Quantum Chemical and Kinetic Study on Polychlorinated Naphthalene Formation from 3-Chlorophenol Precursor
Abstract
:1. Introduction
 .
. ) produced by CO loss of CPRs [26,27,28,29], based on the naphthalene formation mechanism proposed by Melius [41]. Recombination of two chloro-CPDyl radicals to form chlorinated dihydrofulvene (
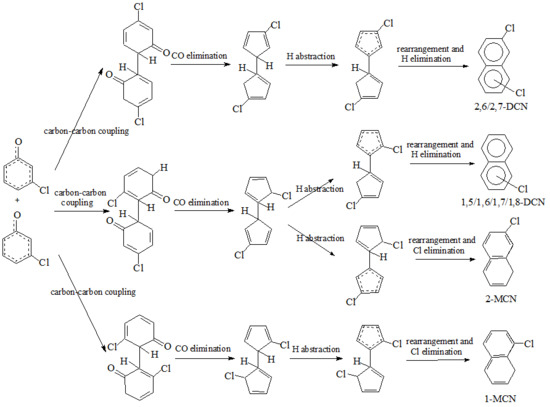
) produced by CO loss of CPRs [26,27,28,29], based on the naphthalene formation mechanism proposed by Melius [41]. Recombination of two chloro-CPDyl radicals to form chlorinated dihydrofulvene (  ), followed by rearrangement and H/Cl elimination can result in the PCN’s formation [25,26,27,28]. In this mechanism, 2-CP, 3-CP, and 4-CP were speculated to form the same chloro-CPDyl radical and exactly the same PCN isomer patterns [26,27,28,29,32,33]. However, this hypothesis was experimentally opposed by Kim via obtaining completely different PCN isomer distributions from 2-CP, 3-CP, and 4-CP (Scheme 1) [32,33]. For example, in Kim’s experiment, 2-CP produced mostly 1-MCN and 1,5/1,6/1,7-DCNs; 4-CP produced mostly 2-MCN and 2,6/2,7-DCNs; 3-CP produced nearely equivalent 1-MCN and 2-MCN, and nearly equal 1,5/1,6/1,7-DCNs and 2,6/2,7-DCNs [32,33]. In addition, this hypothesis was, again, denied by some experimental observations in thermal processes that the correlation between PCN and PCDF isomer distributions or mass concentrations is more closer than that between PCN and PCDD, which indicate a new PCN formation mechanism more similar with PCDF formation than PCDD [30,31,42,43,44,45,46]. In this situation, Kim proposed an alternative PCN formation mechanism based on experimental results [32,33,34]; in his scheme, PCNs are formed via carbon-carbon coupling at ortho-sites of CPR pairs, resulting in an intermediate chlorinated o,oʹ-dihydroxybiphenyl (chloro-DOHB) [32,33,34]. Chlorinated dihydrofulvene are formed from chloro-DOHB by two CO loss and ring close steps, not by condensation of two chloro-CPDyl radicals [32,33,34]. Both PCDF and PCN can be produced from chloro-DOHB by different tautomerization steps [32,33,34].
), followed by rearrangement and H/Cl elimination can result in the PCN’s formation [25,26,27,28]. In this mechanism, 2-CP, 3-CP, and 4-CP were speculated to form the same chloro-CPDyl radical and exactly the same PCN isomer patterns [26,27,28,29,32,33]. However, this hypothesis was experimentally opposed by Kim via obtaining completely different PCN isomer distributions from 2-CP, 3-CP, and 4-CP (Scheme 1) [32,33]. For example, in Kim’s experiment, 2-CP produced mostly 1-MCN and 1,5/1,6/1,7-DCNs; 4-CP produced mostly 2-MCN and 2,6/2,7-DCNs; 3-CP produced nearely equivalent 1-MCN and 2-MCN, and nearly equal 1,5/1,6/1,7-DCNs and 2,6/2,7-DCNs [32,33]. In addition, this hypothesis was, again, denied by some experimental observations in thermal processes that the correlation between PCN and PCDF isomer distributions or mass concentrations is more closer than that between PCN and PCDD, which indicate a new PCN formation mechanism more similar with PCDF formation than PCDD [30,31,42,43,44,45,46]. In this situation, Kim proposed an alternative PCN formation mechanism based on experimental results [32,33,34]; in his scheme, PCNs are formed via carbon-carbon coupling at ortho-sites of CPR pairs, resulting in an intermediate chlorinated o,oʹ-dihydroxybiphenyl (chloro-DOHB) [32,33,34]. Chlorinated dihydrofulvene are formed from chloro-DOHB by two CO loss and ring close steps, not by condensation of two chloro-CPDyl radicals [32,33,34]. Both PCDF and PCN can be produced from chloro-DOHB by different tautomerization steps [32,33,34].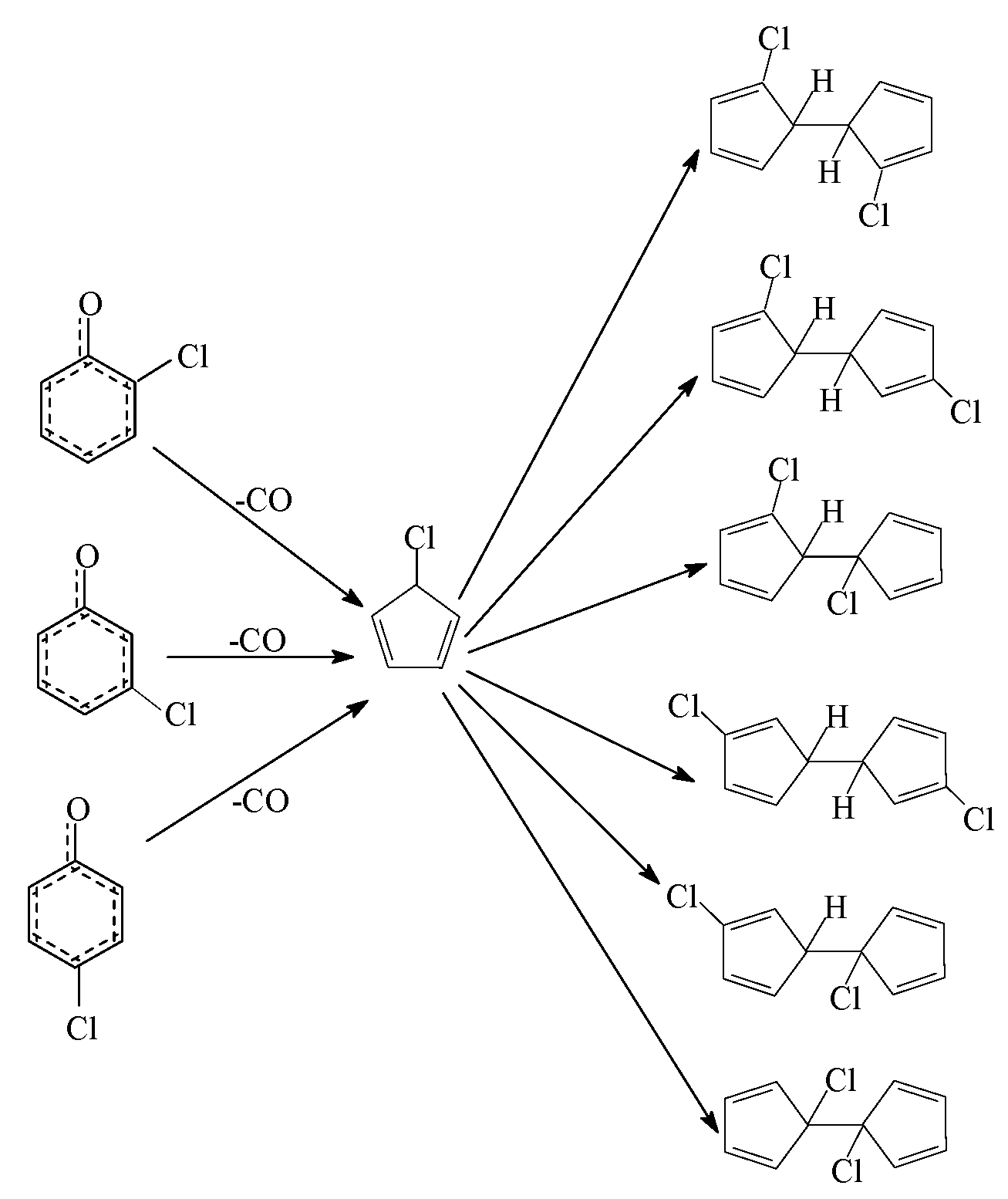
2. Results and Discussion
- 3-CP + H → 3-CPR + H2 ΔE = 12.51 kcal/mol ΔH = −12.94 kcal/mol
- 3-CP + OH → 3-CPR + H2O ΔE = 0.17 kcal/mol ΔH = −27.81 kcal/mol
- 3-CP + O(3P) → 3-CPR + OH ΔE = 8.20 kcal/mol ΔH = −12.04 kcal/mol
- 3-CP + Cl → 3-CPR + HCl ΔE = −5.87 kcal/mol ΔH = −15.66 kcal/mol
2.1. Formation of Chloro-Dihydrofulvene from Dimerization of 3-CPRs
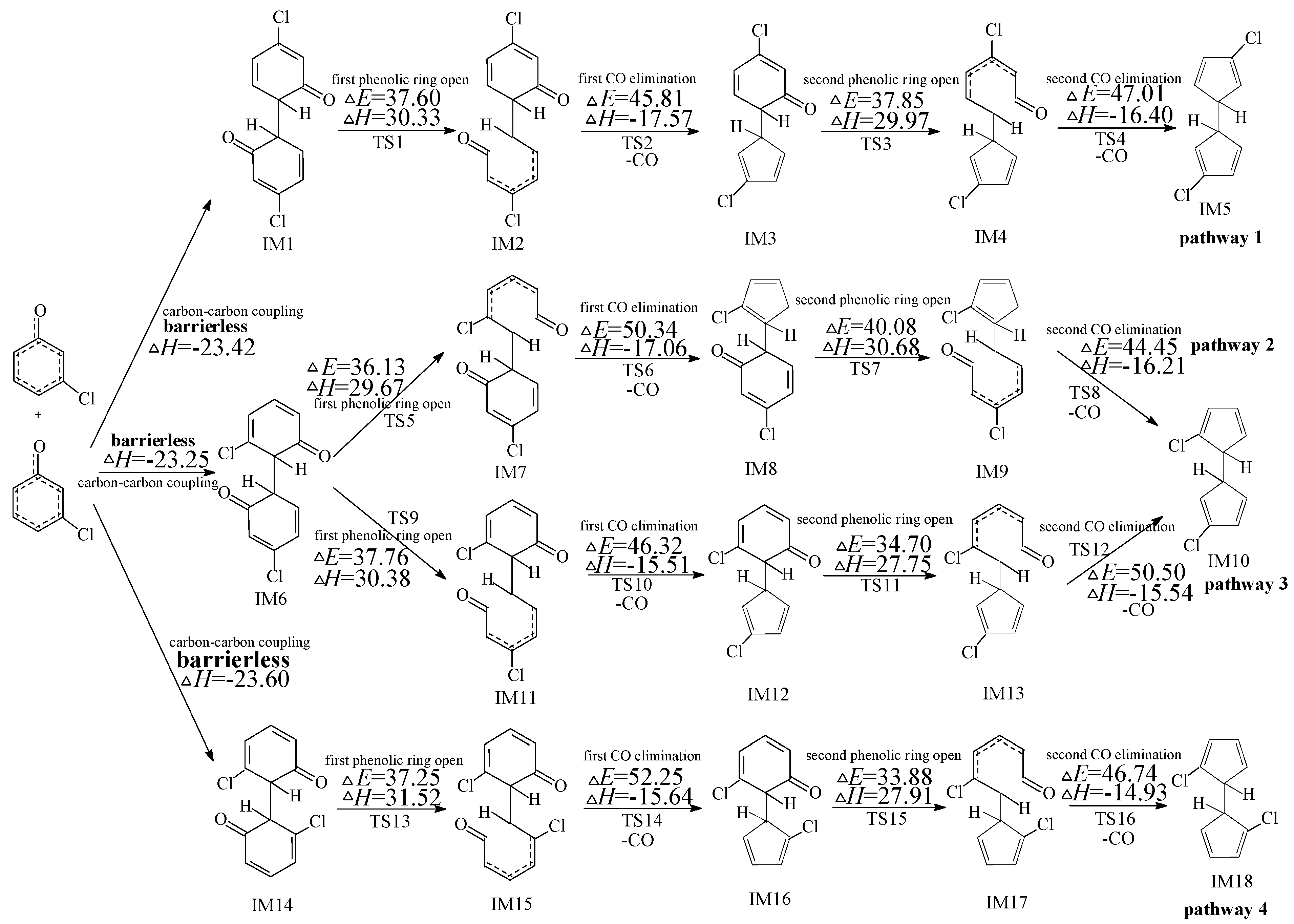
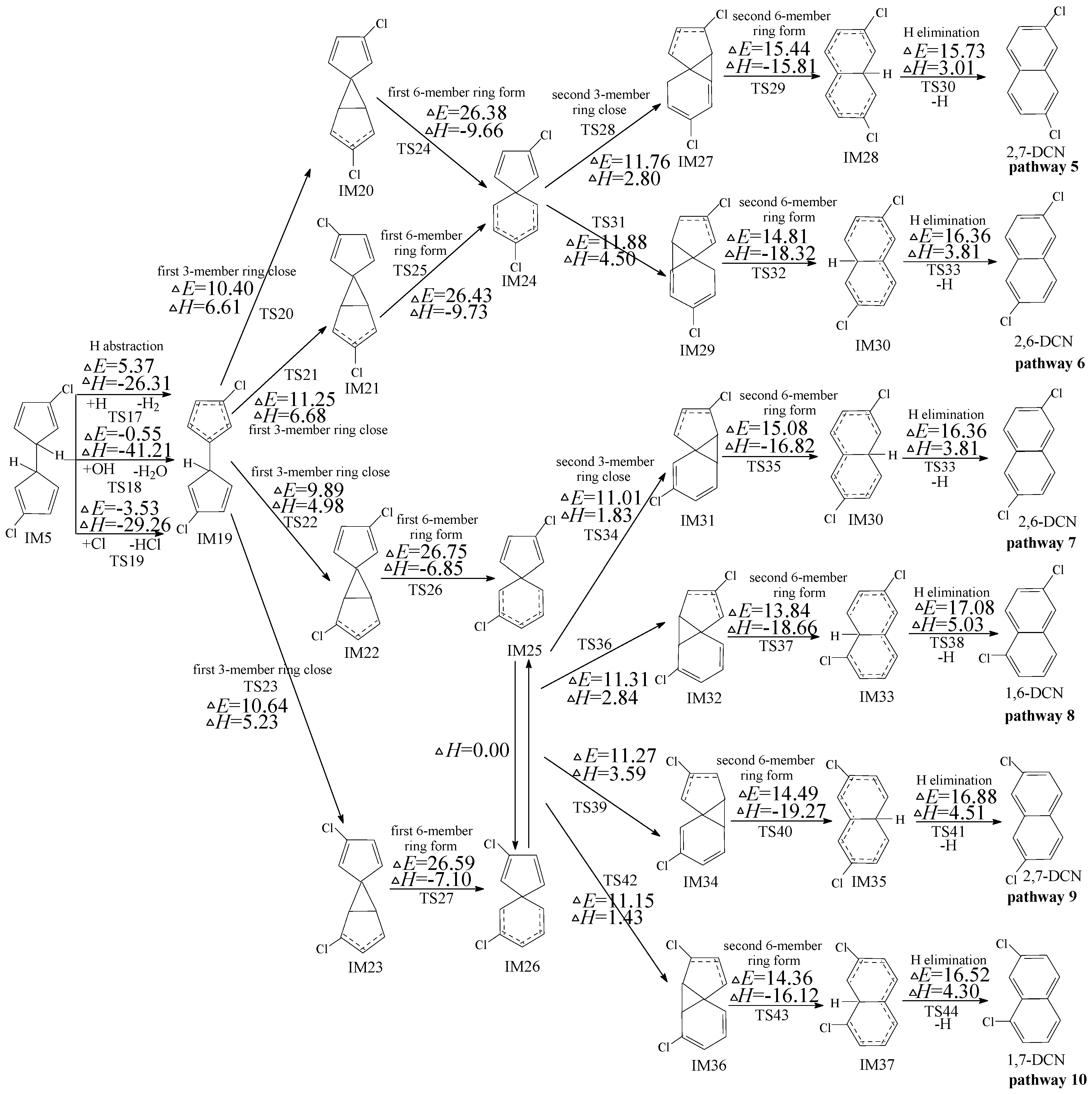
2.2. Formation of PCNs from Subsequent Reactions of IM5
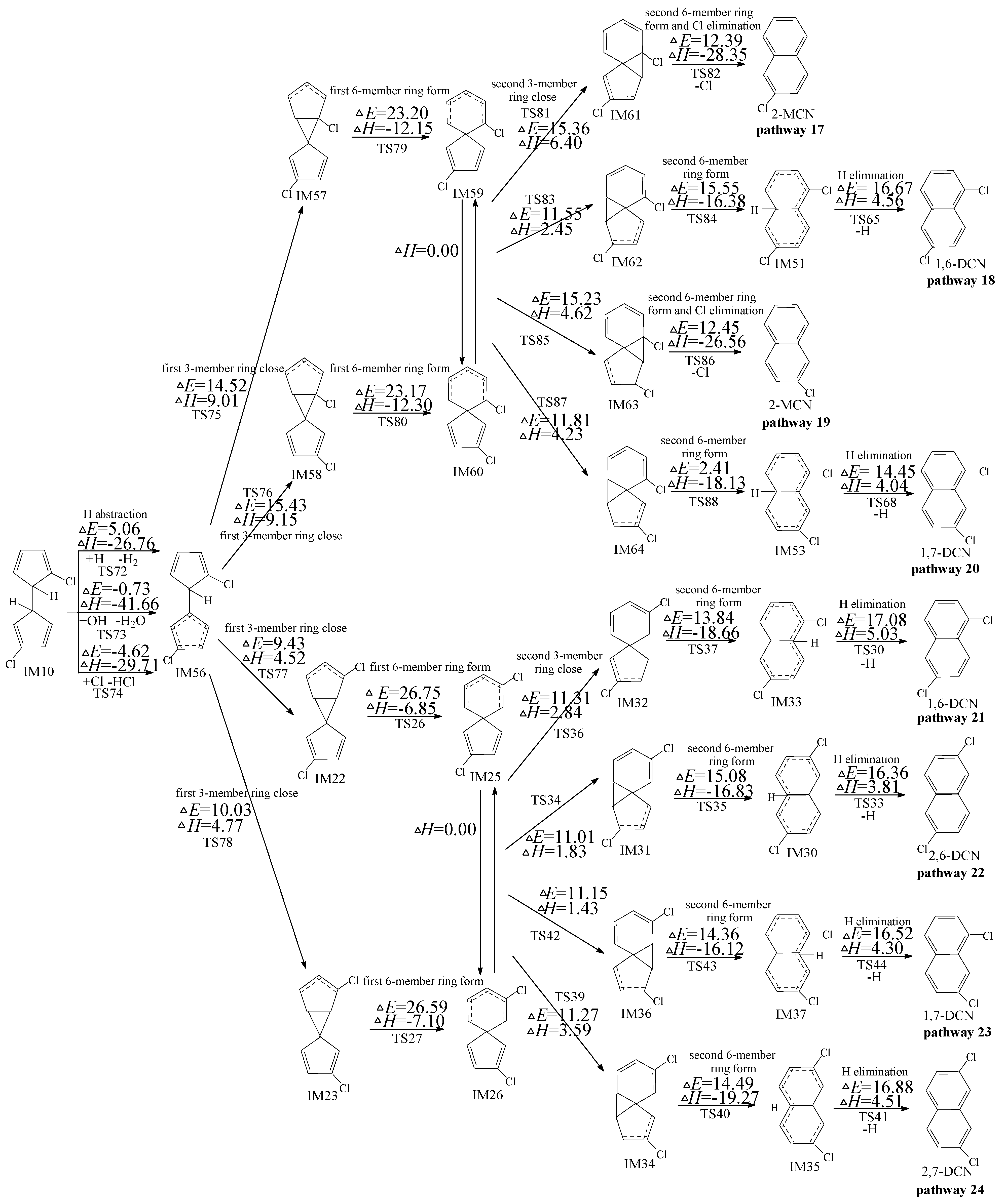
2.3. Formation of PCNs from Subsequent Reactions of IM10

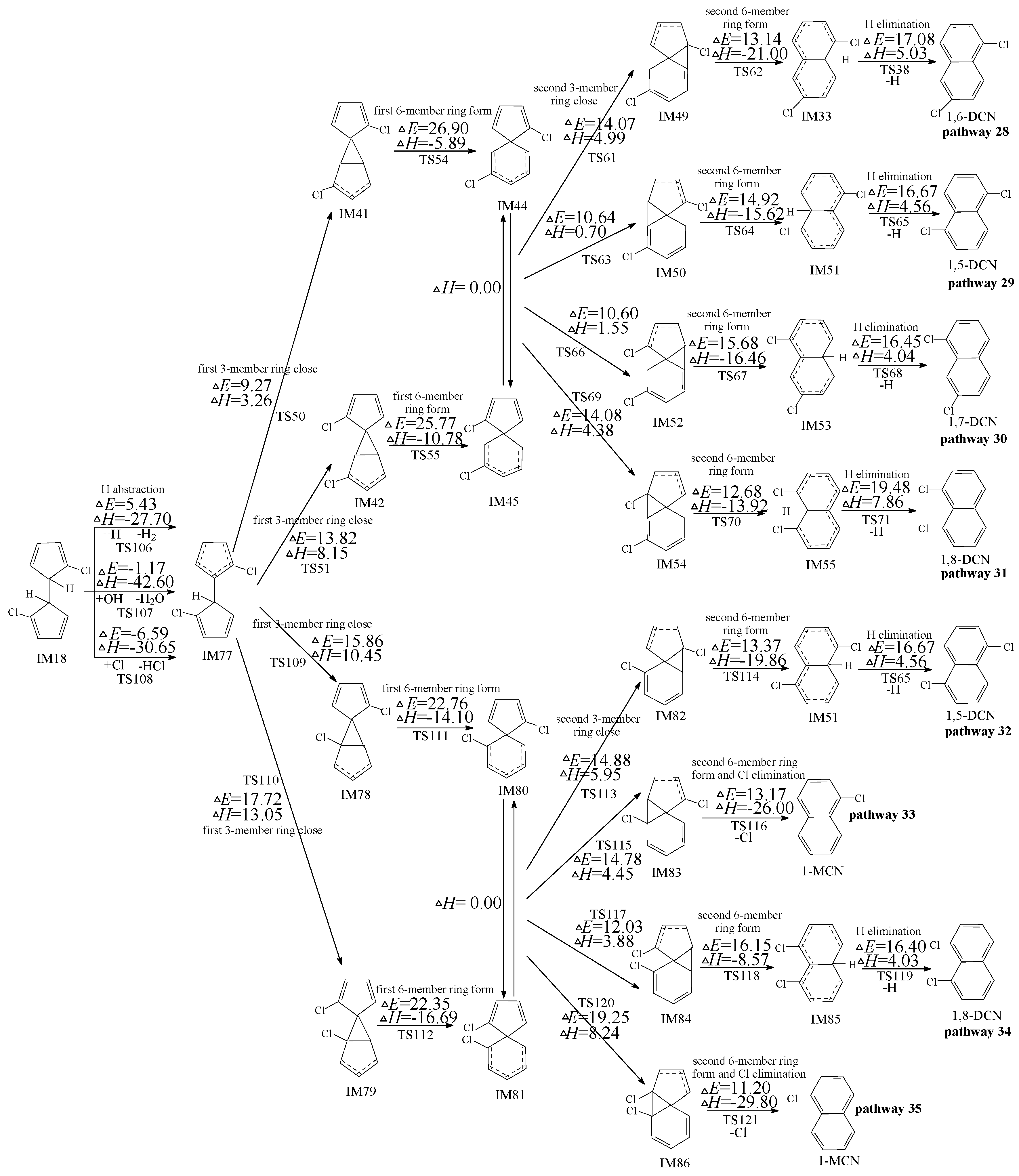
2.4. Formation of PCNs from Subsequent Reactions of IM18
2.5. Comparison of PCN Formation Mechanism with the Experimental Observation
2.6. Comparison of PCN Formation from 2-CP, 3-CP, and 4-CP
2.7. Rate Constant Calculations
 are matches well with the available literature rate constant values [59,60] for structurally-similar compounds
are matches well with the available literature rate constant values [59,60] for structurally-similar compounds  . Actually, the CVT/SCT method has been successfully performed for the the rate calculations in our previous studies involved in the formation of PCDD/Fs from various CP precursors [55,56,57,58] and PCN formation from 2-CP and 4-CP precursors [47,48], and are clarified to be an efficient method to calculate the rate constants.
. Actually, the CVT/SCT method has been successfully performed for the the rate calculations in our previous studies involved in the formation of PCDD/Fs from various CP precursors [55,56,57,58] and PCN formation from 2-CP and 4-CP precursors [47,48], and are clarified to be an efficient method to calculate the rate constants.| Reactions | Arrhenius Formulas |
|---|---|
| 3-TCP + H → 3-MCPR + H2 | k(T) = (2.36 × 10−12) exp (−6579.12/T) |
| 3-TCP + OH → 3-MCPR + H2O | k(T) = (3.12 × 10−12) exp (−1512.16/T) |
| 3-TCP + O(3P) → 3-MCPR + OH | k(T) = (3.50 × 10−11) exp (−4659.85/T) |
| IM1 → IM2 via TS1 | k(T) = (4.06 × 1013) exp (−20,030.86/T) |
| IM2 → IM3 + CO via TS2 | k(T) = (9.43 × 109) exp (−24,696.87/T) |
| IM3 → IM4 via TS3 | k(T) = (5.13 × 1013) exp (−20,235.56/T) |
| IM4 → IM5 + CO via TS4 | k(T) = (2.51 × 1011) exp (−23,661.43/T) |
| IM6 → IM7 via TS5 | k(T) = (3.64 × 1013) exp (−20,292.91/T) |
| IM7 → IM8 + CO TS6 | k(T) = (1.68 × 1011) exp (−25,390.77/T) |
| IM8 → IM9 via TS7 | k(T) = (2.35 × 1013) exp (−36,819.88/T) |
| IM9 → IM10 + CO via TS8 | k(T) = (4.10 × 1012) exp (−22,081.08/T) |
| IM6 → IM11 via TS9 | k(T) = (1.10 × 1014) exp (−19,945.07/T) |
| IM11 → IM12 + CO via TS10 | k(T) = (4.43 × 1011) exp (−23,531.77/T) |
| IM12 → IM13 via TS11 | k(T) = (1.14 × 105) exp (−18,756.53/T) |
| IM13 → IM10 + CO via TS12 | k(T) = (5.95 × 109) exp (−22,995.33/T) |
| IM14 → IM15 via TS13 | k(T) = (5.70 × 1013) exp (−19,945.04/T) |
| IM15 → IM16 + CO via TS14 | k(T) = (7.21 × 101°) exp (−26,688.66/T) |
| IM16 → IM17 via TS15 | k(T) = (1.53 × 1013) exp (−17,862.44/T) |
| IM17 → IM18 + CO via TS16 | k(T) = (3.25 × 1011) exp (−23,917.06/T) |
| IM10 + H → IM56 + H2 via TS72 | k(T) = (6.92 × 10−11) exp (−2926.10/T) |
| IM56 → IM57 via TS75 | k(T) = (1.37 × 1012) exp (−7741.24/T) |
| IM56 → IM58 via TS76 | k(T) = (1.08 × 1012) exp (−8010.75/T) |
| IM57 → IM59 via TS79 | k(T) = (1.67 × 1013) exp (−11,934.50/T) |
| IM58 → IM60 via TS80 | k(T) = (6.96 × 1013) exp (−11,934.96/T) |
| IM59/IM60 → IM61 via TS81 | k(T) = (2.24 × 1011) exp (−9660.05/T) |
| IM61 → 2-MCN + Cl via TS82 | k(T) = (2.21 × 1013) exp (−6651.99/T) |
| IM59/IM60 → IM63 via TS85 | k(T) = (3.02 × 1012) exp (−7870.98/T) |
| IM63 → 2-MCN + Cl via TS86 | k(T) = (1.89 × 1013) exp (−6658.32/T) |
| IM18 + H→IM77 + H2 via TS106 | k(T)= (6.77 × 10−13) exp (−3847.80/T) |
| IM77 → IM78 via TS109 | k(T) = (3.10 × 1012) exp (−8185.85/T) |
| IM77→ IM79 via TS110 | k(T) = (4.05 × 1012) exp (−9142.08/T) |
| IM78 → IM80 via TS111 | k(T) = (1.70 × 1013) exp (−11,846.10/T) |
| IM79 → IM81 via TS112 | k(T) = (1.82 × 1013) exp (−11,683.31/T) |
| IM80/IM81 → IM83 via TS115 | k(T) = (2.73 × 1012) exp (−7528.18/T) |
| IM83 → 1-MCN + Cl via TS116 | k(T) = (1.98 × 1013) exp (−6998.83/T) |
| IM80/IM81 → IM86 via TS120 | k(T) = (7.40 × 1011) exp (−8061.95/T) |
| IM86 → 1-MCN + Cl via TS121 | k(T) = (3.52 × 1013) exp (−6073.38/T) |
3. Experimental Section
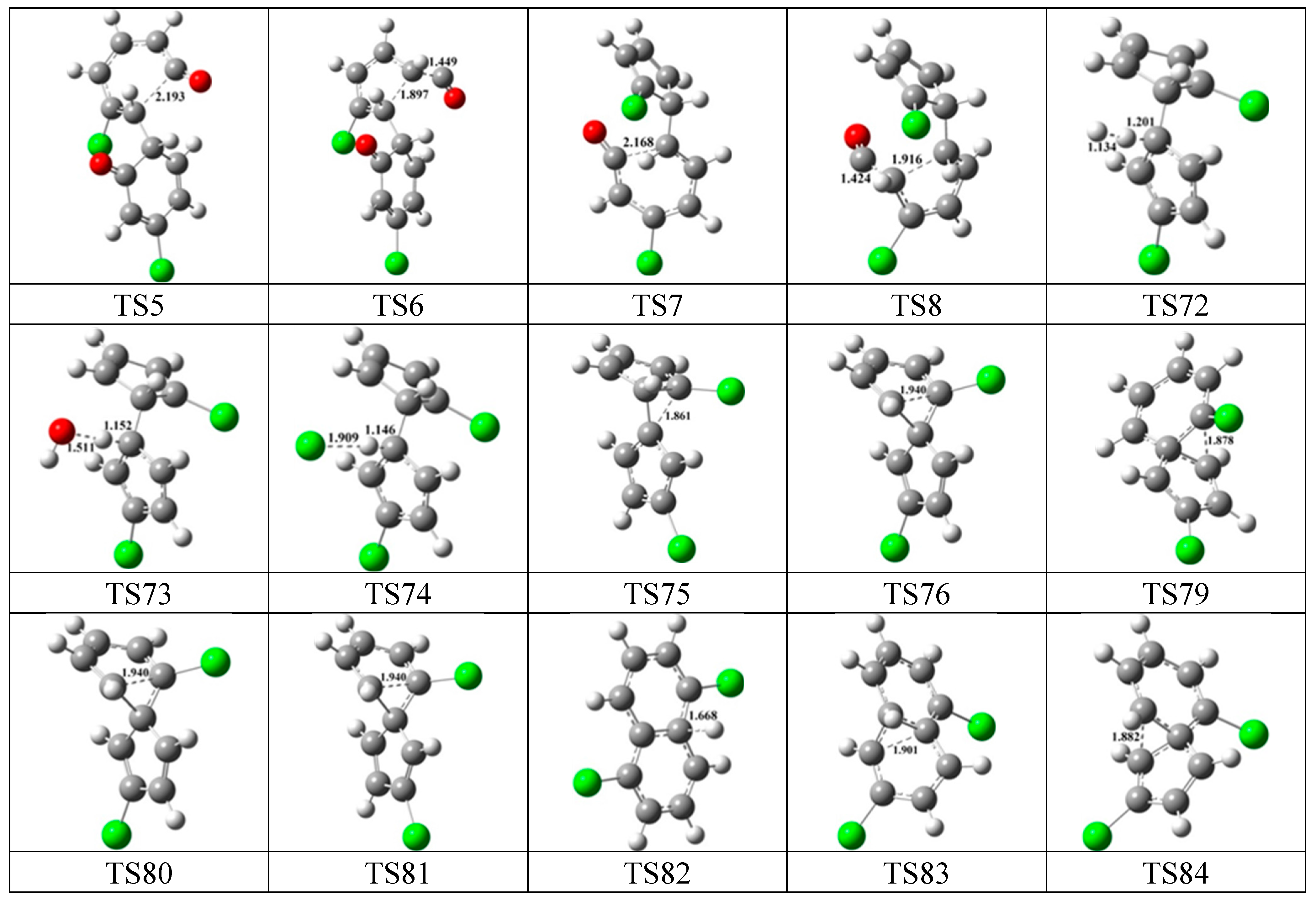
3.1. Density Functional Theory
3.2. Kinetic Calculation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohura, T. Environmental behavior, sources, and effects of chlorinated polycyclic aromatic hydrocarbons. Sci. World J. 2007, 7, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, Z.; Wu, M.; Feng, J.; Horii, Y.; Ohura, T.; Kannan, K. Airborne PM 2.5/PM 10-associated chlorinated polycyclic aromatic hydrocarbons and their parent compounds in a suburban area in Shanghai, China. Environ. Sci. Technol. 2013, 47, 7615–7623. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Morita, M.; Makino, M.; Amagai, T.; Shimoi, K. Aryl hydrocarbon receptor-mediated effects of chlorinated polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2007, 20, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Khim, J.S.; Higley, E.B.; Giesy, J.P.; Ohura, T.; Kannan, K. Relative potencies of individual chlorinated and brominated polycyclic aromatic hydrocarbons for induction of aryl hydrocarbon receptor-mediated responses. Environ. Sci. Technol. 2009, 43, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Kitazawa, A.; Amagai, T.; Makino, M. Occurrence, profiles, and photostabilities of chlorinated polycyclic aromatic hydrocarbons associated with particulates in urban air. Environ. Sci. Technol. 2005, 39, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, A.; Amagai, T.; Ohura, T. Temporal trends and relationships of particulate chlorinated polycyclic aromatic hydrocarbons and their parent compounds in urban air. Environ. Sci. Technol. 2006, 40, 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Suuberg, E.M. Thermochemical properties and phase behavior of halogenated polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 2012, 31, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Sakakibara, H.; Ohura, T.; Guruge, K.S.; Kojima, M.; Hasegawa, J.; Iwamura, T.; Yamanaka, N.; Masuda, S.; Sakaguchi, M.; et al. Evaluation of chlorinated benz[a]anthracene on hepatic toxicity in rats and mutagenic activity in Salmonella typhimurium. Environ. Toxicol. 2013, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Colmsjo, A.; Rannug, A.; Rannug, U. Some chloro derivatives of polynuclear aromatic hydrocarbons are potent mutagens in Salmonella typhimurium. Mutat. Res. 1984, 135, 21–29. [Google Scholar] [CrossRef]
- Hu, J.; Jin, X.; Kunikane, S.; Terao, Y.; Aizawa, T. Transformation of pyrene in aqueous chlorination in the presence and absence of bromide ion: Kinetics, products, and their aryl hydrocarbon receptormediated activities. Environ. Sci. Technol. 2006, 40, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Sankoda, K.; Nomiyama, K.; Yonehara, T.; Kuribayashi, T.; Shinohara, R. Evidence for in situ production of chlorinated polycyclic aromatic hydrocarbons on tidal flats: Environmental monitoring and laboratory scale experiment. Chemosphere 2012, 88, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Katagiri, Y.; Kaneko, M.; Watanabe, T.; Hirayama, T. Chlorination of pyrene in soil components with sodium chloride under xenon irradiation. Chemosphere 1999, 38, 1937–1945. [Google Scholar] [CrossRef]
- Nilsson, U.L.; Oestman, C.E. Chlorinated polycyclic aromatic hydrocarbons: Method of analysis and their occurrence in urban air. Environ. Sci. Technol. 1993, 27, 1826–1831. [Google Scholar] [CrossRef]
- Ishaq, R.; Naf, C.; Yngve, Z.; Broman, D.; Jarnberg, U. PCBs, PCNs, PCDD/Fs, PAHs and Cl-PAHs in air and water particulate samples–Patterns and variations. Chemosphere 2003, 50, 1131–1150. [Google Scholar] [CrossRef]
- Sankoda, K.; Kuribayashi, T.; Nomiyama, K.; Shinohara, R. Occurrence and source of chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) in tidal flats of the ariake bay, Japan. Environ. Sci. Technol. 2013, 47, 7037–7044. [Google Scholar] [CrossRef] [PubMed]
- Brodskv, E.S.; Klyuev, N.A.; Soyfer, V.S.; Ibragimov, V.A. Chlorinated polycyclic aromatic hydrocarbons in the waste water of pulp and paper mills. Organohalogen Compd. 1999, 43, 127–130. [Google Scholar]
- Altwicker, E.R. Relative rates of formation of polychlorinated dioxins and furans from precursor and de novo reactions. Chemosphere 1996, 33, 1897–1904. [Google Scholar] [CrossRef]
- Nielson, A.H.; Allard, A.S.; Hynning, P.A.; Rememberger, M. Distribution, fate and persistence of organochlorine compounds formed during production of bleached pulp. Toxicol. Environ. Chem. 1991, 30, 3–41. [Google Scholar] [CrossRef]
- Ohlenbusch, G.; Kumke, M.U.; Frimmel, F.H. Sorption of phenols to dissolved organic matter investigated by solid phase microextraction. Sci. Total Environ. 2000, 253, 63–74. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.F.; Dong, W.B.; Pan, X.X.; Hou, H.Q. Reaction mechanism of 3-chlorophenol with OH, H in aqueous solution. J. Environ. Sci. 2003, 15, 55–59. [Google Scholar]
- Her, T.M.; Lee, L.S.; Hsum, S.C. Solid-liquid equilibria of mixtures containing tert-butanol, m-chlorophenol, and pchlorophenol and development of adductive crystallization processes. Fluid Phase Equilib. 2005, 237, 152–161. [Google Scholar] [CrossRef]
- Hazardous Substance Fact Sheet. Available online: http://nj.gov/health/eoh/rtkweb/documents/fs/0402.pdf (accessed on 31 August 2015).
- Olie, K.; Vermeulen, P.L.; Hutzinger, O. Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some incinerators in the Netherlands. Chemosphere 1977, 6, 455–459. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Clement, R.E.; Karasek, F.W. Analysis of fly ash from municipal incinerators for trace organic compounds. Anal. Chem. 1979, 51, 2343–2350. [Google Scholar] [CrossRef]
- Imagawa, T.; Takeuchi, M. Relation between isomer compositions of polychlorinated naphthalens and congener compositions of PCDDs/PCDFs from incinerators. Organohalogen Compd. 1995, 23, 487–490. [Google Scholar]
- Akki, U.; Mulholland, J.A. Gas-phase formation of dioxin and other aromatic products from 2,6-dichlorophenol pyrolysis. Organohalogen Compd. 1997, 31, 475–479. [Google Scholar]
- Yang, Y.; Mulholland, J.A.; Akki, U. Formation of furans by gasphase reactions of chlorophenols. Proc. Combust. Inst. 1998, 27, 1761–1768. [Google Scholar] [CrossRef]
- Evans, C.S.; Dellinger, B. Mechanisms of dioxin formation from the high-temperature pyrolysis of 2-chlorophenol. Environ. Sci. Technol. 2003, 37, 1325–1330. [Google Scholar] [CrossRef]
- Evans, C.S.; Dellinger, B. Mechanisms of dioxin formation from the high-temperature oxidation of 2-chlorophenol. Environ. Sci. Technol. 2005, 39, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.L.; Thomas, G.O.; Bailey, R.; Kerstiens, G.; Jones, K.C. Exchange of Polychlorinated Biphenyls (PCBs) and Polychlorinated Naphthalenes (PCNs) between Air and a Mixed Pasture Sward. Environ. Sci. Technol. 2004, 38, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.N.C.; Jansson, S.; Marklund, S. Effects of regional differences in waste composition on the thermal formation of polychlorinated aromatics during incineration. Chemosphere 2013, 93, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Mulholland, J.A. Temperature-dependent formation of polychlorinated naphthalenes and dihenzofurans from chlorophenols. Environ. Sci. Technol. 2005, 39, 5831–5836. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Mulholland, J.A.; Ryu, J.Y. Formation of polychlorinated naphthalenes from chlorophenols. Proc. Combust. Inst. 2005, 30, 1245–1253. [Google Scholar] [CrossRef]
- Kim, D.H.; Mulholland, J.A.; Ryu, J.Y. Chlorinated naphthalene formation from the oxidation of dichlorophenols. Chemosphere 2007, 67, S135–S143. [Google Scholar] [CrossRef] [PubMed]
- Cypres, R.; Bettens, B. La formation de la plupart des composes aromatiques produits lors de la pyrolyse du phenol, ne fait pas intervenir le carbone porteur de la fonction hydroxyle. Tetrahedron 1975, 31, 359–365. [Google Scholar] [CrossRef]
- Manion, J.A.; Louw, R. Rates, products, and mechanisms in the gas-phase hydrogenolysis of phenol between 922 and 1175 K. J. Phys. Chem. 1989, 3, 3563–3574. [Google Scholar] [CrossRef]
- Nich, M.; Marinov, M.J.; Castaldi, C.F.M.; Wing, T. Aromatic and polycyclic aromatic hydrocarbon formation in a premixed propane flame. Combust. Sci. Technol. 1997, 128, 295–342. [Google Scholar]
- Friderichsen, A.V.; Shin, E.J.; Evans, R.J.; Nimlos, M.R.; Dayton, D.C.; Ellison, G.B. The pyrolysis of anisole (C6H5OCH3) using a hyperthermal nozzle. Fuel 2001, 80, 1747–1755. [Google Scholar] [CrossRef]
- Lu, M.M; Mulholland, J.A. PAH growth from the pyrolysis of CPD, indene and naphthalene mixture. Chemosphere 2004, 55, 605–610. [Google Scholar]
- Kim, D.H.; Mulholland, J.A.; Wang, D.; Violi, A. Pyrolytic hydrocarbon growth from cyclopentadiene. J. Phys. Chem. A 2010, 114, 12411–12416. [Google Scholar] [CrossRef] [PubMed]
- Melius, C.F.; Colvin, M.E.; Marinov, N.M.; Pitz, W.J.; Senkan, S.M. Reaction mechanisms in aromatic hydrocarbon formation involving the C5H5 cyclopentadienyl moiety. Symp. Int. Combust.Proc. 1996, 26, 685–692. [Google Scholar] [CrossRef]
- Imagawa, T.; Lee, C.W. Correlation of polychlorinated naphthalenes with polychlorinated dibenzofurans formed from waste incineration. Chemosphere 2001, 44, 1511–1520. [Google Scholar] [CrossRef]
- Weber, R.; Iino, F.; Imagawa, T.; Takeuchi, M.; Sakurai, T.; Sadakata, M. Formation of PCDF, PCDD, PCB, and PCN in de novo synthesis from PAH: Mechanistic aspects and correlation to fluidized bed incinerators. Chemosphere 2001, 44, 1429–1438. [Google Scholar] [CrossRef]
- Oh, J.E.; Gullett, B.; Ryan, S.; Touati, A. Mechanistic relationships among PCDDs/Fs, PCNs, PAHs, CIPhs, and CIBzs in municipal waste incineration. Environ. Sci. Technol. 2007, 41, 4705–4710. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.J.; Russell, D.K. Role of hydrogen abstraction acetylene addition mechanisms in the formation of chlorinated naphthalenes. 1. A quantum chemical investigation. J. Phys. Chem. A 2014, 118, 12192–12204. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.J.; Russell, D.K. Role of hydrogen abstraction acetylene addition mechanisms in the formation of chlorinated naphthalenes. 2. kinetic modeling and the detailed mechanism of ring closure. J. Phys. Chem. A 2014, 118, 12205–2220. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, R.M.; Li, Y.F.; Zhang, Q.Z.; Wang, W.X. Mechanistic and kinetic studies on homogeneous gas-phase formation of 2-chlorophenol as precursor. Int. J. Mol. Sci. 2015. reviesed version review. [Google Scholar]
- Xu, F.; Zhang, R.M.; Li, Y.F.; Zhang, Q.Z. Homogeneous gas-phase formation of polychlorinated naphthalene from dimerization of 4-CPRs and cross-condensation of PhR with 4-CPR: Mechanism and kinetics Study. Comput. Theor. Chem. 2015. reviesed version review. [Google Scholar]
- Zhang, Q.Z.; Qu, X.H.; Xu, F.; Shi, X.Y.; Wang, W.X. Mechanism and thermal rate constants for the complete series reactions of chlorophenols with H. Environ. Sci. Technol. 2009, 43, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, H.; Zhang, Q.Z.; Zhang, R.X.; Qu, X.H.; Wang, W.X. Kinetic properties for the complete series reactions of chlorophenols with OH radicals-relevance for dioxin formation. Environ. Sci. Technol. 2010, 44, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, M.S.; Gordon, R.; Steckler, R.; Truhlar, D.G. Ab initio reaction paths and direct dynamics calculations. J. Phys. Chem. 1989, 93, 5107–5119. [Google Scholar] [CrossRef]
- Gonzalez-Lafont, A.; Truong, T.N.; Truhlar, D.G. Interpolated variational transition-state theory: Practical methods for estimating variational transition-state properties and tunneling contributions to chemical reaction rates from electronic structure calculations. J. Chem. Phys. 1991, 95, 8875–8894. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Generalized transition state theory. Classical mechanical theory and applications to collinear reactions of hydrogen molecules. J. Phys. Chem. 1979, 83, 1052–1079. [Google Scholar] [CrossRef]
- Fernandez-Ramos, A.; Ellingson, B.A.; Garret, B.C.; Truhlar, D.G. Variational transition state theory with multidimensional tunneling. In Reviews in Computational Chemistry; Lipkowitz, K.B., Cundari, T.R., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zhang, Q.Z.; Li, S.Q.; Qu, X.H.; Shi, X.Y.; Wang, W.X. A quantum mechanical study on the formation of PCDD/Fs from 2-chlorophenol as precursor. Environ. Sci. Technol. 2008, 42, 7301–7308. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.H.; Wang, H.; Zhang, Q.Z.; Shi, X.Y.; Xu, F.; Wang, W.X. Mechanistic and kinetic studies on the homogeneous gas-phase formation of PCDD/Fs from 2,4,5-trichlorophenol. Environ. Sci. Technol. 2009, 43, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, W.N.; Gao, R.; Zhou, Q.; Zhang, Q.Z.; Wang, W.X. Dioxin formations from the radical/radical cross-condensation of phenoxy radicals with 2-chlorophenoxy radicals and 2,4,6-trichlorophenoxy radicals. Environ. Sci. Technol. 2010, 44, 6745–6751. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, W.N.; Zhou, Q.; Gao, R.; Sun, X.Y.; Zhang, Q.Z.; Wang, W.X. Mechanism and direct kinetic study of the polychlorinated dibenzo-p-dioxin and dibenzofuran formations from the radical/radical cross-condensation of 2,4-dichlorophenoxy with 2-chlorophenoxy and 2,4,6-trichlorophenoxy. Environ. Sci. Technol. 2011, 45, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Colussi, A.; Zabel, F.; Benson, S.W. The very low-pressure pyrolysis of phenyl ethyl ether, phenyl allyl ether, and benzyl methyl ether and the enthalpy of formation of the phenoxy radical. Int. J. Chem. Kinet. 1977, 9, 161–178. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, M.C. Thermal decomposition of methyl phenyl ether in shock waves: The kinetics of phenoxy radical reactions. J. Phys. Chem. 1986, 90, 425–431. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Hybrid meta density functional theory methods for therochemistry, thermochemical kinetics, and noncovalent interactions: The MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der waals interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions–the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Corchado, J.C.; Chuang, Y.Y.; Fast, P.L.; Villa, J.; Hu, W.P.; Liu, Y.P.; Lynch, G.C.; Nguyen, K.A.; Jackels, C.F.; Melissas, V.S.; et al. POLYRATE Version 9.7; University of Minnesota: Minneapolis, Minnesota, MN, USA, 2007. [Google Scholar]
- Garrett, B.C.; Truhlar, D.G.; Wagner, A.F.; Dunning, T.H., Jr. Variational transition state theory and tunneling for a heavy-light-heavy reaction using an ab initio potential energy surface. 37Cl + H(D) 35Cl→H(D) 37Cl + 35Cl. J. Chem. Phys. 1983, 78, 4400–4413. [Google Scholar] [CrossRef]
- Skodje, R.T.; Truhlar, D.G.; Garrett, B.C. Vibrationally adiabatic models for reactive tunneling. J. Chem. Phys. 1982, 77, 5955–5976. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Shi, X.; Zhang, Q. Quantum Chemical and Kinetic Study on Polychlorinated Naphthalene Formation from 3-Chlorophenol Precursor. Int. J. Mol. Sci. 2015, 16, 20620-20640. https://doi.org/10.3390/ijms160920620
Xu F, Shi X, Zhang Q. Quantum Chemical and Kinetic Study on Polychlorinated Naphthalene Formation from 3-Chlorophenol Precursor. International Journal of Molecular Sciences. 2015; 16(9):20620-20640. https://doi.org/10.3390/ijms160920620
Chicago/Turabian StyleXu, Fei, Xiangli Shi, and Qingzhu Zhang. 2015. "Quantum Chemical and Kinetic Study on Polychlorinated Naphthalene Formation from 3-Chlorophenol Precursor" International Journal of Molecular Sciences 16, no. 9: 20620-20640. https://doi.org/10.3390/ijms160920620
APA StyleXu, F., Shi, X., & Zhang, Q. (2015). Quantum Chemical and Kinetic Study on Polychlorinated Naphthalene Formation from 3-Chlorophenol Precursor. International Journal of Molecular Sciences, 16(9), 20620-20640. https://doi.org/10.3390/ijms160920620