Treadmill Exercise Attenuates Retinal Oxidative Stress in Naturally-Aged Mice: An Immunohistochemical Study
Abstract
:1. Introduction
2. Results
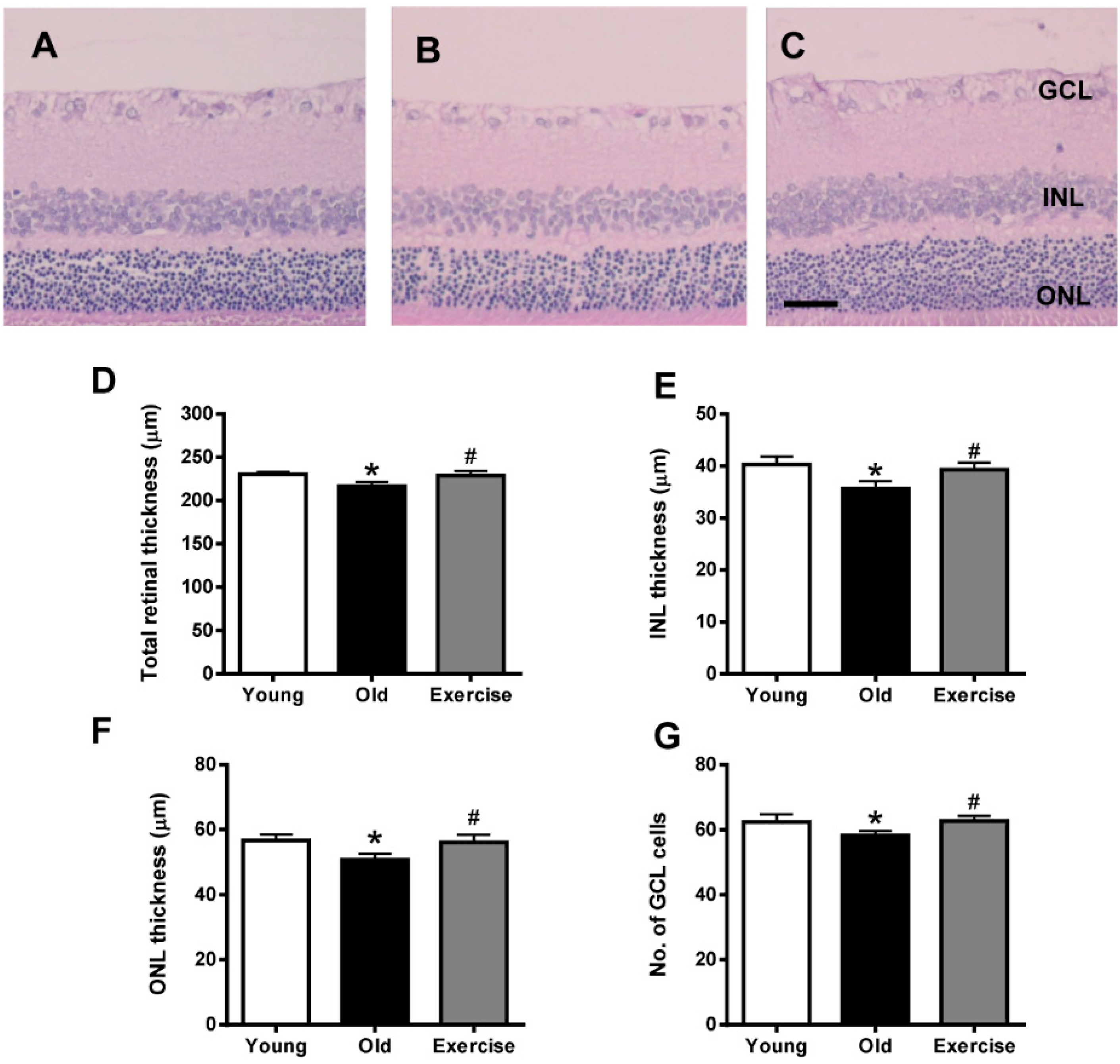
2.1. Histopathology
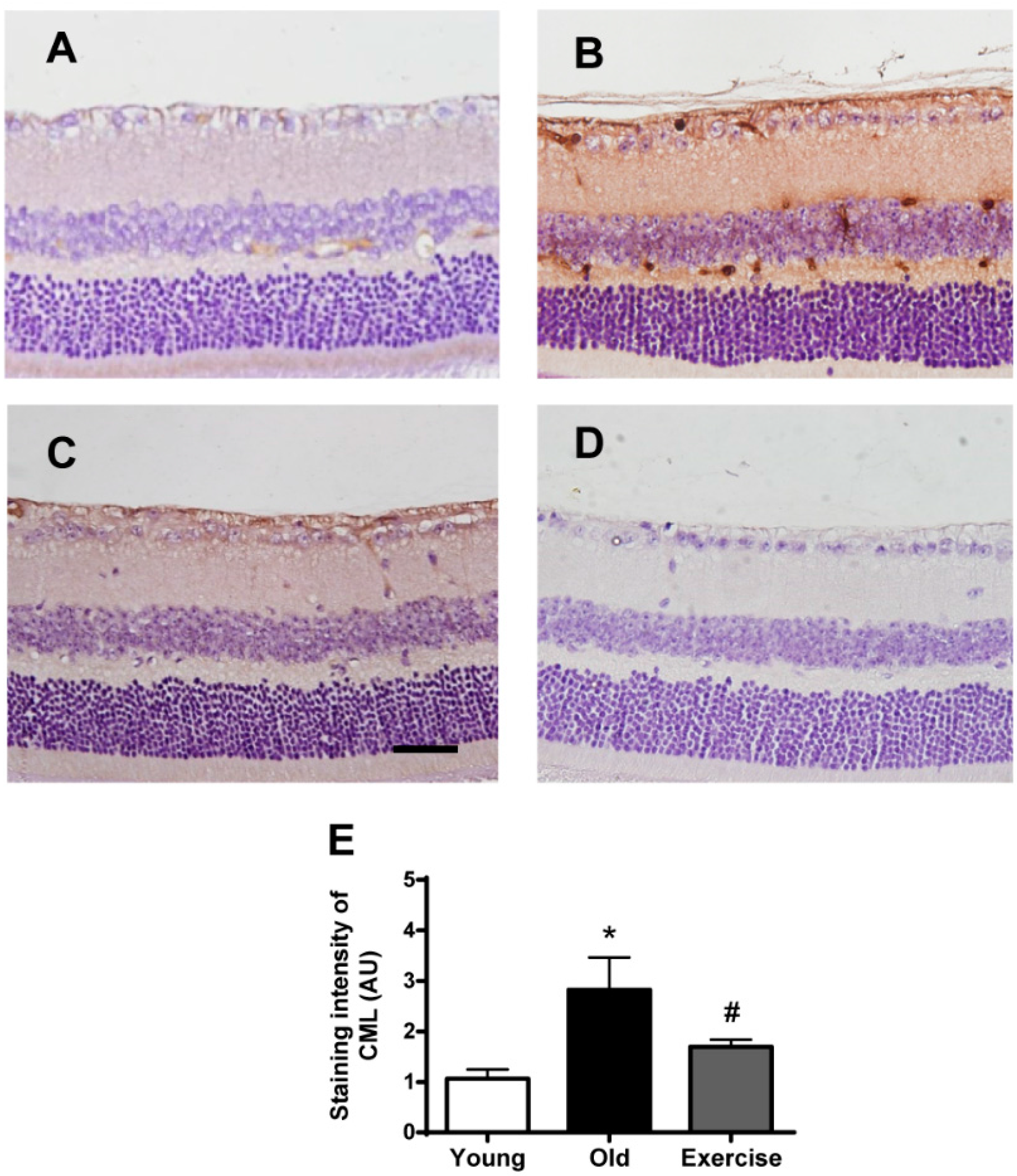
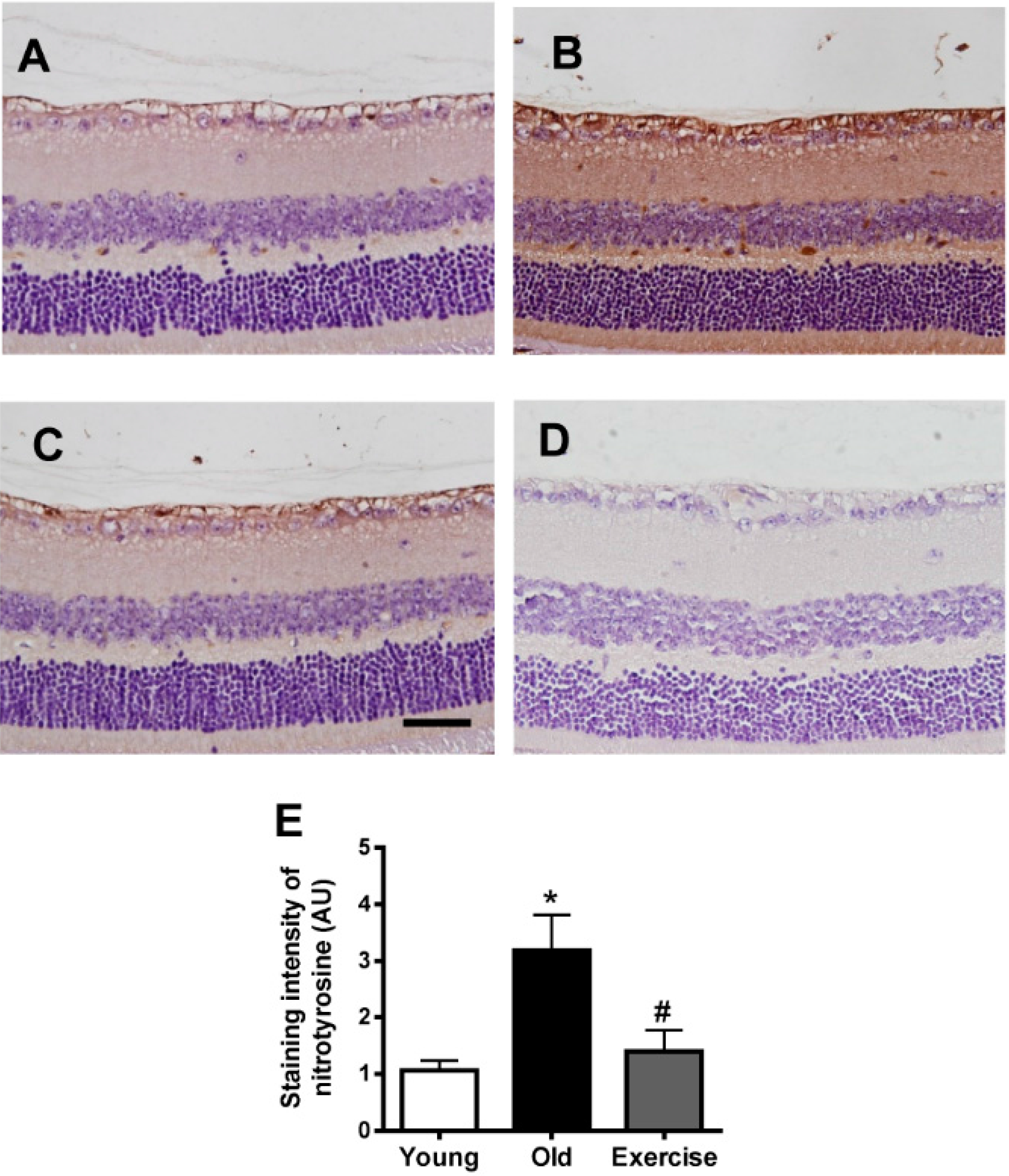
2.2. Changes in Oxidative Stress Markers in the Renal Tissues

3. Discussion
4. Experimental Section
4.1. Animals and Experimental Design
4.2. Histopathology
4.3. Immunohistochemical Staining for Oxidative Stress Markers
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hollyfield, J.G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-related alterations in neurons of the mouse retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef] [PubMed]
- Doly, M.; Droy-Lefaix, M.T.; Braquet, P. Oxidative stress in diabetic retina. In Free Radicals and Aging; Emerit, I., Chance, B., Eds.; Birkhäuser Basel: Basel, Switzerland, 1992; pp. 299–307. [Google Scholar]
- Sanvicens, N.; Cotter, T.G. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J. Neurochem. 2006, 98, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M. Ageing of the retina and retinal pigment epithelium. In Age-Related Macular Degeneration; Holz, F.G., Pauleikhoff, D., et al., Eds.; Springer: Heidelberg, Germany, 2013; pp. 45–63. [Google Scholar]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Forrester, J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009, 28, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.; George, L.; Lokhandwala, M.F. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am. J. Physiol. Ren. Physiol. 2007, 293, F914–F919. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Gomez, C.; Lopez-Cepero, J.M.; Boveris, A. Beneficial effects of moderate exercise on mice aging: Survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Siol. Regul. Integr. Comp. Physiol. 2004, 286, R505–R511. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Vina, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, S.Y.; Kim, C.J.; Sung, Y.H.; Kim, J.D. Treadmill exercise ameliorates apoptotic cell death in the retinas of diabetic rats. Mol. Med. Rep. 2013, 7, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.C.; Han, M.K.; Sellers, J.T.; Chrenek, M.A.; Hanif, A.; Gogniat, M.A.; Boatright, J.H.; Pardue, M.T. Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J. Neurosci. 2014, 34, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Neufeld, A.H. Increased mitochondrial DNA damage and down-regulation of DNA repair enzymes in aged rodent retinal pigment epithelium and choroid. Mol. Vis. 2008, 14, 644–651. [Google Scholar] [PubMed]
- Chrysostomou, V.; Kezic, J.M.; Trounce, I.A.; Crowston, J.G. Forced exercise protects the aged optic nerve against intraocular pressure injury. Neurobiol. Aging 2014, 35, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.D.; Wagner, E.; Nerlich, A.G. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J. Clin. Investig. 1997, 99, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. Oxidative decay of DNA. J. Biol. Chem. 1997, 272, 19633–19636. [Google Scholar] [CrossRef] [PubMed]
- Turko, I.V.; Murad, F. Protein nitration in cardiovascular diseases. Pharmacol. Rev. 2002, 54, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Verzijl, N.; Matsunaga, H.; Aotaki-Keen, A.; Lutty, G.A.; te Koppele, J.M.; Miyata, T.; Hjelmeland, L.M. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Investig. Ophthalmol. Vis. Sci. 1999, 40, 775–779. [Google Scholar]
- Imamura, Y.; Noda, S.; Hashizume, K.; Shinoda, K.; Yamaguchi, M.; Uchiyama, S.; Shimizu, T.; Mizushima, Y.; Shirasawa, T.; Tsubota, K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Thornalley, P.J. Mechanism of the degradation of non-enzymatically glycated proteins under physiological conditions. Studies with the model fructosamine, Nɛ-(1-deoxy-d-fructos-1-yl)hippuryl-lysine. Eur. J. Biochem. 1992, 210, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Ricci, C.; Scipioni, A.; Sansoni, V.; Mazzitelli, G.; Cordone, S.; Pesce, C.; Pugliese, F.; Pricci, F.; et al. Advanced lipoxidation end-products mediate lipid-induced glomerular injury: Role of receptor-mediated mechanisms. J. Pathol. 2009, 218, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.I.; Liu, C.J.; Wei, Y.H. Increase of 8-hydroxy-2ʹ-deoxyguanosine in aqueous humor of patients with exudative age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5486–5490. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ohshima, T.; Ishida, Y. Age-dependent expression of 8-hydroxy-2ʹ-deoxyguanosine in human pituitary gland. Histochem. J. 2001, 33, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Fano, G.; Fulle, S.; MacGarvey, U.; Shinobu, L.; Polidori, M.C.; Cherubini, A.; Vecchiet, J.; Senin, U.; Beal, M.F. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic. Biol. Med. 1999, 26, 303–308. [Google Scholar] [CrossRef]
- Liles, M.R.; Newsome, D.A.; Oliver, P.D. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch. Ophthalmol. 1991, 109, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, R.S.; Fu, W.; Xu, L.; Lam, P.K. Production of reactive oxygen species and 8-hydroxy-2ʹdeoxyguanosine in KB cells co-exposed to benzo[a]pyrene and UV-A radiation. Chemosphere 2004, 55, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Pechter, U.; Maaroos, J.; Mesikepp, S.; Veraksits, A.; Ots, M. Regular low-intensity aquatic exercise improves cardio-respiratory functional capacity and reduces proteinuria in chronic renal failure patients. Nephrol. Dial. Transplant. 2003, 18, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin, I.; Leehey, D.J. A comparison of aerobic exercise and resistance training in patients with and without chronic kidney disease. Adv. Chronic Kidney Dis. 2008, 15, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Coelho, B.L.; Rocha, L.G.; Scarabelot, K.S.; Scheffer, D.L.; Ronsani, M.M.; Silveira, P.C.; Silva, L.A.; Souza, C.T.; Pinho, R.A. Physical exercise prevents the exacerbation of oxidative stress parameters in chronic kidney disease. J. Ren. Nutr. 2010, 20, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.; Celec, P.; Behuliak, M.; Grancic, P.; Kebis, A.; Kukan, M.; Pronayova, N.; Liptaj, T.; Ostendorf, T.; Sebekova, K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism 2009, 58, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.H.; Shih, C.T.; Ching, C.H.; Huang, J.Y.; Jen, C.J.; Yu, L.; Kuo, Y.M.; Wu, F.S.; Chuang, J.I. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp. Neurol. 2015, 263, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.W.; Park, C.H.; Kang, S.S.; Cho, G.J.; Yoo, J.M.; Song, J.K.; Choi, W.S. Nitric oxide synthase expression in the transient ischemic rat retina: Neuroprotection of betaxolol. Neurosci. Lett. 2002, 330, 265–269. [Google Scholar] [CrossRef]
- Goto, R.; Doi, M.; Ma, N.; Semba, R.; Uji, Y. Contribution of nitric oxide-producing cells in normal and diabetic rat retina. Jpn. J. Ophthalmol. 2005, 49, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.H.; Kim, Y.H.; Park, C.K. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vis. Res. 2007, 47, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.K.; Chung, I.W.; Kim, K.Y.; Gwon, J.S.; Lee, M.Y.; Oh, S.J.; Chun, M.H. Sodium nitroprusside selectively induces apoptotic cell death in the outer retina of the rat. Neuroreport. 2001, 12, 4075–4079. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; van Zanten, J.J.C.S.V.; Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Smith, J.P.; Kitas, G.D.; Aldred, S. Three months of moderate-intensity exercise reduced plasma 3-nitrotyrosine in rheumatoid arthritis patients. Eur. J. Appl. Physiol. 2014, 114, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Adams, V.; Schulze, P.C.; Erbs, S.; Gielen, S.; Fiehn, E.; Mobius-Winkler, S.; Schubert, A.; Schuler, G.; Hambrecht, R. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 2005, 111, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Jadhav, K.S.; Williamson, D.L.; Rideout, T.C. Treadmill exercise training modulates hepatic cholesterol metabolism and circulating PCSK9 concentration in high-fat-fed mice. J. Lipids 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.L.; Mouisel, E.; Roblot, N.; Melki, J. Inter- and intrastrain variation in mouse critical running speed. J. Appl. Physiol. 2005, 98, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, C.S.; Lee, J.; Suk Kim, J.; Kim, J. Effect of regular exercise on the histochemical changes of d-galactose-induced oxidative renal injury in high-fat diet-fed rats. Acta Histochem. Cytochem. 2013, 46, 111–119. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-S.; Park, S.; Chun, Y.; Song, W.; Kim, H.-J.; Kim, J. Treadmill Exercise Attenuates Retinal Oxidative Stress in Naturally-Aged Mice: An Immunohistochemical Study. Int. J. Mol. Sci. 2015, 16, 21008-21020. https://doi.org/10.3390/ijms160921008
Kim C-S, Park S, Chun Y, Song W, Kim H-J, Kim J. Treadmill Exercise Attenuates Retinal Oxidative Stress in Naturally-Aged Mice: An Immunohistochemical Study. International Journal of Molecular Sciences. 2015; 16(9):21008-21020. https://doi.org/10.3390/ijms160921008
Chicago/Turabian StyleKim, Chan-Sik, Sok Park, Yoonseok Chun, Wook Song, Hee-Jae Kim, and Junghyun Kim. 2015. "Treadmill Exercise Attenuates Retinal Oxidative Stress in Naturally-Aged Mice: An Immunohistochemical Study" International Journal of Molecular Sciences 16, no. 9: 21008-21020. https://doi.org/10.3390/ijms160921008





