Economic Assessment of Supercritical CO2 Extraction of Waxes as Part of a Maize Stover Biorefinery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Maize Stover Wax Composition
| Compound | Quantity (μg/g of Plant) |
|---|---|
| Hexanoic acid | 1 ± 0.06 |
| Heptanoic acid | 0.3 ± 0.07 |
| Octanoic acid | 4.1 ± 0.3 |
| Nonanoic acid | 3 ± 0.3 |
| Decanoic acid | 4.1 ± 0.1 |
| Dodecanoic acid | 13.5 ± 0.6 |
| Tetradecanoic acid | 23.4 ± 1.1 |
| Pentadecanoic acid | 5.2 ± 0.2 |
| Hexadecanoic acid | 579 ± 20.9 |
| Heptadecanoic acid | 13.5 ± 0.6 |
| Octadecanoic acid | 206.2 ± 10.8 |
| Nonadecanoic acid | 5.1 ± 0.8 |
| Eicosanoic acid | 90.7 ± 5.9 |
| Heneicosanoic acid | 11.4 ± 1.6 |
| Docosanoic acid | 55.6 ± 4.2 |
| Tricosanoic acid | 46.7 ± 3.9 |
| Tetracosanoic acid | 76.8 ± 8.3 |
| Pentacosanoic acid | 18.1 ± 1.4 |
| Hexacosanoic acid | 38.8 ± 4 |
| Octacosanoic acid | 6.3 ± 0.8 |
| Total saturated fatty acids | 1202.8 ± 65.9 |
| 9-hexadecenoic acid | 56.5 ± 1.8 |
| C18 unsaturated fatty acids | 1410.2 ± 82 |
| Total unsaturated fatty acids | 1466.7 ± 83.8 |
| Hexacosanol | 13.4 ± 1.7 |
| Octacosanol | 25.2 ± 3.3 |
| Triacontanol | 123.5 ± 9.4 |
| Dotriacontanol | 84.7 ± 8.3 |
| Total fatty alcohols | 246.8 ± 22.7 |
| Hexacosanal | 63.3 ± 6.4 |
| Octacosanal | 47.6 ± 2.8 |
| Triacontanal | 72.8 ± 8.2 |
| Total fatty aldehydes | 183.7 ± 17.4 |
| Pentacosane | 2.2 ± 0.1 |
| Heptacosane | 9.3 ± 0.4 |
| Nonacosane | 24.7 ± 0.9 |
| Hentriacosane | 49.2 ± 4.2 |
| Triatriacontane | 48 ± 1.6 |
| Total alkanes | 133.4 ± 7.2 |
| Campesterol | 226.4 ± 9.1 |
| Stigmasterol | 319.6 ± 13.6 |
| Β-sitosterol | 735.6 ± 15.8 |
| Stigmastanol | 226.4 ±9.1 |
| Total Sterols | 1358.6 ± 44.3 |
| Stigma-4-en-3-one | 95.8 ± 2.5 |
| 5α-stigmastan-3,6-dione | 42.6 ± 3.2 |
| Total steroid ketones | 138.4 ± 5.7 |
| Wax ester 40 | 13.9 ± 1 |
| Wax ester 42 | 24.9 ± 1.5 |
| Wax ester 43 | 1.4 ± 0.3 |
| Wax ester 44 | 29.1 ± 6.5 |
| Wax ester 45 | 2 ± 0.7 |
| Wax ester 46 | 23.4 ± 7.8 |
| Wax ester 47 | 1.4 ± 0.7 |
| Wax ester 48 | 13 ± 4.2 |
| Wax ester 49 | 1.5 ± 0.4 |
| Wax ester 50 | 10.2 ± 2.1 |
| Wax ester 52 | 5.9 ± 0.5 |
| Wax ester 53 | 0.8 ± 0.1 |
| Wax ester 54 | 5 ± 0.5 |
| Wax ester 55 | 0.5 ± 0.05 |
| Wax ester 56 | 2.9 ± 0.4 |
| Wax ester 58 | 1 ± 0.06 |
| Total Wax esters | 137.7 ± 26.9 |
| Phytol | 8.4 ± 1.1 |
| 2-Pentadecanone-6,10,14-trimethyl | 90.1 ± 3.7 |
| Total “other” compounds | 98.5 ± 4.8 |
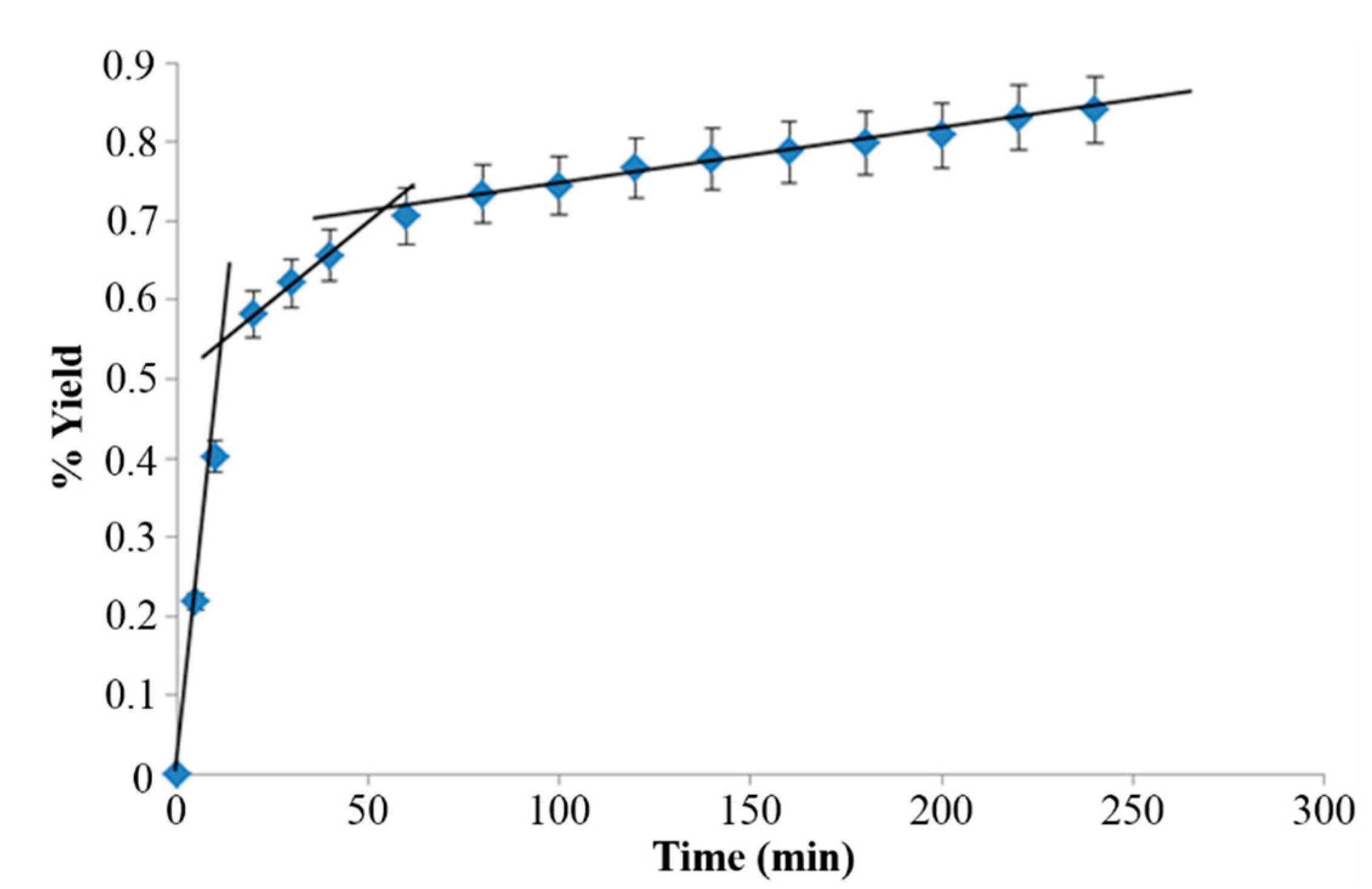
2.2. Extraction Kinetics
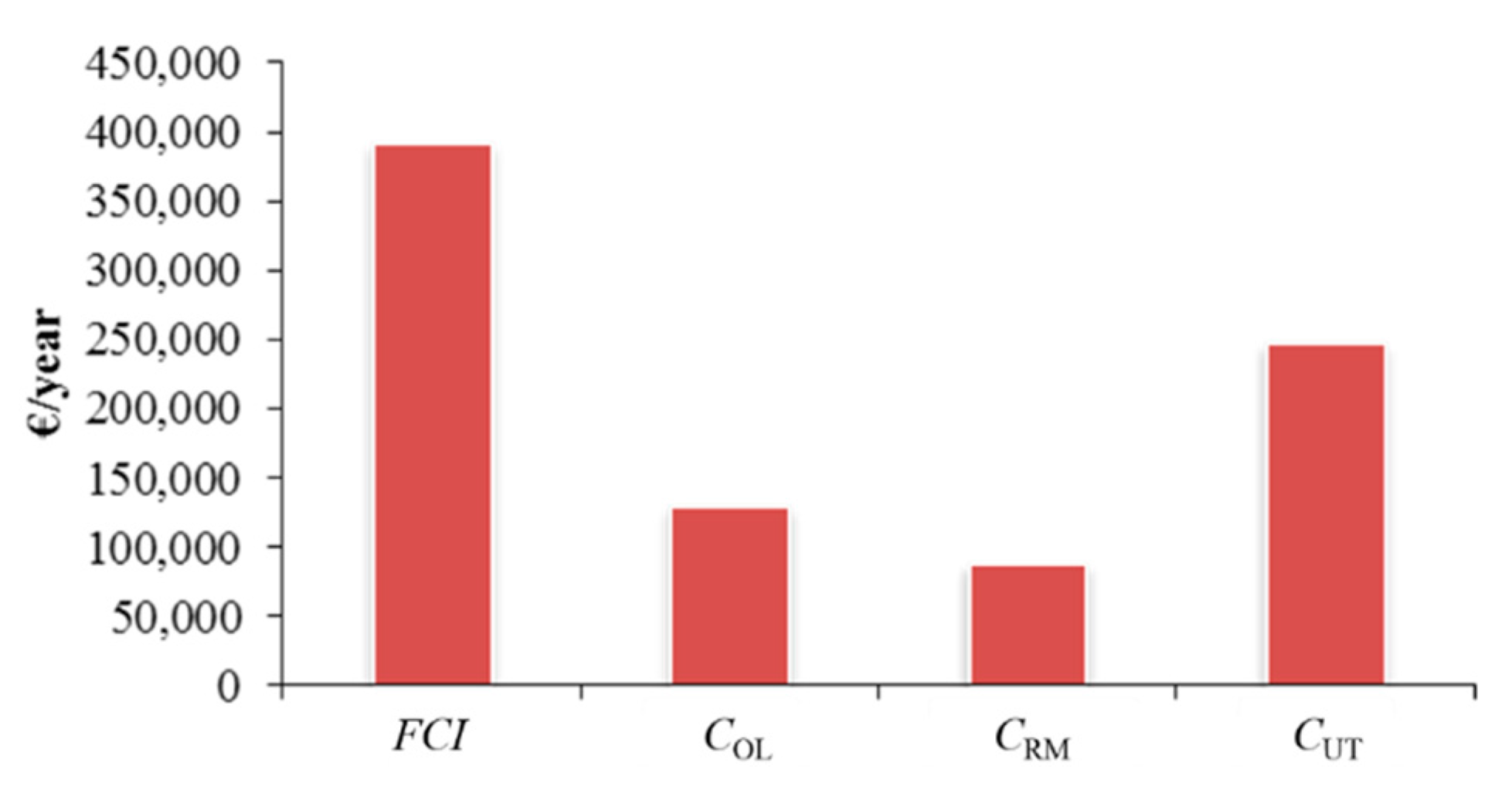
2.3. Economic Assessment of Maize Stover Wax Extraction: Cost of Manufacture (COM)
2.3.1. Fixed Capital Investment FCI
2.3.2. Operational Labour Costs (COL)
2.3.3. Raw Material Costs (CRM)
| Study | Cost of Stover |
|---|---|
| Perlack et al., 2003 [41] | $43.10–$56.10/dry metric tonne (Mid-point $49.60) |
| Eggeman et al., 2005 [42] | $35/dry metric tonne |
| Graham et al., 2007 [5] | $33/dry metric tonne |
| Sendich et al., 2008 [43] | $40/dry metric tonne |
| Dutta et al., 2009 [44] | $60.10/dry metric tonne |
| Sokhansanj et al., 2010 [45] | $74/dry metric tonne (baled), $84/dry metric tonne (chopped) and $86/dry metric tonne (pelletised) (assumed pelletised in this calculation) |
| Kazi et al., 2010 [46] | $83/dry tonne |
| Humbird et al., 2011 [47] | $58.50/dry tonne |
| Gonzalez et al., 2012 [48] | $80.3/dry tonne |
| Fiegel et al., 2012 [49] | $85.40/dry tonne |
| Vadas et al., 2013 [51] | $44.09/dry tonne (most expensive) |
| Tao et al., 2013 [52] | $58.50/dry tonne |
| Meyer et al., 2013 [50] | $58.50/dry tonne |
| Petrou et al., 2014 [53] | $58.50/dry tonne |
| Ou et al., 2014 [54] | $83/dry tonne |
| Thompson et al., 2014 [4] | $88.19/dry tonne |
2.3.4. Cost of Waste (CWT)
2.3.5. Cost of Utilities (CUT)
Costs Associated with the Electric Power Used in the CO2 Pump
Costs Associated with the CO2 Heater
Costs Associated with Refrigeration
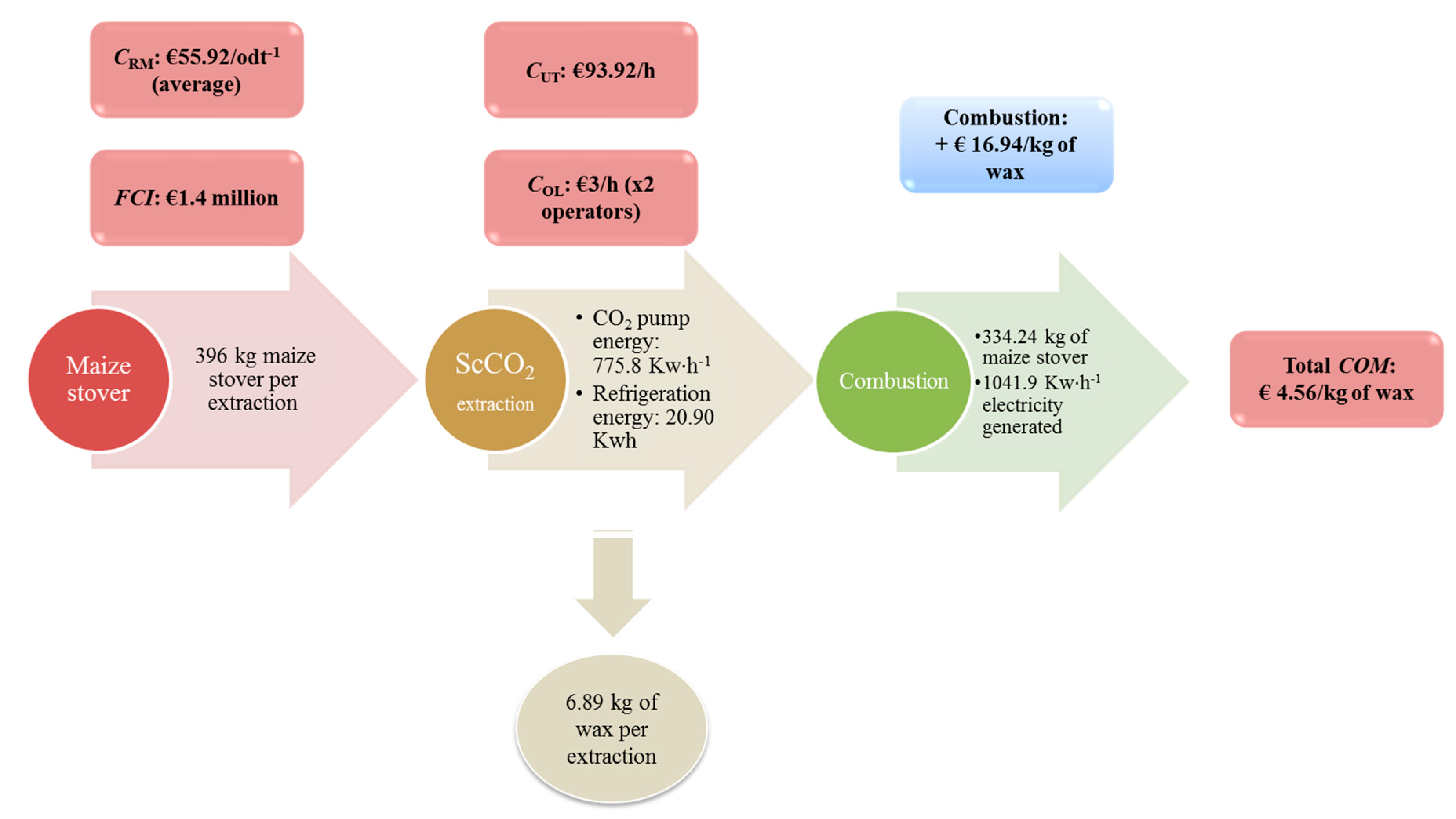
2.3.6. Total COM Calculation
2.3.7. Utilization of Maize Stover Biomass for Electricity Generation
Calculation Assuming Use of Most Efficient Technology (combustion)
Calculation Assuming Average Efficiency of All Technologies
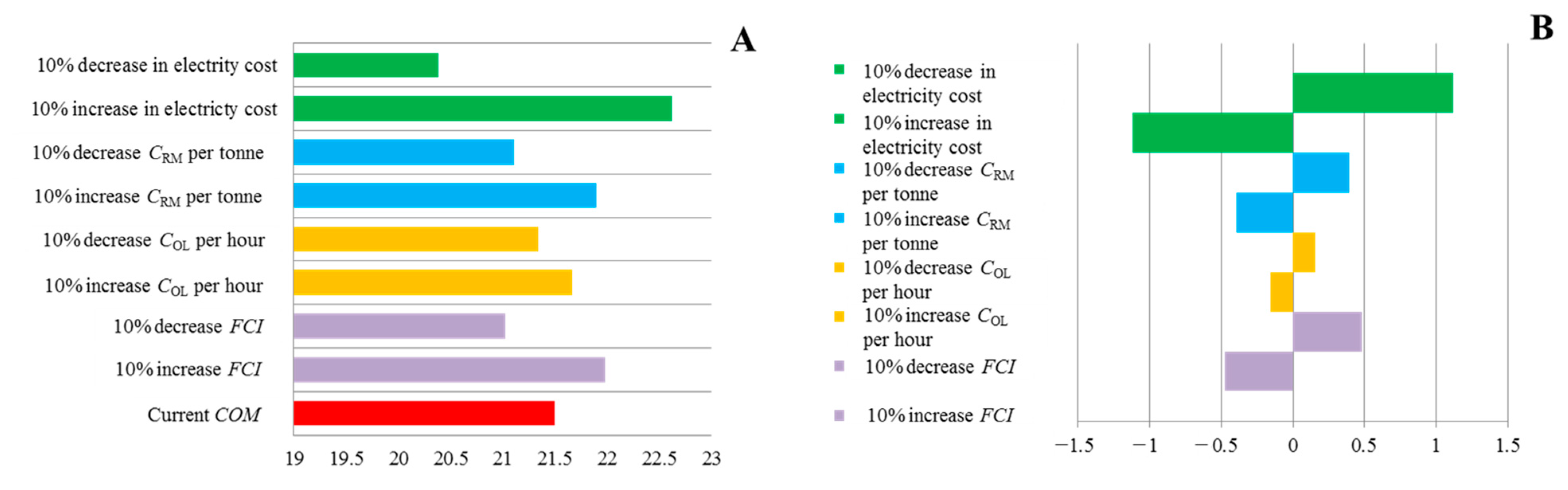
2.3.8. Sensitivity Analysis
3. Experimental Section
3.1. Material
3.2. Supercritical Fluid Extraction of Maize Stover Wax for Analysis
3.3. Supercritical Extraction of Maize Stover Extraction Kinetics
3.4. Derivitisation Prior to HT-GC (High Temperature-Gas Chromatography) Analysis
3.5. HT-GC Procedure for Analysis of Wax
3.6. HT-GC-MS (High Temperature-Gas Chromatography Mass Spectrometry) Procedure for Analysis of Wax
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pereira, C.G.; Meireles, M.A.A. Economic analysis of rosemary, fennel and anise essential oils obtained by supercritical fluid extraction. Flavour Fragr. J. 2007, 22, 407–413. [Google Scholar] [CrossRef]
- Attard, T.M.; Watterson, B.; Budarin, V.L.; Clark, J.H.; Hunt, A.J. Microwave assisted extraction as an important technology for valorising orange waste. New J. Chem. 2014, 38, 2278–2283. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.L.; Tyner, W.E. Corn stover for bioenergy production: Cost estimates and farmer supply response. Biomass Bioenergy 2014, 62, 166–173. [Google Scholar] [CrossRef]
- Graham, R.L.; Nelson, R.; Sheehan, J.; Perlack, R.D.; Wright, L.L. Current and potential U.S. Corn stover supplies. Agron. J. 2007, 99, 1–11. [Google Scholar] [CrossRef]
- Johnson, J.M.-F.; Reicosky, D.; Allmaras, R.; Archer, D.; Wilhelm, W. A matter of balance: Conservation and renewable energy. J. Soil Water Conserv. 2006, 61, 120A–125A. [Google Scholar]
- Wilhelm, W.W.; Johnson, J.M.F.; Hatfield, J.L.; Voorhees, W.B.; Linden, D.R. Crop and soil productivity response to corn residue removal. Agron. J. 2004, 96, 1–17. [Google Scholar] [CrossRef]
- Sin, E.H.K.; Marriott, R.; Hunt, A.J.; Clark, J.H. Identification, quantification and chrastil modelling of wheat straw wax extraction using supercritical carbon dioxide. Comptes Rendus Chimie 2014, 17, 293–300. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer: New York, NY, USA, 1994. [Google Scholar]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Hunt, A.J.; Sin, E.H.K.; Marriott, R.; Clark, J.H. Generation, capture, and utilization of industrial carbon dioxide. ChemSusChem 2010, 3, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit. Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Reverchon, E.; de Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Catchpole, O.J.; Simôes, P.; Grey, J.B.; Nogueiro, E.M.M.; Carmelo, P.J.; Nunes da Ponte, M. Fractionation of lipids in a static mixer and packed column using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2000, 39, 4820–4827. [Google Scholar] [CrossRef]
- Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Hunt, A.J.; Lanigan, B.; Marriott, R.; Milkowski, K.J.; Wilson, A.J.; Breeden, S.W.; Fan, J.; et al. Use of green chemical technologies in an integrated biorefinery. Energy Environ. Sci. 2011, 4, 471–479. [Google Scholar] [CrossRef]
- Attard, T.M.; Theeuwes, E.; Gomez, L.D.; Johansson, E.; Dimitriou, I.; Wright, P.C.; Clark, J.H.; McQueen-Mason, S.J.; Hunt, A.J. Supercritical extraction as an effective first-step in a maize stover biorefinery. RSC Adv. 2015, 5, 43831–43838. [Google Scholar] [CrossRef]
- Deswarte, F.E.I.; Clark, J.H.; Hardy, J.J.E.; Rose, P.M. The fractionation of valuable wax products from wheat straw using CO2. Green Chem. 2006, 8, 39–42. [Google Scholar] [CrossRef]
- Attard, T.M.; McElroy, C.R.; Rezende, C.A.; Polikarpov, I.; Clark, J.H.; Hunt, A.J. Sugarcane waste as a valuable source of lipophilic molecules. Ind. Crops Prod. 2015, 76, 95–103. [Google Scholar] [CrossRef]
- Leal, P.; Maia, N.; Carmello, Q.C.; Catharino, R.; Eberlin, M.; Meireles, M.A. Sweet basil (Ocimum Basilicum) extracts obtained by supercritical fluid extraction (SFE): Global yields, chemical composition, antioxidant activity, and estimation of the cost of manufacturing. Food Bioprocess Technol. 2008, 1, 326–338. [Google Scholar] [CrossRef]
- Perrut, M. Supercritical fluid applications: Industrial developments and economic issues. Ind. Eng. Chem. Res. 2000, 39, 4531–4535. [Google Scholar] [CrossRef]
- Rosa, P.T.V.; Meireles, M.A.A. Rapid estimation of the manufacturing cost of extracts obtained by supercritical fluid extraction. J. Food Eng. 2005, 67, 235–240. [Google Scholar] [CrossRef]
- Teja, A.S.; Eckert, C.A. Commentary on supercritical fluids: Research and applications. Ind. Eng. Chem. Res. 2000, 39, 4442–4444. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes; Prentice Hall: Upper Saddle River, NJ, USA, 2013. [Google Scholar]
- Pereira, C.G.; Rosa, P.T.V.; Meireles, M.A.A. Extraction and isolation of indole alkaloids from tabernaemontana catharinensis A.DC: Technical and economical analysis. J. Supercrit. Fluids 2007, 40, 232–238. [Google Scholar] [CrossRef]
- Bradford, P.G.; Awad, A.B. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007, 51, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H.; Frohlich, J.J. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: Clinical and experimental evidence. Am. J. Med. 1999, 107, 588–594. [Google Scholar] [CrossRef]
- Shepherd, J.; Packard, C.J.; Patsch, J.R.; Gotto, A.M., Jr.; Taunton, D.O. Effects of dietary polyunsaturated and saturated fat on the properties of high density lipoproteins and the metabolism of apolipoprotein AI. J. Clin. Investig. 1978, 61, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, E. Carbohydrate degradation products from alkaline treatment of biomass. Biomass Bioenergy 1991, 1, 61–64. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997, 15, 401–409. [Google Scholar] [CrossRef]
- Hill, K. Fats and oils as oleochemical raw materials. Pure Appl. Chem. 2000, 72, 1255–1264. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Löfstedt, C.; Hansson, B.S.; Ibarra, F.; Francke, W. Orchid pollination by sexual swindle. Nature 1999, 399, 421–421. [Google Scholar] [CrossRef]
- Gunawan, E.R.; Basri, M.; Rahman, M.B.A.; Salleh, A.B.; Rahman, R.N.Z.A. Study on response surface methodology (RSM) of lipase-catalyzed synthesis of palm-based wax ester. Enzym. Microb. Technol. 2005, 37, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Gangadharan, G.K.; Prakash, S. Compositions and Methods Containing High Purity Fatty Alcohol C24 to C36 for Cosmetic Applications. U.S. Patent 20070196507 A1, 23 August 2007. [Google Scholar]
- Marinangeli, C.P.F.; Jones, P.J.H.; Kassis, A.N.; Eskin, M.N.A. Policosanols as nutraceuticals: Fact or fiction. Crit. Rev. Food Sci. Nutr. 2010, 50, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E.J. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004, 15, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Bruce, V.M.; McDonald, B.E. Dietary α-linolenic acid is as effective as oleic acid and linoleic acid in lowering blood cholesterol in normolipidemic men. Am. J. Clin. Nutr. 1991, 53, 1230–1234. [Google Scholar] [PubMed]
- Clark, J.H.; Deswarte, F. Introduction to Chemicals from Biomass, 2nd ed.; Wiley: Chichester, UK, 2015. [Google Scholar]
- Ferreira, S.R.S.; Meireles, M.A.A. Modeling the supercritical fluid extraction of black pepper (Piper nigrum L.) essential oil. J. Food Eng. 2002, 54, 263–269. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Barbosa, H.M.A.; Passos, C.P.; Silva, C.M. Supercritical fluid extraction of spent coffee grounds: Measurement of extraction curves, oil characterization and economic analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Perlack, R.D.; Turhollow, A.F. Feedstock cost analysis of corn stover residues for further processing. Energy 2003, 28, 1395–1403. [Google Scholar] [CrossRef]
- Eggeman, T.; Elander, R.T. Process and economic analysis of pretreatment technologies. Bioresour. Technol. 2005, 96, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Sendich, E.; Laser, M.; Kim, S.; Alizadeh, H.; Laureano-Perez, L.; Dale, B.; Lynd, L. Recent process improvements for the ammonia fiber expansion (AFEX) process and resulting reductions in minimum ethanol selling price. Bioresour. Technol. 2008, 99, 8429–8435. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Dowe, N.; Ibsen, K.N.; Schell, D.J.; Aden, A. An economic comparison of different fermentation configurations to convert corn stover to ethanol using Z. Mobilis and Saccharomyces. Biotechnol. Prog. 2010, 26, 64–72. [Google Scholar] [PubMed]
- Sokhansanj, S.; Mani, S.; Tagore, S.; Turhollow, A.F. Techno-economic analysis of using corn stover to supply heat and power to a corn ethanol plant—Part 1: Cost of feedstock supply logistics. Biomass Bioenergy 2010, 34, 75–81. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M. Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: Dilute-acid pretreatment and enzymatic hydrolysis of corn stover. Available online: http://www.nrel.gov/biomass/pdfs/47764.pdf (accessed on 14 January 2015).
- Gonzalez, R.; Daystar, J.; Jett, M.; Treasure, T.; Jameel, H.; Venditti, R.; Phillips, R. Economics of cellulosic ethanol production in a thermochemical pathway for softwood, hardwood, corn stover and switchgrass. Fuel Proc. Technol. 2012, 94, 113–122. [Google Scholar] [CrossRef]
- Fiegel, J.; Taheripour, F.; Tyner, W.E. Development of a Viable Corn Stover Market: Impacts on Corn and Soybean Markets. Avaliable Online: http://www.extension.iastate.edu/stover/sites/www.extension.iastate.edu/files/stover/Additional_Resources/RE-6-W.pdf (accessed on 16 January 2015).
- Meyer, P.A.; Tews, I.J.; Magnuson, J.K.; Karagiosis, S.A.; Jones, S.B. Techno-economic analysis of corn stover fungal fermentation to ethanol. Appl. Energy 2013, 111, 657–668. [Google Scholar] [CrossRef]
- Vadas, P.A.; Digman, M.F. Production costs of potential corn stover harvest and storage systems. Biomass Bioenergy 2013, 54, 133–139. [Google Scholar] [CrossRef]
- Tao, L.; He, X.; Tan, E.C.D.; Zhang, M.; Aden, A. Comparative techno-economic analysis and reviews of n-butanol production from corn grain and corn stover. Biofuels Bioprod. Biorefin. 2014, 8, 342–361. [Google Scholar] [CrossRef]
- Petrou, E.C.; Pappis, C.P. Sustainability of systems producing ethanol, power, and lignosulfonates or lignin from corn stover: A comparative assessment. ACS Sustain. Chem. Eng. 2014, 2, 2527–2535. [Google Scholar] [CrossRef]
- Ou, L.; Brown, T.R.; Thilakaratne, R.; Hu, G.; Brown, R.C. Techno-economic analysis of co-located corn grain and corn stover ethanol plants. Biofuels Bioprod. Biorefin. 2014, 8, 412–422. [Google Scholar] [CrossRef]
- Webbook, N. Thermophysical Properties of Fluid Systems. Avaliable Online: http://webbook.nist.gov/ chemistry/fluid/ (access on 18 January 2015).
- GOV.UK. Statistical Data Set: Industrial Energy Price Indices. Avaliable Online: https://www.gov.uk/ government/statistical-data-sets/industrial-energy-price-indices (access on 18 January 2015).
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]
- Morissette, R.; Savoie, P.; Villeneuve, J. Combustion of corn stover bales in a small 146-kw boiler. Energies 2011, 4, 1102–1111. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Assessing the potential for biofuel production of corn stover pyrolysis using a pressurized batch reactor. Fuel 2012, 95, 563–572. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Kuan, W.-H.; Chang, C.-C.; Tzou, Y.-M. Catalytic and atmospheric effects on microwave pyrolysis of corn stover. Bioresour. Technol. 2013, 131, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Morissette, R.; Savoie, P.; Villeneuve, J. Corn stover and wheat straw combustion in a 176-kw boiler adapted for round bales. Energies 2013, 6, 5760–5774. [Google Scholar] [CrossRef]
- Deswarte, F.E.I.; Clark, J.H.; Wilson, A.J.; Hardy, J.J.E.; Marriott, R.; Chahal, S.P.; Jackson, C.; Heslop, G.; Birkett, M.; Bruce, T.J.; et al. Toward an integrated straw-based biorefinery. Biofuels Bioprod. Biorefin. 2007, 1, 245–254. [Google Scholar] [CrossRef]
- Attard, T.M. Supercritical CO2 Extraction of Waxes as Part of a Holistic Biorefinery; University of York: New York, NY, USA, 2015. [Google Scholar]
- Gustavsson, L.; Madlener, R. CO2 mitigation costs of large-scale bioenergy technologies in competitive electricity markets. Energy 2003, 28, 1405–1425. [Google Scholar] [CrossRef]
- Evans, A.; Strezov, V.; Evans, T.J. Sustainability considerations for electricity generation from biomass. Renew. Sustain. Energy Rev. 2010, 14, 1419–1427. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attard, T.M.; McElroy, C.R.; Hunt, A.J. Economic Assessment of Supercritical CO2 Extraction of Waxes as Part of a Maize Stover Biorefinery. Int. J. Mol. Sci. 2015, 16, 17546-17564. https://doi.org/10.3390/ijms160817546
Attard TM, McElroy CR, Hunt AJ. Economic Assessment of Supercritical CO2 Extraction of Waxes as Part of a Maize Stover Biorefinery. International Journal of Molecular Sciences. 2015; 16(8):17546-17564. https://doi.org/10.3390/ijms160817546
Chicago/Turabian StyleAttard, Thomas M., Con Robert McElroy, and Andrew J. Hunt. 2015. "Economic Assessment of Supercritical CO2 Extraction of Waxes as Part of a Maize Stover Biorefinery" International Journal of Molecular Sciences 16, no. 8: 17546-17564. https://doi.org/10.3390/ijms160817546