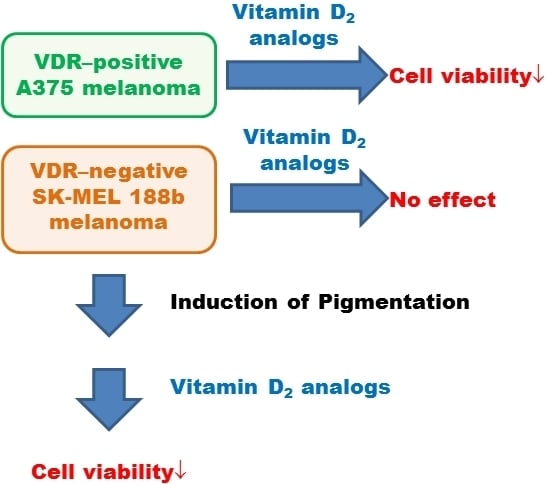
Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D2 Against Human Malignant Melanoma Cell Lines
Abstract
:1. Introduction
2. Results
2.1. New Vitamin D Analogs Effectively Inhibit A375 Cell Proliferation
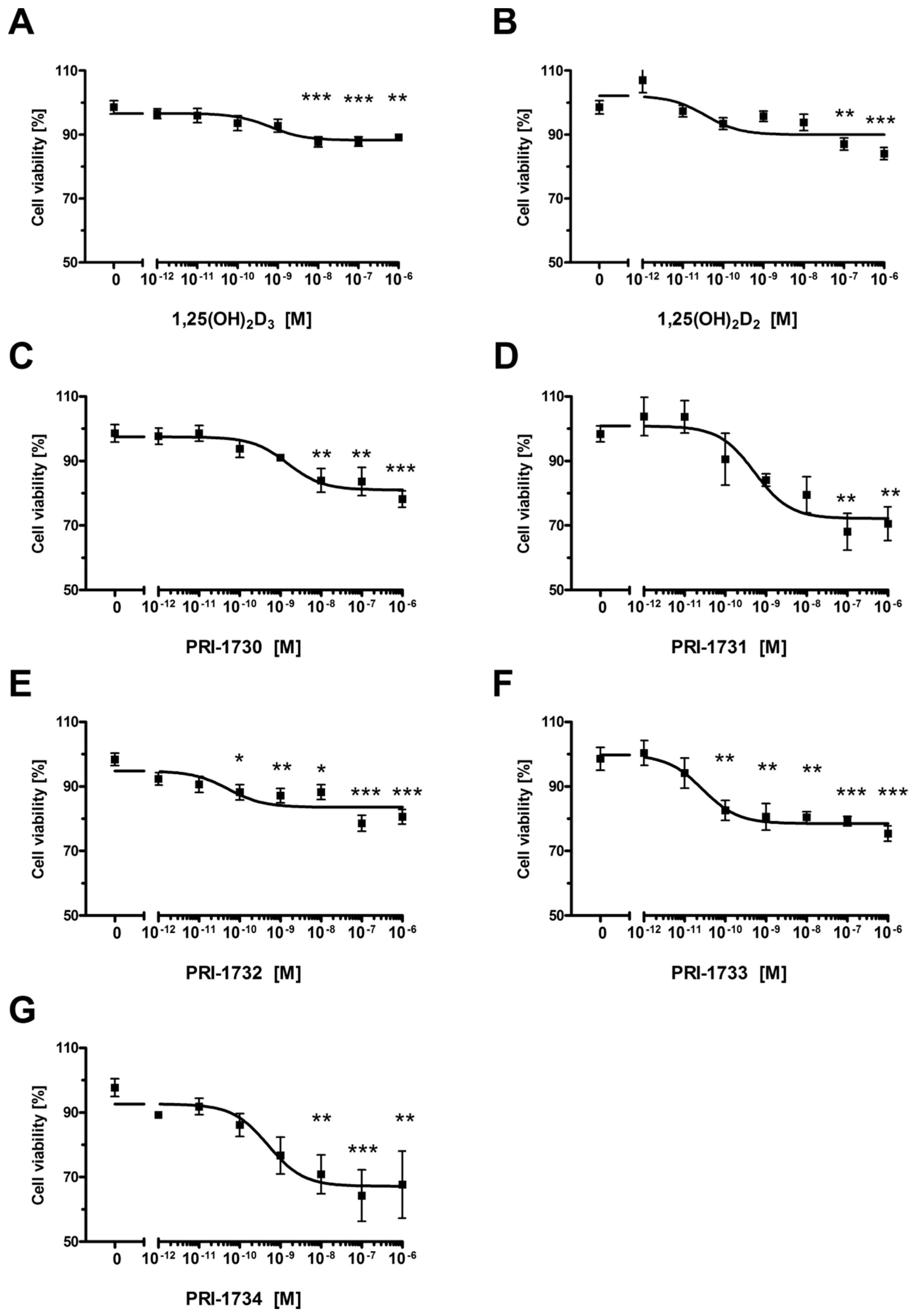
| Compound | IC50 (nM) |
|---|---|
| 1,25(OH)2D3 | 0.656 |
| 1,25(OH)2D2 | 0.036 |
| PRI-1730 | 1.500 |
| PRI-1731 | 0.524 |
| PRI-1732 | 0.053 |
| PRI-1733 | 0.028 |
| PRI-1734 | 0.497 |
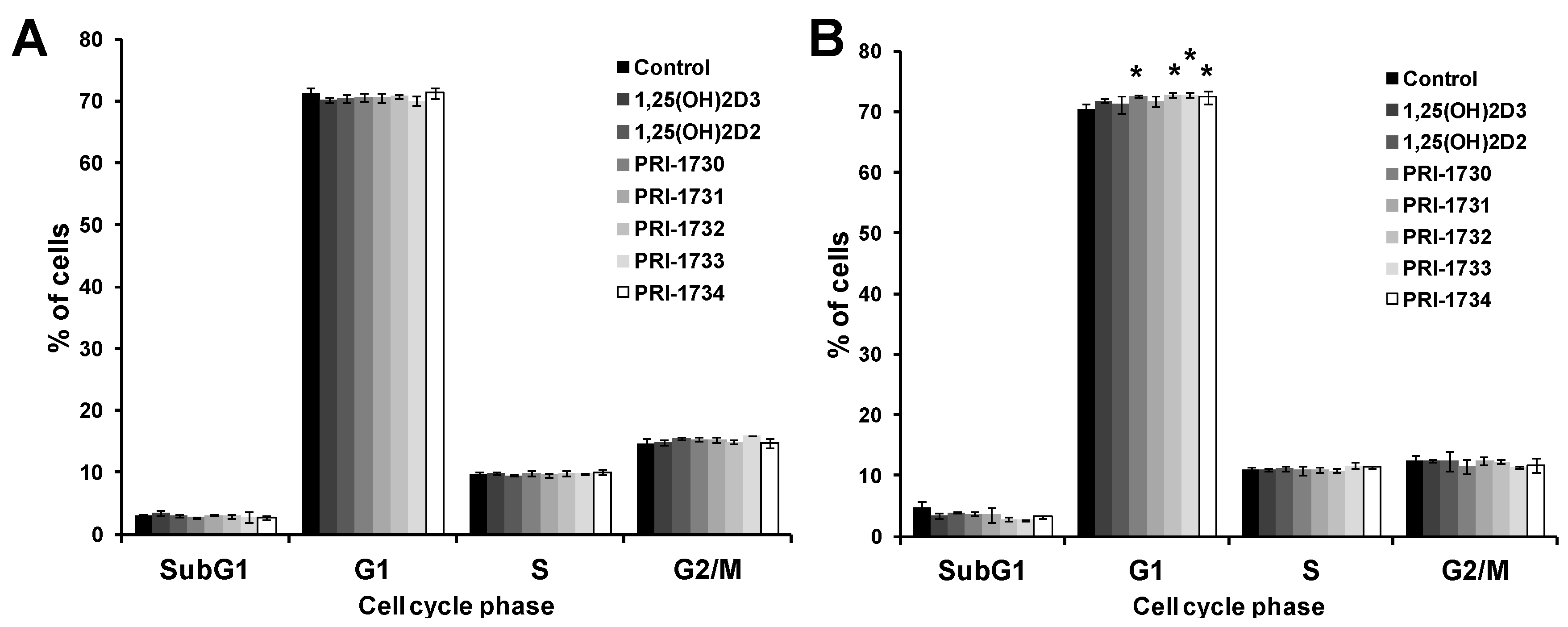
2.2. Treatment of A375 Human Malignant Melanoma Cells with the New Vitamin D Analogs Resulted in G0/G1 Arrest

2.3. SK-MEL 188b Melanoma Cells Do Not Express VDR Receptor and Vitamin D 24-Hydroxylase (CYP24A1)
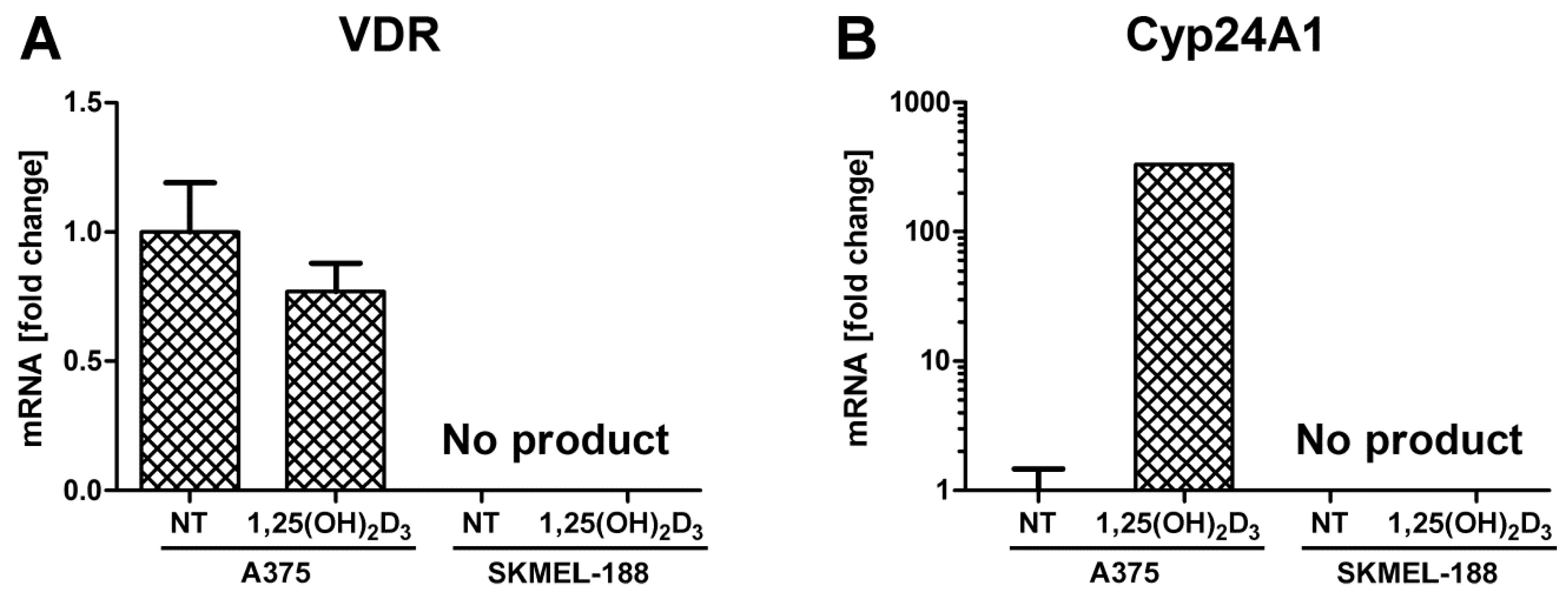
2.4. Inhibition of Melanoma Proliferation by Novel Vitamin D Analogs Is Reliant on VDR
| Compound | IC50 (nM) |
|---|---|
| 1,25(OH)2D3 | NS |
| 1,25(OH)2D2 | NS |
| PRI-1730 | NS |
| PRI-1731 | 0.408 |
| PRI-1732 | NS |
| PRI-1733 | NS |
| PRI-1734 | NS |
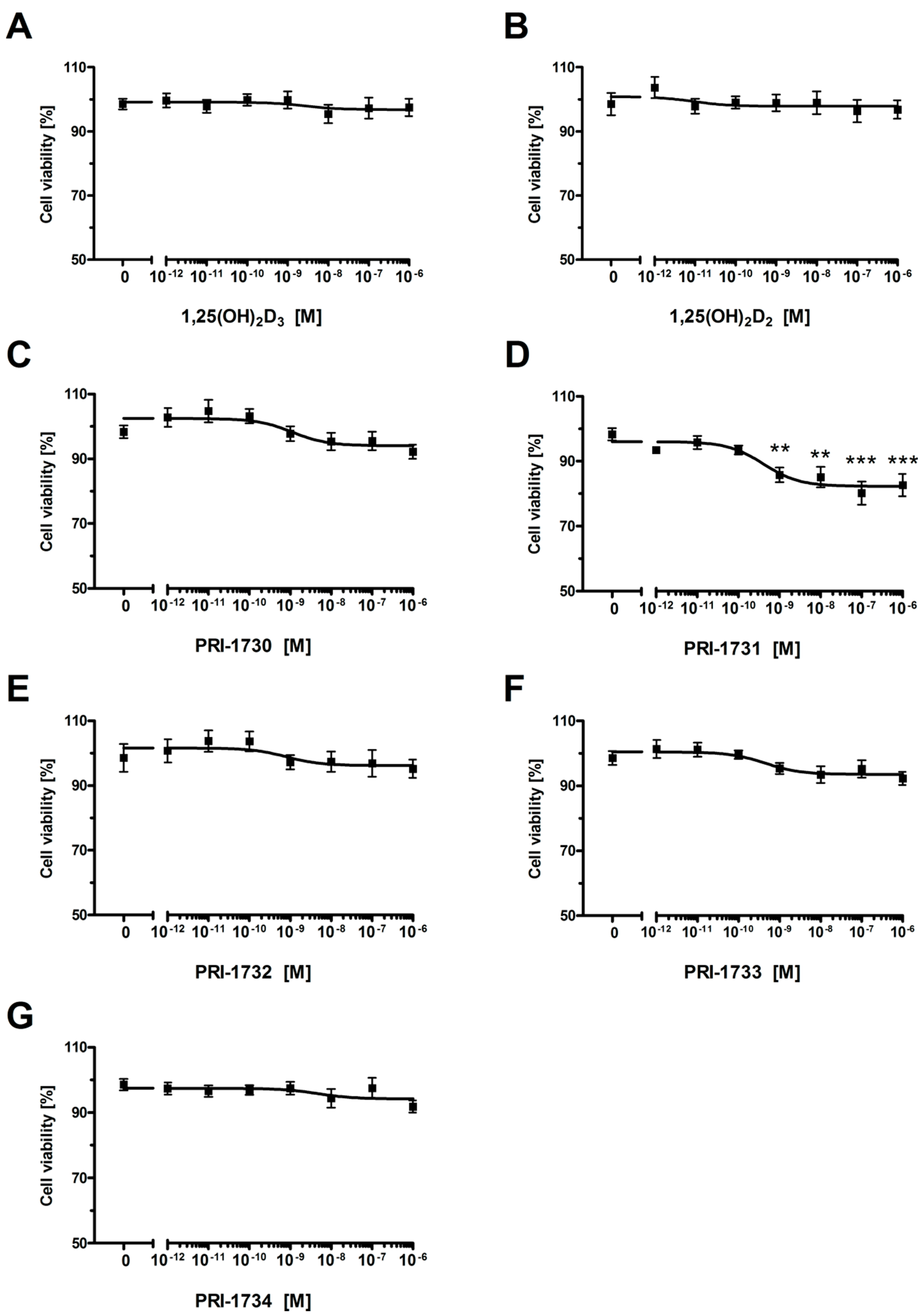
2.5. New Vitamin D Analogs Had Only a Very Limited Effect on Non-Pigmented SK-MEL 188b Melanoma Cells as to the Distribution of Cells in Phases of the Cell Cycle. NS—Not Significant

2.6. Pigmentation of SK-MEL 188 Cells Sensitizes Them to the Effects of the New Vitamin D Analogs
| Compound | IC50 (nM) |
|---|---|
| 1,25(OH)2D3 | 0.0087 |
| 1,25(OH)2D2 | 0.0158 |
| PRI-1730 | 0.0011 |
| PRI-1731 | 0.0004 |
| PRI-1732 | 0.0380 |
| PRI-1733 | 0.0020 |
| PRI-1734 | 0.0054 |
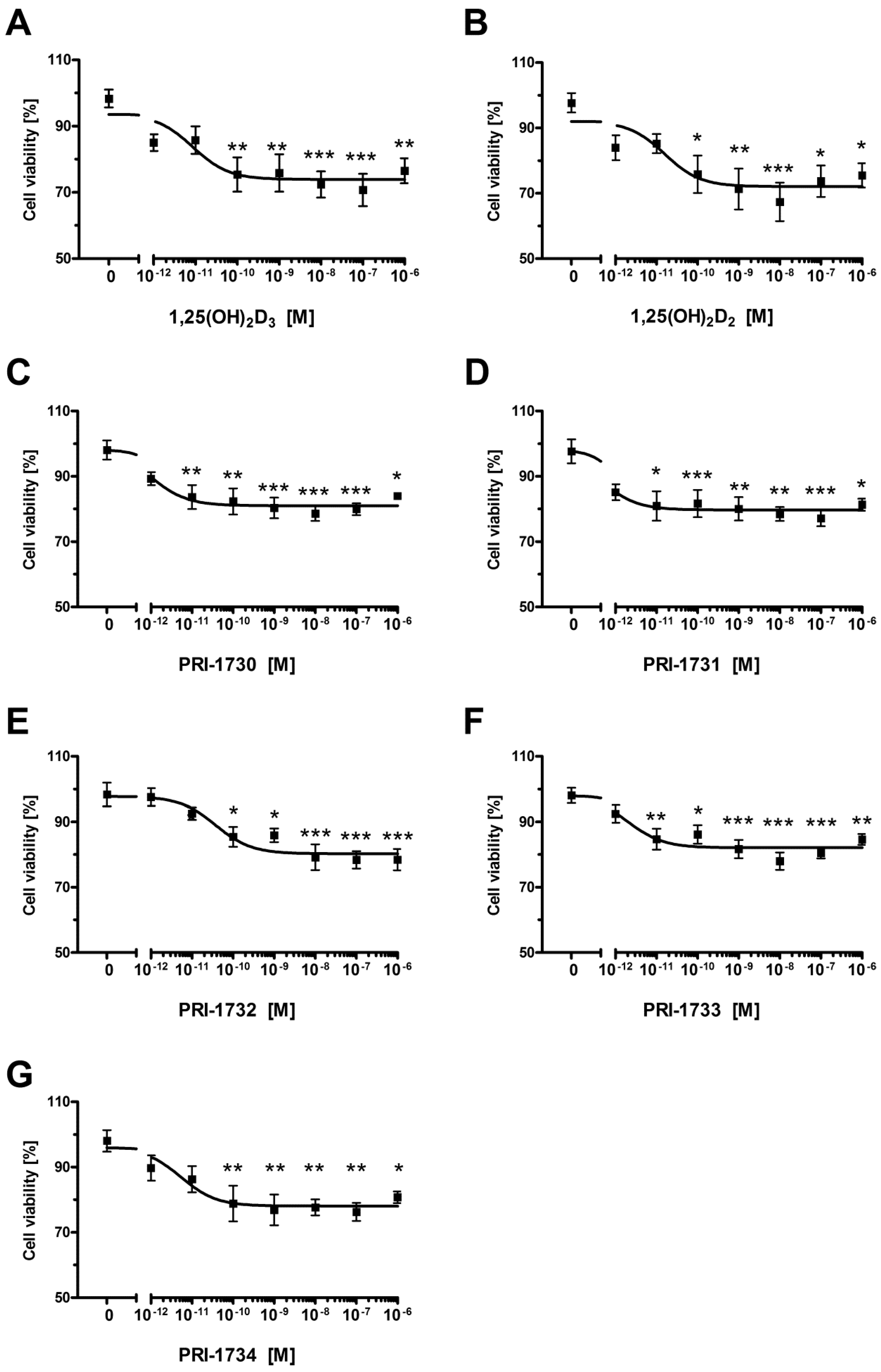
2.7. Pigmentation of SK-MEL 188b Cells Affected Cell Cycle Distribution of Melanoma Cells after Treatment with Vitamin D Analogs
3. Discussion
4. Materials and Methods
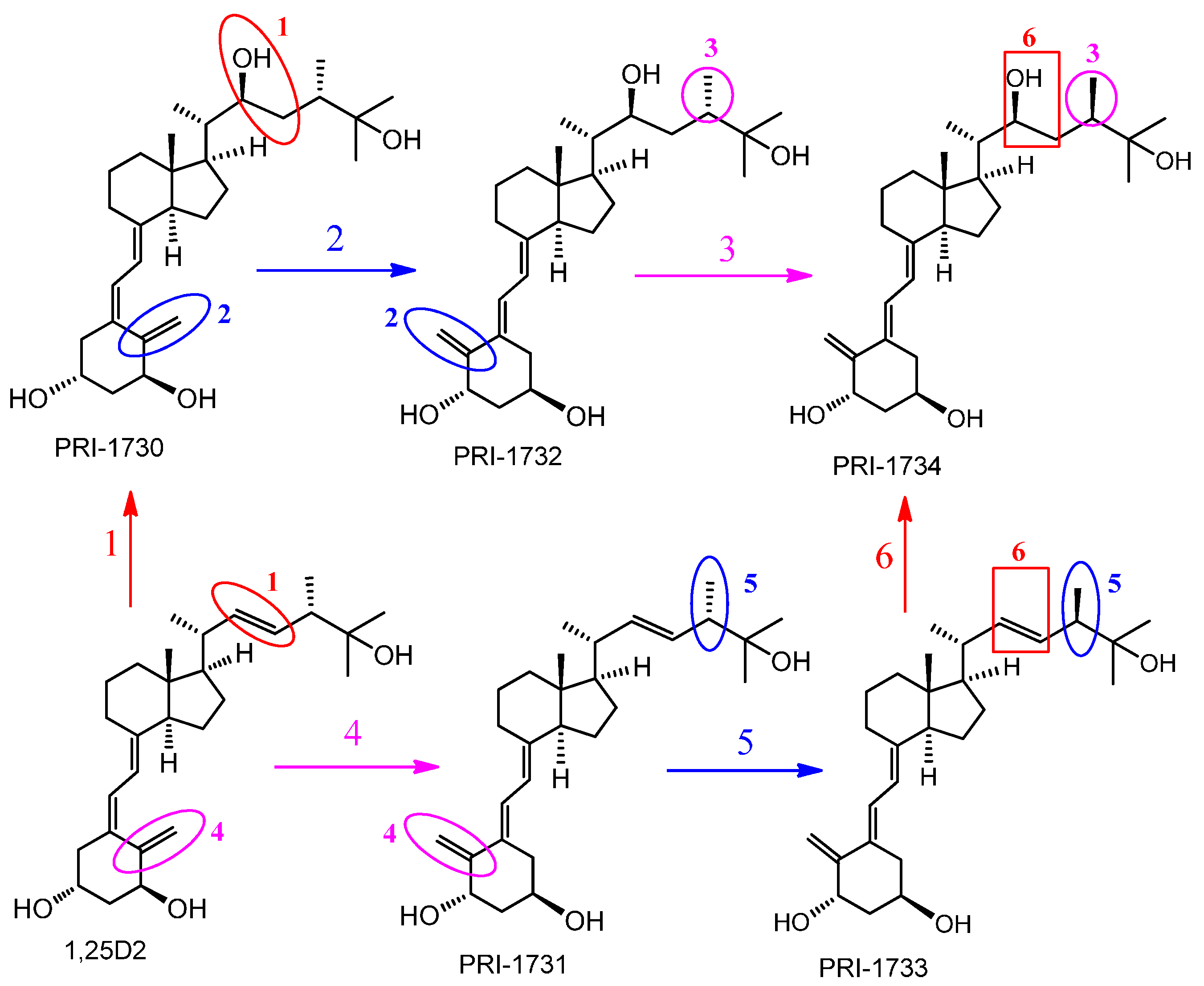
4.1. Vitamin D Analogs
4.2. Cell Culture
4.3. Proliferation Assay
4.4. Cell Cycle Analysis
4.5. cDNA Preparation and PCR Assays
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| RPL37A | TTCTGATGGCGGACTTTACC | CACTTGCTCTTTCTGTGGCA |
| VDR | CCAGTTCGTGTGAATGATGG | GTCGTCCATGGTGAAGGA |
| CYP24A1 | GCAGCCTAGTGCAGATTT | ATTCACCCAGAACTGTTG |
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Bikle, D.D. Vitamin d: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.; Piotrowska, A.; Zmijewski, M.A. The renaissance of vitamin D. Acta Biochim. Pol. 2014, 61, 679–686. [Google Scholar] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Biancuzzo, R.M.; Chen, T.C.; Klein, E.K.; Young, A.; Bibuld, D.; Reitz, R.; Salameh, W.; Ameri, A.; Tannenbaum, A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 2008, 93, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, sufficiency and toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef] [PubMed]
- Grober, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Derm. Endocrinol. 2013, 5, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin d-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin d. Am. J. Clin. Nutr. 2004, 80, 1689–1696. [Google Scholar]
- Mason, R.S.; Sequeira, V.B.; Gordon-Thomson, C. Vitamin D: The light side of sunshine. Eur. J. Clin. Nutr. 2011, 65, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S. Vitamin D: A hormone for all seasons. Climacteric 2011, 14, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Zella, L.A.; Meyer, M.B.; Fretz, J.A.; Kim, S. Molecular actions of 1,25-dihydroxyvitamin D3 on genes involved in calcium homeostasis. J. Bone Miner. Res. 2007, 22, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (vdr)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin d receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. In Endocrinology and Metabolism Clinics of North America; 2010 Elsevier Inc.: Philadelphia, PA, USA, 2010; Volume 39, pp. 255–269, table of contents. [Google Scholar]
- Holick, M.F. Resurrection of vitamin d deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D regulated keratinocyte differentiation. J. Cell. Biochem. 2004, 92, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Oda, Y.; Xie, Z. Calcium and 1,25(OH)2D: Interacting drivers of epidermal differentiation. J. Steroid Biochem. Mol. Biol. 2004, 89, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Diaz-Munoz, M.; Garcia-Gaytan, A.C.; Mendez, I. Mechanistic effects of calcitriol in cancer biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef] [PubMed]
- Kubis, A.M.; Piwowar, A. The new insight on the regulatory role of the vitamin D3 in metabolic pathways characteristic for cancerogenesis and neurodegenerative diseases. Ageing Res. Rev. 2015, 24, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N. Vitamin D-mediated apoptosis in cancer and obesity. Horm. Mol. Biol. Clin. Investig. 2014, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A D-lightful solution for health. J. Investig. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A D-lightful solution for good health. J. Med. Biochem. 2012, 31, 263–264. [Google Scholar] [CrossRef]
- Ma, Y.; Trump, D.L.; Johnson, C.S. Vitamin D in combination cancer treatment. J. Cancer 2010, 1, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.A.; Miller, D.D.; Li, W. The role of vitamin D in cancer prevention. Chin. J. Nat. Med. 2015, 13, 481–497. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Swami, S.; Feldman, D. Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: Importance of mammary cyp27b1 for treatment and prevention. J. Steroid Biochem. Mol. Biol. 2013, 136, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.A. Vitamin D activities for health outcomes. Ann. Lab. Med. 2014, 34, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D. The relationship between vitamin D and cancer. Clin. J. Oncol. Nurs. 2011, 15, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Walentowicz-Sadlecka, M.; Sadlecki, P.; Walentowicz, P.; Grabiec, M. The role of vitamin D in the carcinogenesis of breast and ovarian cancer. Ginekol. Pol. 2013, 84, 305–308. [Google Scholar] [PubMed]
- Schwartz, G.G. Vitamin D and intervention trials in prostate cancer: From theory to therapy. Ann. Epidemiol. 2009, 19, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ordonez-Mena, J.M.; Chen, T.; Schottker, B.; Arndt, V.; Brenner, H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: A systematic review and meta-analysis. Prev. Med. 2013, 57, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.; Wietzke, J.A.; Zinser, G.M.; Byrne, B.; Smith, K.; Narvaez, C.J. Vitamin D-3 receptor as a target for breast cancer prevention. J. Nutr. 2003, 133, 2425S–2433S. [Google Scholar] [PubMed]
- Mehta, R.G.; Hussain, E.A.; Mehta, R.R.; Das Gupta, T.K. Chemoprevention of mammary carcinogenesis by 1alpha-hydroxyvitamin D5, a synthetic analog of vitamin D. Mutat. Res. 2003, 523, 253–264. [Google Scholar] [CrossRef]
- Lamprecht, S.A.; Lipkin, M. Chemoprevention of colon cancer by calcium, vitamin D and folate: Molecular mechanisms. Nat. Rev. Cancer 2003, 3, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kawaura, A.; Kato, S.; Takeda, E.; Okano, T. 1α,25-dihydroxyvitamin d(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis 2005, 26, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Peehl, D.M.; Feldman, D. The role of vitamin D in prostate cancer. Recent Results Cancer Res. 2003, 164, 205–221. [Google Scholar] [PubMed]
- Szyszka, P.; Zmijewski, M.A.; Slominski, A.T. New vitamin D analogs as potential therapeutics in melanoma. Expert Rev. Anticancer Ther. 2012, 12, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Wasiewicz, T.; Szyszka, P.; Cichorek, M.; Janjetovic, Z.; Tuckey, R.C.; Slominski, A.T.; Zmijewski, M.A. Antitumor effects of vitamin D analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation. Int. J. Mol. Sci. 2015, 16, 6645–6667. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.M.; Binek, A.; Ahrends, T.; Nowacka, J.D.; Szydłowska, A.; Turczyk, Ł.; Wąsiewicz, T.; Wierzbicki, P.M.; Sądej, R.; Tuckey, R.C.; et al. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int. J. Oncol. 2015, 47, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Derm. Endocrinol. 2013, 5, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Kotlarz, A.; Przybyszewska, M.; Swoboda, P.; Miloszewska, J.; Grygorowicz, M.A.; Kutner, A.; Markowicz, S. Differential interference of vitamin D analogs PRI-1906, PRI-2191, and PRI-2205 with the renewal of human colon cancer cells refractory to treatment with 5-fluorouracil. Tumour Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Trynda, J.; Turlej, E.; Milczarek, M.; Pietraszek, A.; Chodynski, M.; Kutner, A.; Wietrzyk, J. Antiproliferative activity and in vivo toxicity of double-point modified analogs of 1,25-dihydroxyergocalciferol. Int. J. Mol. Sci. 2015, 16, 24873–24894. [Google Scholar] [CrossRef] [PubMed]
- Huet, T.; Laverny, G.; Ciesielski, F.; Molnar, F.; Ramamoorthy, T.G.; Belorusova, A.Y.; Antony, P.; Potier, N.; Metzger, D.; Moras, D.; et al. A vitamin D receptor selectively activated by gemini analogs reveals ligand dependent and independent effects. Cell Rep. 2015, 10, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Malinska, M.; Kutner, A.; Wozniak, K. Predicted structures of new vitamin D receptor agonists based on available x-ray structures. Steroids 2015, 104, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Pietraszek, A.; Malinska, M.; Chodynski, M.; Krupa, M.; Krajewski, K.; Cmoch, P.; Wozniak, K.; Kutner, A. Synthesis and crystallographic study of 1,25-dihydroxyergocalciferol analogs. Steroids 2013, 78, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Nachliely, M.; Sharony, E.; Kutner, A.; Danilenko, M. Novel analogs of 1,25-dihydroxyvitamin D combined with a plant polyphenol as highly efficient inducers of differentiation in human acute myeloid leukemia cells. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bolla, N.R.; Corcoran, A.; Yasuda, K.; Chodynski, M.; Krajewski, K.; Cmoch, P.; Marcinkowska, E.; Brown, G.; Sakaki, T.; Kutner, A. Synthesis and evaluation of geometric analogs of 1α,25-dihydroxyvitamin D2 as potential therapeutics. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Brozyna, A.A.; Tuckey, R.C.; Kim, T.K.; Nguyen, M.N.; Jozwicki, W.; Pfeffer, S.R.; Pfeffer, L.M.; Slominski, A.T. High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br. J. Cancer 2011, 105, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum. Pathol. 2011, 42, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Slominski, A.T. Decreased vdr expression in cutaneous melanomas as marker of tumor progression: New data and analyses. Anticancer Res. 2014, 34, 2735–2743. [Google Scholar] [PubMed]
- Zmijewski, M.A.; Li, W.; Chen, J.; Kim, T.K.; Zjawiony, J.K.; Sweatman, T.W.; Miller, D.D.; Slominski, A.T. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids 2011, 76, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Seuter, S. A genomic perspective on vitamin D signaling. Anticancer Res. 2009, 29, 3485–3493. [Google Scholar] [PubMed]
- Ingraham, B.A.; Bragdon, B.; Nohe, A. Molecular basis of the potential of vitamin D to prevent cancer. Curr. Med. Res. Opin. 2008, 24, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Zbytek, B.; Pisarchik, A.; Li, W.; Zjawiony, J.; Tuckey, R.C. Cytochromes p450 and skin cancer: Role of local endocrine pathways. Anticancer Agents Med. Chem. 2014, 14, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Perlman, K.; Kutner, A.; Prahl, J.; Smith, C.; Inaba, M.; Schnoes, H.K.; DeLuca, H.F. 24-homologated 1,25-dihydroxyvitamin D3 compounds: Separation of calcium and cell differentiation activities. Biochemistry 1990, 29, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, S.; Chodynski, M.; Corcoran, A.; Marcinkowska, E.; Brown, G.; Kutner, A. Double point modified analogs of vitamin D as potent activators of vitamin D receptor. Curr. Pharm. Des. 2015, 21, 1741–1763. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Bermudez, M.A.; Seoane, S.; Perez-Fernandez, R.; Krupa, M.; Pietraszek, A.; Chodynski, M.; Kutner, A.; Brown, G.; Marcinkowska, E. Biological evaluation of new vitamin D2 analogues. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Rech, M.; Meineke, V.; Tilgen, W.; Reichrath, J. Differential biological effects of 1,25-dihydroxyvitamin D3 on melanoma cell lines in vitro. J. Steroid Biochem. Mol. Biol. 2004, 89, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, S.; Chodynski, M.; Krajewski, K.; Cmoch, P.; Marcinkowska, E.; Brown, G.; Kutner, A. Convergent synthesis of double point modified analogs of 1α,25-dihydroxyvitamin D2 for biological evaluation. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; VanMiddlesworth, L.; Slominski, A.T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer 2008, 123, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, A.; Wierzbicka, J.; Nadkarni, S.; Brown, G.; Kutner, A.; Żmijewski, M.A. Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D2 Against Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2016, 17, 76. https://doi.org/10.3390/ijms17010076
Piotrowska A, Wierzbicka J, Nadkarni S, Brown G, Kutner A, Żmijewski MA. Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D2 Against Human Malignant Melanoma Cell Lines. International Journal of Molecular Sciences. 2016; 17(1):76. https://doi.org/10.3390/ijms17010076
Chicago/Turabian StylePiotrowska, Anna, Justyna Wierzbicka, Sharmin Nadkarni, Geoffrey Brown, Andrzej Kutner, and Michał A. Żmijewski. 2016. "Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D2 Against Human Malignant Melanoma Cell Lines" International Journal of Molecular Sciences 17, no. 1: 76. https://doi.org/10.3390/ijms17010076
APA StylePiotrowska, A., Wierzbicka, J., Nadkarni, S., Brown, G., Kutner, A., & Żmijewski, M. A. (2016). Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D2 Against Human Malignant Melanoma Cell Lines. International Journal of Molecular Sciences, 17(1), 76. https://doi.org/10.3390/ijms17010076