9, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Misof, B.; Fleck, G. Comparative analysis of mt LSU rRNA secondary structures of Odonates: Structural variability and phylogenetic signal. Insect Mol. Biol. 2003, 12, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Schnare, M.N.; Damberger, S.H.; Gray, M.W.; Gutell, R.R. Comprehensive comparison of structural characteristics in eukaryotic cytoplasmic large subunit (23 S-like) ribosomal RNA. J. Mol. Biol. 1996, 256, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, E.M.; Dogan, O.; Budak, M.; Basibuyuk, H.H. Two nearly complete mitogenomes of wheat stem borers, Cephus pygmeus (L.) and Cephus sareptanus Dovnar-Zapolskij (Hymenoptera: Cephidae): An unusual elongation of rrnS gene. Gene 2015, 558, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Boore, J.L.; Lavrov, D.V.; Brown, W.M. Gene translocation links insects and crustaceans. Nature 1998, 392, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Dowton, M. The position of the Hymenoptera within the Holometabola as inferred from the mitochondrial genome of Perga condei (Hymenoptera: Symphyta: Pergidae). Mol. Phylogenet. Evol. 2005, 34, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Flook, P.K.; Rowell, C.H.F.; Gellissen, G. The sequence, organization, and evolution of the Locusta migratoria mitochondrial genome. J. Mol. Evol. 1995, 41, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M.; Castro, L.R.; Campbell, S.L.; Bargon, S.D.; Austin, A.D. Frequent mitochondrial gene rearrangements at the Hymenopteran nad3-nad5 junction. J. Mol. Evol. 2003, 56, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Dowton, M.; Campbell, N.J. Intramitochondrial recombination - is it why some mitochondrial genes sleep around? Trends Ecol. Evol. 2001, 16, 269–271. [Google Scholar] [CrossRef]
- Lunt, D.H.; Hyman, B.C. Animal mitochondrial DNA recombination. Nature 1997, 387, 247. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Mitani, H.; Barker, S.C.; Takahashi, M.; Fukunaga, M. Novel mitochondrial gene content and gene arrangement indicate illegitimate inter-mtDNA recombination in the chigger mite, Leptotrombidium pallidum. J. Mol. Evol. 2005, 60, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, D.V.; Boore, J.L.; Brown, W.M. Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: Duplication and nonrandom loss. Mol. Biol. Evol. 2002, 19, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hong, E.J.; Kim, I. Complete mitochondrial genome of Camponotus atrox (Hymenoptera: Formicidae): A new tRNA arrangement in Hymenoptera. Genome 2016, 59, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Niu, F.F.; Tan, J.L. The mitochondrial genome of the Vespa bicolor Fabricius (Hymenoptera: Vespidae: Vespinae). Mitochondr. DNA 2016, 27, 875–876. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, D.D.; Ahrens, M.E.; Ross, K.G. Molecular phylogeny of fire ants of the Solenopsis saevissima species-group based on mtDNA sequences. Mol. Phylogenet. Evol. 2006, 38, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Bi, G.; Yang, J.; Zhang, Z.; Liu, G.; Du, Q.; Shang, E. Complete mitochondrial genome of the argentine ant, Linepithema humile (Hymenoptera: Formicidae). Mitochondr. DNA 2015. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, E.M.; Dohlen, C.D.V.; Pitts, J.P. Molecular phylogenetics of Vespoidea indicate paraphyly of the superfamily and novel relationships of its component families and subfamilies. Zool. Scr. 2008, 37, 539–560. [Google Scholar] [CrossRef]
- Chen, L.; Li, T. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar]
- Bernt, M.; Donath, A.; Juhling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Putz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Cannone, J.; Subramanian, S.; Schnare, M.; Collett, J.; D’Souza, L.; Du, Y.; Feng, B.; Lin, N.; Madabusi, L.; Müller, K.; et al. The Comparative RNA Web (CRW) Site: An online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinform. 2002, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Mark, P.V.D.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. Partitionfinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Su, T.; Qu, L.; Wu, Y.; Gu, P.; He, B.; Xu, X.; Zhu, C. The complete mitochondrial genome of the Colletes gigas (Hymenoptera: Colletidae: Colletinae). Mitochondr. DNA 2014, 8. [Google Scholar] [CrossRef]
- Babbucci, M.; Basso, A.; Scupola, A.; Patarnello, T.; Negrisolo, E. Is it an ant or a butterfly? Convergent evolution in the mitochondrial gene order of Hymenoptera and Lepidoptera. Genome Biol. Evol. 2014, 6, 3326–3343. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, E.; Kobayashi, K.; Yagi, N.; Tsuji, K. Complete mitochondrial genomes of normal and cheater morphs in the parthenogenetic ant Pristomyrmex punctatus (Hymenoptera: Formicidae). Myrmecol. News 2011, 15, 85–90. [Google Scholar]
- Berman, M.; Austin, C.M.; Miller, A.D. Characterisation of the complete mitochondrial genome and 13 microsatellite loci through next-generation sequencing for the New Caledonian spider-ant Leptomyrmex pallens. Mol. Biol. Rep. 2014, 41, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Rodovalho Cde, M.; Lyra, M.L.; Ferro, M.; Bacci, M., Jr. The mitochondrial genome of the leaf-cutter ant Atta laevigata: A mitogenome with a large number of intergenic spacers. PLoS ONE 2014, 9, e97117. [Google Scholar]
- Yang, S.; Li, X.; Cai, L.G.; Qian, Z.Q. Characterization of the complete mitochondrial genome of Formica selysi (Insecta: Hymenoptera: Formicidae: Formicinae). Mitochondr. DNA 2016, 27, 3378–3380. [Google Scholar]
- Williams, L.E.; Wernegreen, J.J. Sequence context of indel mutations and their effect on protein evolution in a bacterial endosymbiont. Genome Biol. Evol. 2012, 5, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tan, M.H.; Meng, G.L.; Yang, S.Z.; Su, X.; Liu, S.L.; Song, W.H.; Li, Y.Y.; Wu, Q.; Zhang, A.B.; et al. Multiplex sequencing of pooled mitochondrial genomes-a crucial step toward biodiversity analysis using mito-metagenomics. Nucleic Acids Res. 2014, 42, e166. [Google Scholar] [CrossRef] [PubMed]
| Species | Whole Genome | All Protein-Coding Genes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T% | C% | A% | G% | (A + T)% | AT-skew | GC-skew | T% | C% | A% | G% | (A + T)% | AT-skew | GC-skew | |
| Agenioideua sp. | 42.99 | 9.32 | 35.65 | 12.04 | 78.64 | −0.0932 | 0.1272 | 44.95 | 10.66 | 32.76 | 11.62 | 77.72 | −0.1569 | 0.0431 |
| Auplopus sp. | 44.08 | 8.41 | 39.04 | 8.47 | 83.12 | −0.0607 | 0.0036 | 47.47 | 8.39 | 34.85 | 9.29 | 82.33 | −0.1532 | 0.0507 |
| Wallacidia oculata | 33.58 | 14.56 | 43.78 | 8.08 | 77.36 | 0.1318 | −0.2864 | 41.17 | 12.40 | 33.84 | 12.59 | 75.01 | −0.0978 | 0.0075 |
| Solenopsis geminata | 37.94 | 16.95 | 38.60 | 6.51 | 76.54 | 0.0086 | −0.4452 | 42.26 | 14.86 | 31.32 | 11.56 | 73.58 | −0.1487 | −0.1250 |
| Solenopsis invicta | 38.65 | 16.52 | 38.53 | 6.31 | 77.18 | −0.0015 | −0.4472 | 42.93 | 14.29 | 31.31 | 11.47 | 74.24 | −0.1564 | −0.1096 |
| Solenopsis richteri | 38.55 | 16.59 | 38.39 | 6.47 | 76.95 | −0.0021 | −0.4391 | 42.84 | 14.37 | 31.23 | 11.57 | 74.07 | −0.1567 | −0.1079 |
| Myrmica scabrinodis | 42.66 | 11.27 | 39.52 | 6.54 | 82.19 | −0.0382 | −0.2659 | 46.17 | 10.18 | 34.01 | 9.63 | 80.19 | −0.1516 | −0.0279 |
| Pristomyrmex punctatus | 40.65 | 14.28 | 38.98 | 6.09 | 79.64 | −0.0210 | −0.4024 | 43.95 | 11.90 | 33.99 | 10.16 | 77.94 | −0.1278 | −0.0790 |
| Leptomyrmex pallens | 32.60 | 22.01 | 36.94 | 8.45 | 69.54 | 0.0624 | −0.4452 | 38.69 | 17.49 | 28.71 | 15.11 | 67.41 | −0.1480 | −0.0729 |
| Atta laevigata | 43.60 | 14.18 | 37.25 | 4.98 | 80.84 | −0.0785 | −0.4799 | 44.70 | 12.10 | 32.98 | 10.22 | 77.68 | −0.1508 | −0.0842 |
| Formica fusca | 43.07 | 10.96 | 40.35 | 5.63 | 83.42 | −0.0326 | −0.3215 | 46.77 | 9.95 | 34.37 | 8.91 | 81.14 | −0.1528 | −0.0551 |
| Formica selysi vouche | 42.94 | 11.07 | 40.33 | 5.66 | 83.27 | −0.0313 | −0.3236 | 46.81 | 10.06 | 34.20 | 8.92 | 81.01 | −0.1556 | −0.0599 |
| Camponotus chromaiodes | 38.77 | 14.93 | 39.37 | 6.93 | 78.14 | 0.0077 | −0.3661 | 44.53 | 12.39 | 32.58 | 10.49 | 77.12 | −0.1550 | −0.0832 |
| Camponotus atrox | 39.87 | 14.75 | 38.97 | 6.42 | 78.83 | −0.0114 | −0.3933 | 44.06 | 12.73 | 32.34 | 10.86 | 76.41 | −0.1534 | −0.0790 |
| Linepithema humile | 41.27 | 6.23 | 39.05 | 13.45 | 80.32 | −0.0277 | 0.3668 | 45.02 | 11.36 | 33.17 | 10.46 | 78.18 | −0.1516 | −0.0411 |
| Polistes humilis | 41.65 | 9.95 | 43.09 | 5.32 | 84.73 | 0.0170 | −0.3031 | 46.61 | 8.51 | 36.77 | 8.11 | 83.38 | −0.1180 | −0.0244 |
| Polistes jokahamae | 41.45 | 10.79 | 41.97 | 5.80 | 83.41 | 0.0062 | −0.3012 | 45.51 | 9.70 | 36.00 | 8.79 | 81.51 | −0.1167 | −0.0491 |
| Vespidae sp. | 39.46 | 11.19 | 43.07 | 6.28 | 82.53 | 0.0437 | −0.2810 | 44.93 | 9.87 | 35.36 | 9.84 | 80.28 | −0.1192 | −0.0014 |
| Vespa bicolor | 40.98 | 12.81 | 40.74 | 5.47 | 81.72 | −0.0030 | −0.4012 | 44.32 | 11.11 | 35.00 | 9.57 | 79.31 | −0.1175 | −0.0745 |
| Abispa ephippium | 41.05 | 13.38 | 39.55 | 6.02 | 80.61 | −0.0187 | −0.3796 | 43.48 | 11.21 | 35.20 | 10.12 | 78.67 | −0.1052 | −0.0510 |
| Vespa mandarinia | 40.51 | 14.53 | 38.88 | 6.07 | 79.39 | −0.0205 | −0.4104 | 43.37 | 12.35 | 33.73 | 10.56 | 77.09 | −0.1251 | −0.0781 |
| Vespula germanica | 41.47 | 12.39 | 40.21 | 5.94 | 81.67 | −0.0154 | −0.3523 | 45.45 | 10.55 | 33.83 | 10.17 | 79.28 | −0.1465 | −0.0186 |
| GeneSpecies | Agenioideus sp. | Auplopus sp. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T% | C% | A% | G% | (A + T)% | AT-skew | GC-skew | T% | C% | A% | G% | (A + T)% | AT-skew | GC-skew | |
| atp6 | 49.57 | 7.98 | 29.20 | 13.25 | 78.77 | −0.2586 | 0.2483 | 50.00 | 8.86 | 32.58 | 8.56 | 82.58 | −0.2109 | −0.0172 |
| atp8 | 48.00 | 6.80 | 37.20 | 8.00 | 85.20 | −0.1268 | 0.0811 | 47.80 | 5.03 | 44.03 | 3.14 | 91.82 | −0.0411 | −0.2308 |
| cob | 46.07 | 10.88 | 28.56 | 14.50 | 74.62 | −0.2346 | 0.1429 | 45.08 | 10.89 | 32.57 | 11.46 | 77.65 | −0.1611 | 0.0256 |
| cox1 | 45.09 | 12.47 | 26.68 | 15.76 | 71.77 | −0.2565 | 0.1167 | 43.95 | 10.94 | 31.48 | 13.63 | 75.43 | −0.1654 | 0.1094 |
| cox2 | 41.74 | 10.58 | 34.20 | 13.48 | 75.94 | −0.0992 | 0.1205 | 46.09 | 9.42 | 35.22 | 9.28 | 81.30 | −0.1337 | −0.0078 |
| cox3 | 48.23 | 10.10 | 27.40 | 14.27 | 75.63 | −0.2755 | 0.1710 | 48.91 | 9.45 | 31.16 | 10.47 | 80.08 | −0.2217 | 0.0513 |
| nad1 | 43.41 | 12.67 | 34.53 | 9.38 | 77.94 | −0.1140 | −0.1493 | 48.26 | 7.95 | 33.66 | 10.13 | 81.92 | −0.1782 | 0.1205 |
| nad2 | 50.65 | 5.54 | 32.53 | 11.28 | 83.18 | −0.2179 | 0.3413 | 50.70 | 5.62 | 36.55 | 7.13 | 87.25 | −0.1623 | 0.1181 |
| nad3 | 52.42 | 6.84 | 28.21 | 12.54 | 80.63 | −0.3004 | 0.2941 | 56.16 | 4.58 | 30.95 | 8.31 | 87.11 | −0.2895 | 0.2889 |
| nad4 | 39.79 | 12.65 | 39.56 | 8.00 | 79.34 | −0.0029 | −0.2251 | 46.44 | 6.74 | 38.08 | 8.74 | 84.52 | −0.0988 | 0.1287 |
| nad4l | 44.09 | 9.68 | 39.07 | 7.17 | 83.15 | −0.0603 | −0.1489 | 48.55 | 5.43 | 39.49 | 6.52 | 88.04 | −0.1029 | 0.0909 |
| nad5 | 40.97 | 13.24 | 37.67 | 8.12 | 78.64 | −0.0421 | −0.2394 | 46.07 | 8.83 | 37.48 | 7.62 | 83.56 | −0.1027 | −0.0735 |
| nad6 | 46.96 | 7.03 | 32.89 | 13.12 | 79.85 | −0.1762 | 0.3019 | 52.19 | 6.10 | 37.33 | 4.38 | 89.52 | −0.1660 | −0.1636 |
| rrnL | 37.29 | 8.60 | 43.88 | 10.23 | 81.16 | 0.0812 | 0.0864 | 38.44 | 5.78 | 47.61 | 8.17 | 86.06 | 0.1065 | 0.1713 |
| rrnS | 34.64 | 8.78 | 46.38 | 10.20 | 81.02 | 0.1449 | 0.0750 | 37.27 | 6.37 | 47.35 | 9.02 | 84.62 | 0.1191 | 0.1724 |
| Agenioideus sp. | Auplopus sp. | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | Codon | No. | RSCU | AA | Codon | No. | RSCU | AA | Codon | No. | RSCU | AA | Codon | No. | RSCU | AA | Codon | No. | RSCU | AA | Codon | No. | RSCU |
| Phe | UUU | 335 | 1.83 | Ser | UCU | 125 | 2.44 | Tyr | UAU | 163 | 1.71 | Phe | UUU | 395 | 1.96 | Ser | UCU | 107 | 2.32 | Tyr | UAU | 175 | 1.91 |
| UUC | 31 | 0.17 | UCC | 17 | 0.33 | UAC | 28 | 0.29 | UUC | 9 | 0.04 | UCC | 3 | 0.07 | UAC | 8 | 0.09 | ||||||
| Leu | UUA | 408 | 4.6 | UCA | 115 | 2.24 | Cys | UGU | 43 | 1.87 | Leu | UUA | 502 | 5.68 | UCA | 140 | 3.04 | Cys | UGU | 41 | 1.95 | ||
| UUG | 35 | 0.39 | UCG | 5 | 0.1 | UGC | 3 | 0.13 | UUG | 8 | 0.09 | UCG | 5 | 0.11 | UGC | 1 | 0.05 | ||||||
| CUU | 35 | 0.39 | Pro | CCU | 67 | 2.29 | His | CAU | 52 | 1.68 | CUU | 17 | 0.19 | Pro | CCU | 66 | 2.38 | His | CAU | 60 | 1.9 | ||
| CUC | 4 | 0.05 | CCC | 12 | 0.41 | CAC | 10 | 0.32 | CUC | 0 | 0 | CCC | 2 | 0.07 | CAC | 3 | 0.1 | ||||||
| CUA | 48 | 0.54 | CCA | 33 | 1.13 | Gln | CAA | 43 | 1.79 | CUA | 3 | 0.03 | CCA | 40 | 1.44 | Gln | CAA | 50 | 1.92 | ||||
| CUG | 2 | 0.02 | CCG | 5 | 0.17 | CAG | 5 | 0.21 | CUG | 0 | 0 | CCG | 3 | 0.11 | CAG | 2 | 0.08 | ||||||
| Ile | AUU | 389 | 1.87 | Thr | ACU | 76 | 2.01 | Asn | AAU | 154 | 1.58 | Ile | AUU | 439 | 1.98 | Thr | ACU | 82 | 2.58 | Asn | AAU | 199 | 1.91 |
| AUC | 26 | 0.13 | ACC | 7 | 0.19 | AAC | 41 | 0.42 | AUC | 5 | 0.02 | ACC | 3 | 0.09 | AAC | 9 | 0.09 | ||||||
| Met | AUA | 306 | 1.8 | ACA | 63 | 1.67 | Lys | AAA | 113 | 1.71 | Met | AUA | 315 | 1.9 | ACA | 37 | 1.17 | Lys | AAA | 121 | 1.92 | ||
| AUG | 34 | 0.2 | ACG | 5 | 0.13 | AAG | 19 | 0.29 | AUG | 16 | 0.1 | ACG | 5 | 0.16 | AAG | 5 | 0.08 | ||||||
| Val | GUU | 99 | 2.04 | Ala | GCU | 55 | 2.22 | Asp | GAU | 65 | 1.91 | Val | GUU | 79 | 2.36 | Ala | GCU | 42 | 2.05 | Asp | GAU | 58 | 2 |
| GUC | 6 | 0.12 | GCC | 9 | 0.36 | GAC | 3 | 0.09 | GUC | 1 | 0.03 | GCC | 3 | 0.15 | GAC | 0 | 0 | ||||||
| GUA | 76 | 1.57 | GCA | 33 | 1.33 | Glu | GAA | 55 | 1.45 | GUA | 52 | 1.55 | GCA | 37 | 1.8 | Glu | GAA | 73 | 1.95 | ||||
| GUG | 13 | 0.27 | GCG | 2 | 0.08 | GAG | 21 | 0.55 | GUG | 2 | 0.06 | GCG | 0 | 0 | GAG | 2 | 0.05 | ||||||
| Gly | GGU | 57 | 1.56 | Arg | CGU | 20 | 1.74 | Ser | AGU | 48 | 0.94 | Gly | GGU | 48 | 1.21 | Arg | CGU | 20 | 1.7 | Ser | AGU | 24 | 0.52 |
| GGC | 7 | 0.19 | CGC | 0 | 0 | AGC | 3 | 0.06 | GGC | 0 | 0 | CGC | 0 | 0 | AGC | 2 | 0.04 | ||||||
| GGA | 47 | 1.29 | CGA | 20 | 1.74 | AGA | 80 | 1.56 | GGA | 107 | 2.69 | CGA | 26 | 2.21 | AGA | 87 | 1.89 | ||||||
| GGG | 35 | 0.96 | CGG | 6 | 0.52 | AGG | 17 | 0.33 | GGG | 4 | 0.1 | CGG | 1 | 0.09 | AGG | 1 | 0.02 | ||||||
| Trp | UGA | 71 | 1.41 | – | – | – | – | – | – | – | – | Trp | UGA | 83 | 1.91 | – | – | – | – | – | – | – | – |
| UGG | 30 | 0.59 | – | – | – | – | – | – | – | UGG | 4 | 0.09 | – | – | – | – | – | – | |||||
| Species | Superfamily | Family | Accession Number | References |
|---|---|---|---|---|
| Agenioideua sp. | Vespoidea | Pompilidae | KX584356 | This study |
| Auplopus sp. | Vespoidea | Pompilidae | KX584357 | This study |
| Wallacidia oculata | Vespoidea | Mutillidae | FJ611801 | [22] |
| Solenopsis geminata | Vespoidea | Formicidae | HQ215537 | [67] |
| Solenopsis invicta | Vespoidea | Formicidae | HQ215538 | [67] |
| Solenopsis richteri | Vespoidea | Formicidae | HQ215539 | [67] |
| Myrmica scabrinodis | Vespoidea | Formicidae | LN607806 | [80] |
| Pristomyrmex punctatus | Vespoidea | Formicidae | AB556946 | [81] |
| Leptomyrmex pallens | Vespoidea | Formicidae | KC160533 | [82] |
| Atta laevigata | Vespoidea | Formicidae | KC346251 | [83] |
| Formica fusca | Vespoidea | Formicidae | LN607805 | [80] |
| Formica selysi | Vespoidea | Formicidae | KP670862 | [84] |
| Camponotus chromaiodes | Vespoidea | Formicidae | JX966368 | [85] |
| Camponotus atrox | Vespoidea | Formicidae | KT159775 | [65] |
| Linepithema humile | Vespoidea | Formicidae | KT428891 | [68] |
| Polistes humilis | Vespoidea | Vespidae | EU024653 | [40] |
| Polistes jokahamae | Vespoidea | Vespidae | KR052468 | [35] |
| Vespidae sp. | Vespoidea | Vespidae | KM244667 | [86] |
| Vespa bicolor | Vespoidea | Vespidae | KJ735511 | [66] |
| Abispa ephippium | Vespoidea | Vespidae | NC011520 | [40] |
| Vespa mandarinia | Vespoidea | Vespidae | KR059904 | [36] |
| Vespula germanica | Vespoidea | Vespidae | KR703587 | [34] |
| Optimal Partition | Model | Initial Partition |
|---|---|---|
| Partition 1 | GTR + I + G | a6p1, c2p1, c3p1, cbp1, n3p1 |
| Partition 2 | HKY + I + G | a8p1, n2p1, n6p1 |
| Partition 3 | GTR + I + G | a6p2, c2p2, c3p2, cbp2, n3p2 |
| Partition 4 | GTR + I + G | n1p2, n4lp2, n4p2, n5p2 |
| Partition 5 | GTR + G | c1p2 |
| Partition 6 | GTR + G | a8p2, n2p2, n6p2 |
| Partition 7 | HKY + G | a8p3, n2p3, n6p3 |
| Partition 8 | GTR + I + G | n1p1, n4lp1, n4p1, n5p1 |
| Partition 9 | HKY + G | n1p3, n4lp3, n4p3, n5p3 |
| Partition 10 | GTR + G | a6p3, c1p3, c2p3, c3p3, cbp3, n3p3 |
| Partition 11 | GTR + I + G | c1p1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Zheng, B.-Y.; Liu, J.-X.; Wei, S.-J. Next-Generation Sequencing of Two Mitochondrial Genomes from Family Pompilidae (Hymenoptera: Vespoidea) Reveal Novel Patterns of Gene Arrangement. Int. J. Mol. Sci. 2016, 17, 1641. https://doi.org/10.3390/ijms17101641
Chen P-Y, Zheng B-Y, Liu J-X, Wei S-J. Next-Generation Sequencing of Two Mitochondrial Genomes from Family Pompilidae (Hymenoptera: Vespoidea) Reveal Novel Patterns of Gene Arrangement. International Journal of Molecular Sciences. 2016; 17(10):1641. https://doi.org/10.3390/ijms17101641
Chicago/Turabian StyleChen, Peng-Yan, Bo-Ying Zheng, Jing-Xian Liu, and Shu-Jun Wei. 2016. "Next-Generation Sequencing of Two Mitochondrial Genomes from Family Pompilidae (Hymenoptera: Vespoidea) Reveal Novel Patterns of Gene Arrangement" International Journal of Molecular Sciences 17, no. 10: 1641. https://doi.org/10.3390/ijms17101641
APA StyleChen, P.-Y., Zheng, B.-Y., Liu, J.-X., & Wei, S.-J. (2016). Next-Generation Sequencing of Two Mitochondrial Genomes from Family Pompilidae (Hymenoptera: Vespoidea) Reveal Novel Patterns of Gene Arrangement. International Journal of Molecular Sciences, 17(10), 1641. https://doi.org/10.3390/ijms17101641








