Comparative Proteomic Analysis of Mature and Immature Oocytes of the Swamp Buffalo (Bubalus bubalis)
Abstract
:1. Introduction
2. Results
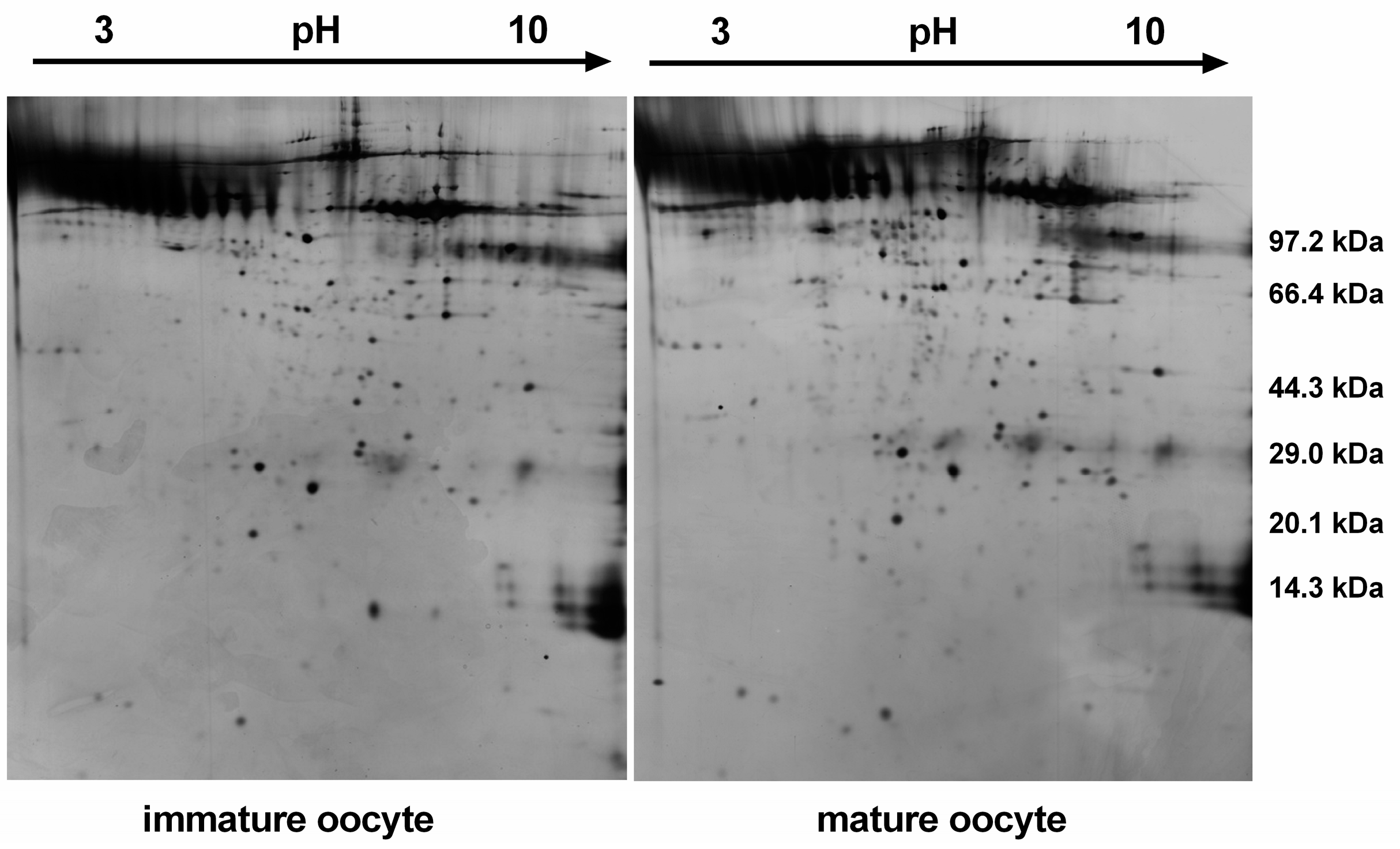
2.1. Two-Dimensional Gel Electrophoresis Profile
2.2. MS Identification and Bioinformatics Analysis

| Spot No. | Protein Name | Gene Symbol | Calc.MW (Da) | Uniprot Accession No. | Peptide Count | Mascot Score * | Regulation Profile | Subcellular Location |
|---|---|---|---|---|---|---|---|---|
| 1 | Ras-responsive element-binding protein 1 | RREB1 | 181,420 | Q92766 | 1 | 76 (99%) | mature up | Nucleus |
| 2 | Hemoglobin subunit α | HBA | 15,184 | P01966 | 1 | 85 (99%) | mature up | Unknown |
| 3 | Heat shock cognate 71 kDa protein | HSC71 | 71,241 | P19120 | 2 | 66 (99%) | mature up | Cytoplasm/Nucleus |
| 4 | 60kDa heat shock protein, mitochondrial | HSP60 | 61,108 | P31081 | 3 | 117 (100%) | mature up | Mitochondrion |
| 5 | N-acetyllactosaminide β-1,6-N-acetylglucosaminyl-transferase, isoform C | GCNT2 | 46,531 | Q8NFS9 | 5 | 63 (98%) | immature up | Golgi apparatus/Membrane |
| 6 | Major vault protein | MVP | 98,924 | Q3SYU9 | 11 | 190 (100%) | immature up | Cytoplasm/Nucleus |
| 7 | BMP-2-inducible protein kinase | BMP2K | 129,172 | Q9NSY1 | 4 | 83 (99%) | mature up | Nucleus |
| 8 | Gem-associated protein 8 | GEMIN8 | 26,744 | Q1LZ79 | 3 | 86 (99%) | immature up | Cytoplasm/Nucleus |
2.3. Western Blot Validation
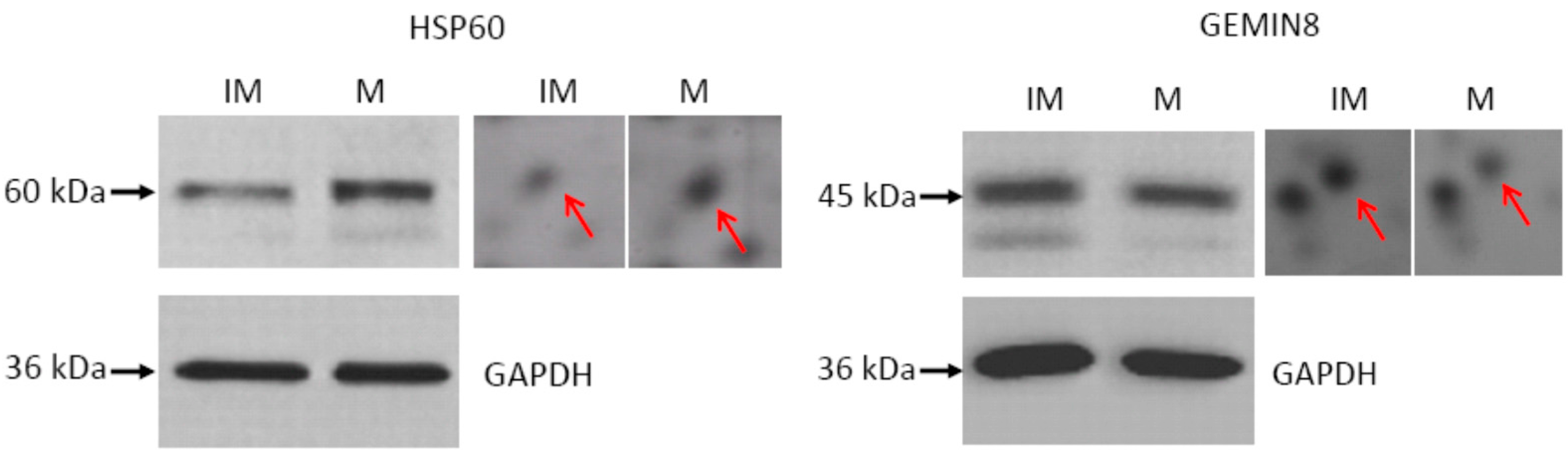
3. Discussion
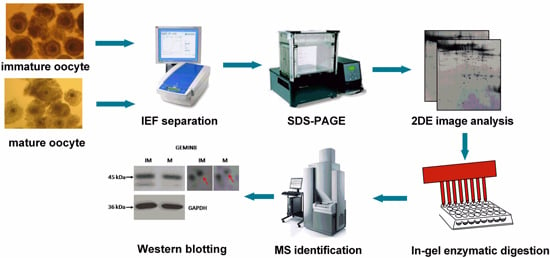
4. Materials and Methods
4.1. Oocyte in Vitro Maturation
4.2. Sample Perparation and Protein Extraction
4.3. Two-Dimensional Electrophoresis
4.4. Silver Staining and Image Analysis
4.5. In-Gel Digestion and Mass Spectrometry Identification
4.6. Protein Bioinformation Analysis
4.7. Western Blot Validation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vitale, A.M.; Calvert, M.E.; Mallavarapu, M.; Yurttas, P.; Perlin, J.; Herr, J.; Coonrod, S. Proteomic profiling of murine oocyte maturation. Mol. Reprod. Dev. 2007, 74, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Motlik, J.; Fulka, J. In vitro maturation of mammalian oocytes. Ontogenez 1981, 12, 435–442. [Google Scholar] [PubMed]
- Payer, B.; Saitou, M.; Barton, S.C.; Thresher, R.; Dixon, J.P.; Zahn, D.; Colledge, W.H.; Carlton, M.B.; Nakano, T.; Surani, M.A. Stella is a maternal effect gene required for normal early development in mice. Curr. Biol. 2003, 13, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Viveiros, M.M.; Eppig, J.J.; Bai, Y.; Fitzpatrick, S.L.; Matzuk, M.M. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat. Genet. 2003, 33, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.B.; Gold, L.; Pfeifer, K.E.; Dorward, H.; Lee, E.; Bondy, C.A.; Dean, J.; Nelson, L.M. Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 2000, 26, 267–268. [Google Scholar] [PubMed]
- Calvert, M.E.; Digilio, L.C.; Herr, J.C.; Coonrod, S.A. Oolemmal proteomics—Identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane. Reprod. Biol. Endocrinol. 2003, 1, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Liu, X.H.; Ma, X.; Shen, Y.; Fan, L.; Leng, J.; Liu, J.Y.; Sha, J.H. The protein profile of mouse mature cumulus-oocyte complex. Biochim. Biophys. Acta 2007, 1774, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Ellederova, Z.; Halada, P.; Man, P.; Kubelka, M.; Motlik, J.; Kovarova, H. Protein patterns of pig oocytes during in vitro maturation. Biol. Reprod. 2004, 71, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Memili, E.; Peddinti, D.; Shack, L.A.; Nanduri, B.; McCarthy, F.; Sagirkaya, H.; Burgess, S.C. Bovine germinal vesicle oocyte and cumulus cell proteomics. Reproduction 2007, 133, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ni, X.; Guo, Y.; Guo, X.; Wang, Y.; Zhou, Z.; Huo, R.; Sha, J. Proteomic-based identification of maternal proteins in mature mouse oocytes. BMC Genom. 2009, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kou, Z.; Jing, Z.; Zhang, Y.; Guo, X.; Dong, M.; Wilmut, I.; Gao, S. Proteome of mouse oocytes at different developmental stages. Proc. Natl. Acad. Sci. USA 2010, 107, 17639–17644. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.J.; Siatkowski, M.; Paudel, Y.; Balbach, S.T.; Baeumer, N.; Crosetto, N.; Drexler, H.C.; Fuellen, G.; Boiani, M. Proteomic analysis of mouse oocytes reveals 28 candidate factors of the “reprogrammome”. J. Proteome Res. 2011, 10, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Graumann, J.; Hubner, N.C.; Kim, J.B.; Ko, K.; Moser, M.; Kumar, C.; Cox, J.; Scholer, H.; Mann, M. Stable isotope labeling by amino acids in cell culture (SILAC) and proteome quantitation of mouse embryonic stem cells to a depth of 5111 proteins. Mol. Cell. Proteom. 2008, 7, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Bovine proteome database. Available online: http://www.uniprot.org/proteomes/up000009136 (accessed on 12 January 2016).
- SIB Swiss Institute of Bioinformatics Members. The SIB Swiss Institute of Bioinformatics’ resources: Focus on curated databases. Nucleic Acids Res. 2015. [Google Scholar] [CrossRef]
- Rocken, C.; Ebert, M.P.; Roessner, A. Proteomics in pathology, research and practice. Pathol. Res. Pract. 2004, 200, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Charro, N.; Hood, B.L.; Faria, D.; Pacheco, P.; Azevedo, P.; Lopes, C.; de Almeida, A.B.; Couto, F.M.; Conrads, T.P.; Penque, D. Serum proteomics signature of cystic fibrosis patients: a complementary 2-DE and LC-MS/MS approach. J. Proteom. 2011, 74, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Hammer, E.; Bien, S.; Salazar, M.G.; Steil, L.; Scharf, C.; Hildebrandt, P.; Schroeder, H.W.; Kroemer, H.K.; Volker, U.; Ritter, C.A. Proteomic analysis of doxorubicin-induced changes in the proteome of HepG2cells combining 2-D DIGE and LC-MS/MS approaches. Proteomics 2010, 10, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Hwang, S.Y.; Solter, D.; Knowles, B.B. Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development 1997, 124, 493–503. [Google Scholar] [PubMed]
- Novak, S.; Paradis, F.; Savard, C.; Tremblay, K.; Sirard, M.A. Identification of porcine oocyte proteins that are associated with somatic cell nuclei after co-incubation. Biol. Reprod. 2004, 71, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Curci, A.; Bevilacqua, A.; Mangia, F. Lack of heat-shock response in preovulatory mouse oocytes. Dev. Biol. 1987, 123, 154–160. [Google Scholar] [CrossRef]
- Manejwala, F.M.; Logan, C.Y.; Schultz, R.M. Regulation of hsp70 mRNA levels during oocyte maturation and zygotic gene activation in the mouse. Dev. Biol. 1991, 144, 301–308. [Google Scholar] [CrossRef]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—Integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, L. Proteomic analysis of neural differentiation of mouse embryonic stem cells. Proteomics 2005, 5, 4414–4426. [Google Scholar] [CrossRef] [PubMed]
- Kurisaki, A.; Hamazaki, T.S.; Okabayashi, K.; Iida, T.; Nishine, T.; Chonan, R.; Kido, H.; Tsunasawa, S.; Nishimura, O.; Asashima, M.; et al. Chromatin-related proteins in pluripotent mouse embryonic stem cells are downregulated after removal of leukemia inhibitory factor. Biochem. Biophys. Res. Commun. 2005, 335, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Intawicha, P.; Wang, S.H.; Hsieh, Y.C.; Lo, N.W.; Lee, K.H.; Huang, S.Y.; Ju, J.C. Proteomic profiling of rabbit embryonic stem cells derived from parthenotes and fertilized embryos. PLoS ONE 2013, 8, e67772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukau, B.; Weissman, J.; Horwich, A. Molecular chaperones and protein quality control. Cell 2006, 125, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Hansen, P.J. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol. Reprod. Dev. 1997, 46, 138–145. [Google Scholar] [CrossRef]
- Carissimi, C.; Saieva, L.; Baccon, J.; Chiarella, P.; Maiolica, A.; Sawyer, A.; Rappsilber, J.; Pellizzoni, L. Gemin8 is a novel component of the survival motor neuron complex and functions in small nuclear ribonucleoprotein assembly. J. Biol. Chem. 2006, 281, 8126–8134. [Google Scholar] [CrossRef] [PubMed]
- Carissimi, C.; Saieva, L.; Gabanella, F.; Pellizzoni, L. Gemin8 is required for the architecture and function of the survival motor neuron complex. J. Biol. Chem. 2006, 281, 37009–37016. [Google Scholar] [CrossRef] [PubMed]
- Borner, G.H.; Antrobus, R.; Hirst, J.; Bhumbra, G.S.; Kozik, P.; Jackson, L.P.; Sahlender, D.A.; Robinson, M.S. Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J. Cell Biol. 2012, 197, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.L.; Heuser, J.E.; Chugani, D.C.; Rome, L.H. Vaults. III. Vault ribonucleoprotein particles open into flower-like structures with octagonal symmetry. J. Cell Biol. 1991, 112, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Mossink, M.; van Zon, A.; Franzel-Luiten, E.; Schoester, M.; Scheffer, G.L.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A. The genomic sequence of the murine major vault protein and its promoter. Gene 2002, 294, 225–232. [Google Scholar] [CrossRef]
- Sutovsky, P.; Manandhar, G.; Laurincik, J.; Letko, J.; Caamano, J.N.; Day, B.N.; Lai, L.; Prather, R.S.; Sharpe-Timms, K.L.; Zimmer, R.; et al. Expression and proteasomal degradation of the major vault protein (MVP) in mammalian oocytes and zygotes. Reproduction 2005, 129, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, K.H.; Seidel, G.E. Development of bovine embryos after in vitro fertilization of oocytes with flow cytometrically sorted, stained and unsorted sperm from different bulls. Theriogenology 2003, 60, 1657–1663. [Google Scholar] [CrossRef]
- Expert Protein Analysis System (ExPASy). Available online: http://www.expasy.org (accessed on 12 January 2016).
- KEGG Orthology Based Annotation System (KOBAS). Available online: http://kobas.cbi.pku.edu.cn (accessed on 12 January 2016).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Liu, Z.-F.; Huang, Y.-L.; Lu, Y.-Q.; Zhang, M. Comparative Proteomic Analysis of Mature and Immature Oocytes of the Swamp Buffalo (Bubalus bubalis). Int. J. Mol. Sci. 2016, 17, 94. https://doi.org/10.3390/ijms17010094
Fu Q, Liu Z-F, Huang Y-L, Lu Y-Q, Zhang M. Comparative Proteomic Analysis of Mature and Immature Oocytes of the Swamp Buffalo (Bubalus bubalis). International Journal of Molecular Sciences. 2016; 17(1):94. https://doi.org/10.3390/ijms17010094
Chicago/Turabian StyleFu, Qiang, Zhen-Fang Liu, Yu-Lin Huang, Yang-Qing Lu, and Ming Zhang. 2016. "Comparative Proteomic Analysis of Mature and Immature Oocytes of the Swamp Buffalo (Bubalus bubalis)" International Journal of Molecular Sciences 17, no. 1: 94. https://doi.org/10.3390/ijms17010094