DNA Targeting Sequence Improves Magnetic Nanoparticle-Based Plasmid DNA Transfection Efficiency in Model Neurons
Abstract
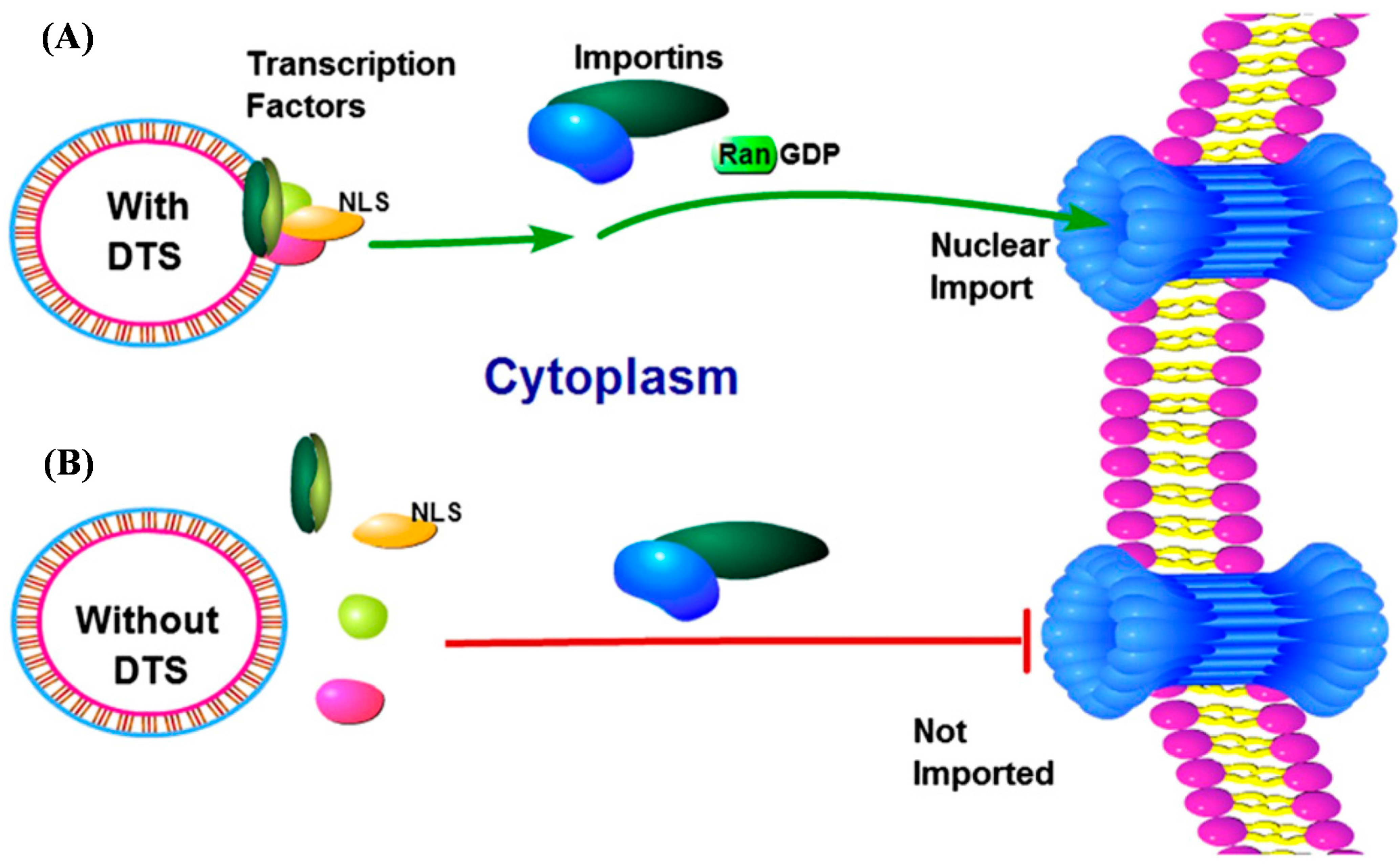
:1. Introduction
2. Results and Discussion
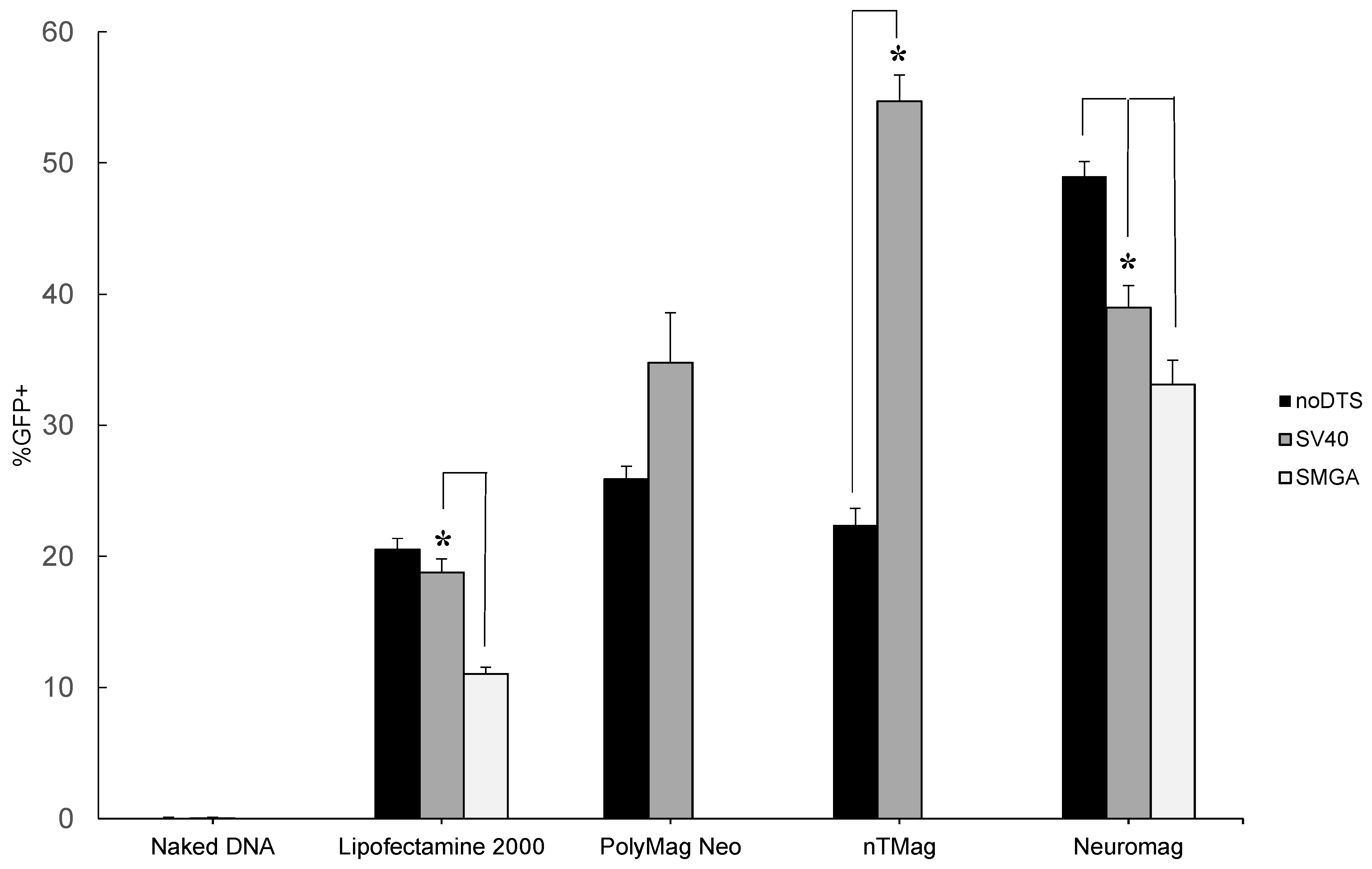
2.1. Transgenic Plasmid Size Primarily Determines Nanomagnetic Transfection Outcome in the Undifferentiated SH-SY5Y Cell Phenotype
| MNP Type | Mode Diameter (nm) | Mean Diameter (nm) | Size Range (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| PolyMag Neo | 146 | 166.21 | 105–225 | +49.29 |
| nTMag | 99 | 119.41 | 70–170 | +31.13 |
| Neuromag | 122 | 151.63 | 80–220 | +48.16 |
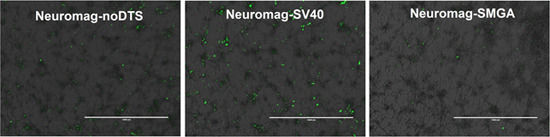
2.2. Incorporation of a DTS Feature within the pDNA Improves Nanomagnetic Transfection Efficiency of the Model Neuron, Differentiated SH-SY5Y
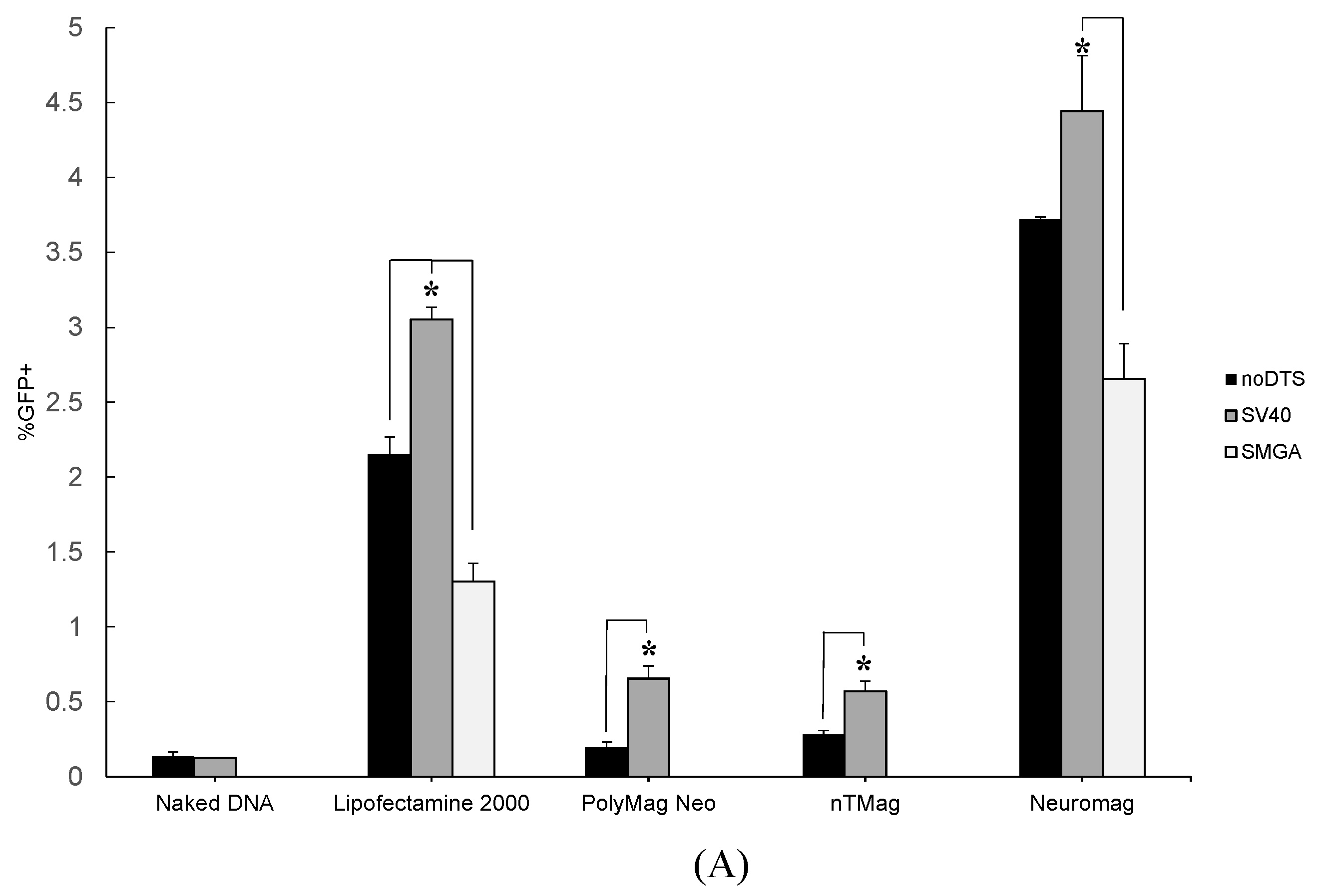
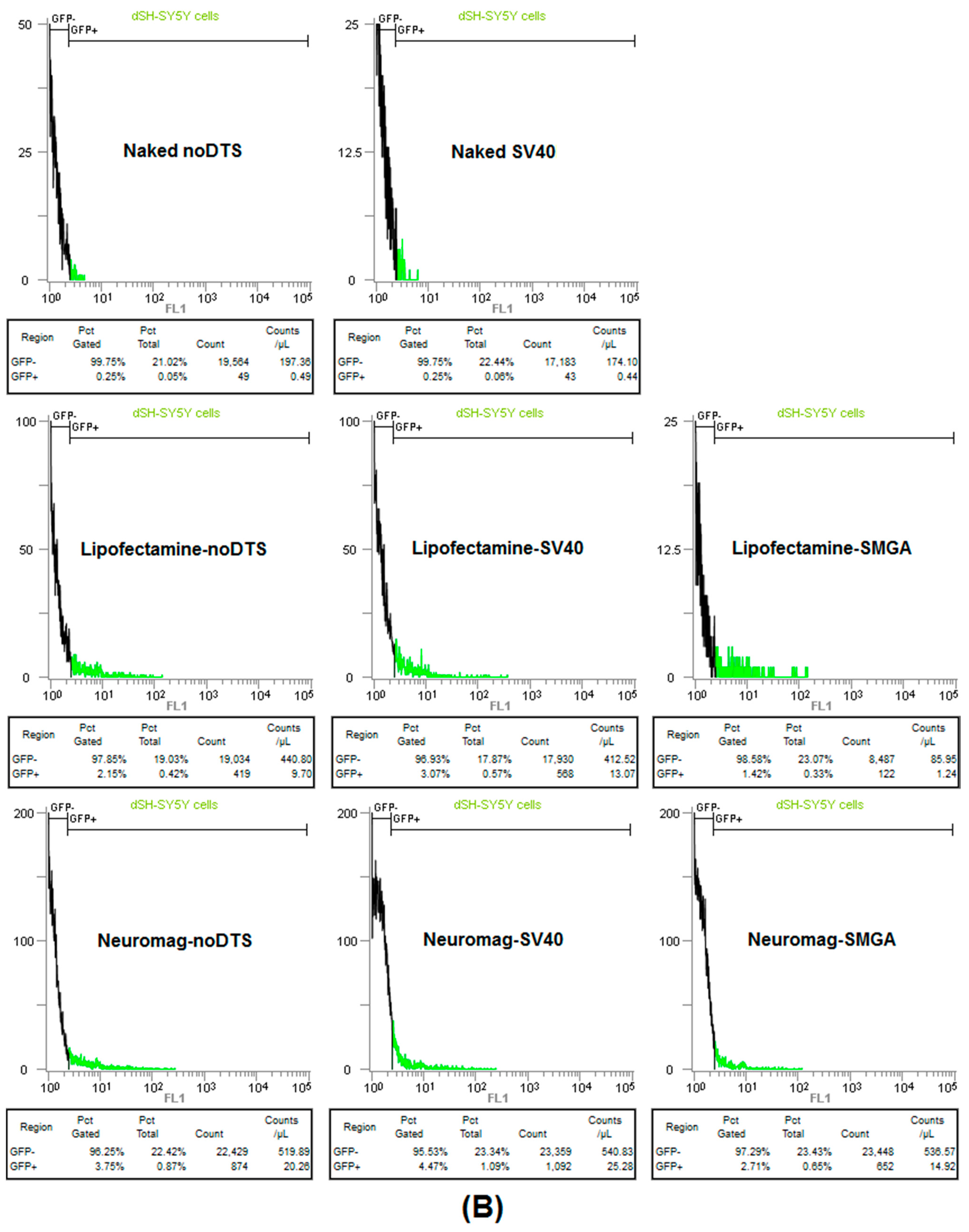
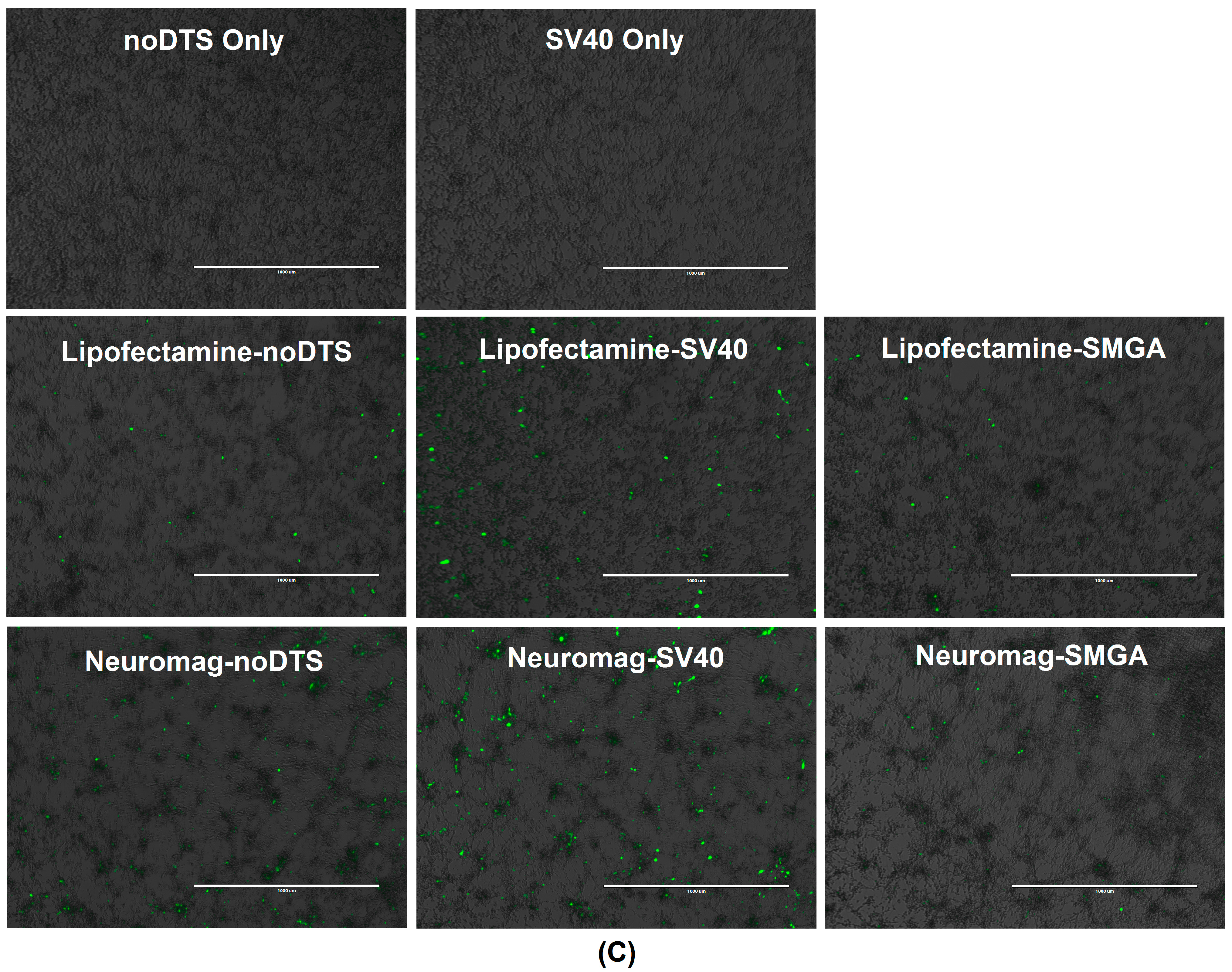
Temporal Response of Transfection: Undifferentiated vs. Differentiated SH-SY5Y Cell Phenotypes


3. Experimental Section
3.1. SH-SY5Y Cell Culture and Differentiation
3.2. SH-SY5Y Cell Characterization
3.3. MNP Vector Characterization
3.3.1. Hydrodynamic Diameter
3.3.2. Zeta Potential
3.4. Magnetic Nanoparticle-Based DNA Transfection
3.5. Transfection Efficiency Quantification
3.6. Statistical Analysis of Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Mellott, A.J.; Forrest, M.L.; Detamore, M.S. Physical non-viral gene delivery methods for tissue engineering. Ann. Biomed. Eng. 2013, 41, 446–468. [Google Scholar] [CrossRef] [PubMed]
- Ohki, E.; Tilkins, M.; Ciccarone, V.; Price, P. Improving the transfection efficiency of post-mitotic neurons. J. Neurosci. Methods 2001, 112, 95–99. [Google Scholar] [CrossRef]
- Hong-Rong, X.; Lin-Sen, H.; Guo-Yi, L. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. (Engl.) 2010, 123, 1086–1092. [Google Scholar]
- McBain, S.C.; Griesenbach, U.; Xenariou, S.; Keramane, A.; Batich, C.D.; Alton, E.W.; Dobson, J. Magnetic nanoparticles as gene delivery agents: Enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.; Fraites, J.T.J.; Zolotukhin, I.; Song, S.; Flotte, T.R.; Dobson, J.; Batich, C.; Byrne, B.J. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther. 2002, 6, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Rosenecker, J. Magnetofection: The use of magnetic nanoparticles for nucleic acid delivery. Cold Spring Harb. Protoc. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Clements, M.A.; Dobson, J. Delivery of short interfering ribonucleic acid-complexed magnetic nanoparticles in an oscillating field occurs via caveolae-mediated endocytosis. PLoS ONE 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42. [Google Scholar] [CrossRef]
- Pollard, H. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J. Biol. Chem. 1998, 273, 7507–7511. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Sauer, T.; Carotta, S.; Cotten, M.; Saltik, M.; Wagner, E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.C. Transfection by cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression. J. Biol. Chem. 1997, 272, 25641–25647. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.P.; Dean, D.A. Progress and prospects: Nuclear import of nonviral vectors. Gene Ther. 2010, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A. Import of plasmid DNA into the nucleus is sequence specific. Exp. Cell Res. 1997, 230, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Dean, D.A. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv. Drug Deliv. Rev. 2009, 61, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Vacik, J.; Dean, B.; Zimmer, W.; Dean, D. Cell-specific nuclear import of plasmid DNA. Gene Ther. 1999, 6, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Zimmer, W.E.; Dean, D.A. Smooth muscle-specific gene delivery in the vasculature based on restriction of DNA nuclear import. Exp. Biol. Med. (Maywood) 2008, 233, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Munkonge, F.M.; Alton, E.W.; Dean, D.A. Identification of protein cofactors necessary for sequence-specific plasmid DNA nuclear import. Mol. Ther. 2009, 17, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Strong, D.; Zimmer, W. Nuclear entry of nonviral vectors. Gene Ther. 2005, 12, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Cheunga, Y.T.; Laua, W.K.W.; Yua, M.S.; Laia, C.S.W.; Yeunga, S.C.; So, K.F.; Changa, R.C.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Cen, V.; Gallego, C.; Comella, J.X. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the role of surface charges in cellular adsorption vs. internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, G.L.; Haggie, P.; Seksek, O.; Lechardeur, D.; Freedman, N.; Verkman, A. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2000, 275, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Ciofani, G.; Raffa, V.; Cuschieri, A.; Menciassi, A.; Dario, P.; Takeoka, S. Evaluation of cationic liposomes composed of an amino acid-based lipid for neuronal transfection. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montanez, E.; Lopez-Tellez, J.; Acevedo, M.; Pavia, J.; Khan, Z. Efficiency of gene transfection reagents in NG108-15, SH-SY5Y and CHO-K1 cell lines. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Herweijer, H.; Zhang, G.; Subbotin, V.M.; Budker, V.; Williams, P.; Wolff, J.A. Time course of gene expression after plasmid DNA gene transfer to the liver. J. Gene Med. 2001, 3, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Herweijer, H.; Wolff, J.A. Progress and prospects: Naked DNA gene transfer and therapy. Gene Ther. 2003, 10, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.; Collins, S.A.; Rajenderan, S.; Casey, G.; O’Sullivan, G.C.; Tangney, M. Plasmid Transgene Expression in vivo: Promoter and Tissue Variables; Molina, F.M., Ed.; InTech: Rijeka, Croatia, 2013; pp. 35–47. [Google Scholar]
- ATCC. Product Information Sheet for ATCC® CRL-2266™; American Type Culture Collection (ATCC): Manassas, VA, USA, 2007; pp. 1–3. [Google Scholar]
- Mastroeni, D.; Grover, A.; Leonard, B.; Joyce, J.N.; Coleman, P.D.; Kozik, B.; Bellinger, D.L.; Rogers, J. Microglial responses to dopamine in a cell culture model of Parkinson’s disease. Neurobiol. Aging 2009, 30, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Biotechnology, S.C. Immuno-Flow Cytometry Protocol; Santa Cruz Biotechnology, Inc.: Santa Cruz, CA, USA, 2007; pp. 1–3. [Google Scholar]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernon, M.M.; Dean, D.A.; Dobson, J. DNA Targeting Sequence Improves Magnetic Nanoparticle-Based Plasmid DNA Transfection Efficiency in Model Neurons. Int. J. Mol. Sci. 2015, 16, 19369-19386. https://doi.org/10.3390/ijms160819369
Vernon MM, Dean DA, Dobson J. DNA Targeting Sequence Improves Magnetic Nanoparticle-Based Plasmid DNA Transfection Efficiency in Model Neurons. International Journal of Molecular Sciences. 2015; 16(8):19369-19386. https://doi.org/10.3390/ijms160819369
Chicago/Turabian StyleVernon, Matthew M., David A. Dean, and Jon Dobson. 2015. "DNA Targeting Sequence Improves Magnetic Nanoparticle-Based Plasmid DNA Transfection Efficiency in Model Neurons" International Journal of Molecular Sciences 16, no. 8: 19369-19386. https://doi.org/10.3390/ijms160819369