Sasa quelpaertensis Leaf Extract Inhibits Colon Cancer by Regulating Cancer Cell Stemness in Vitro and in Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of CD133+CD44+ HT29 and CD133+CD44+ HCT116 Cells by FACS (Flow-Activated Cell Sorting)

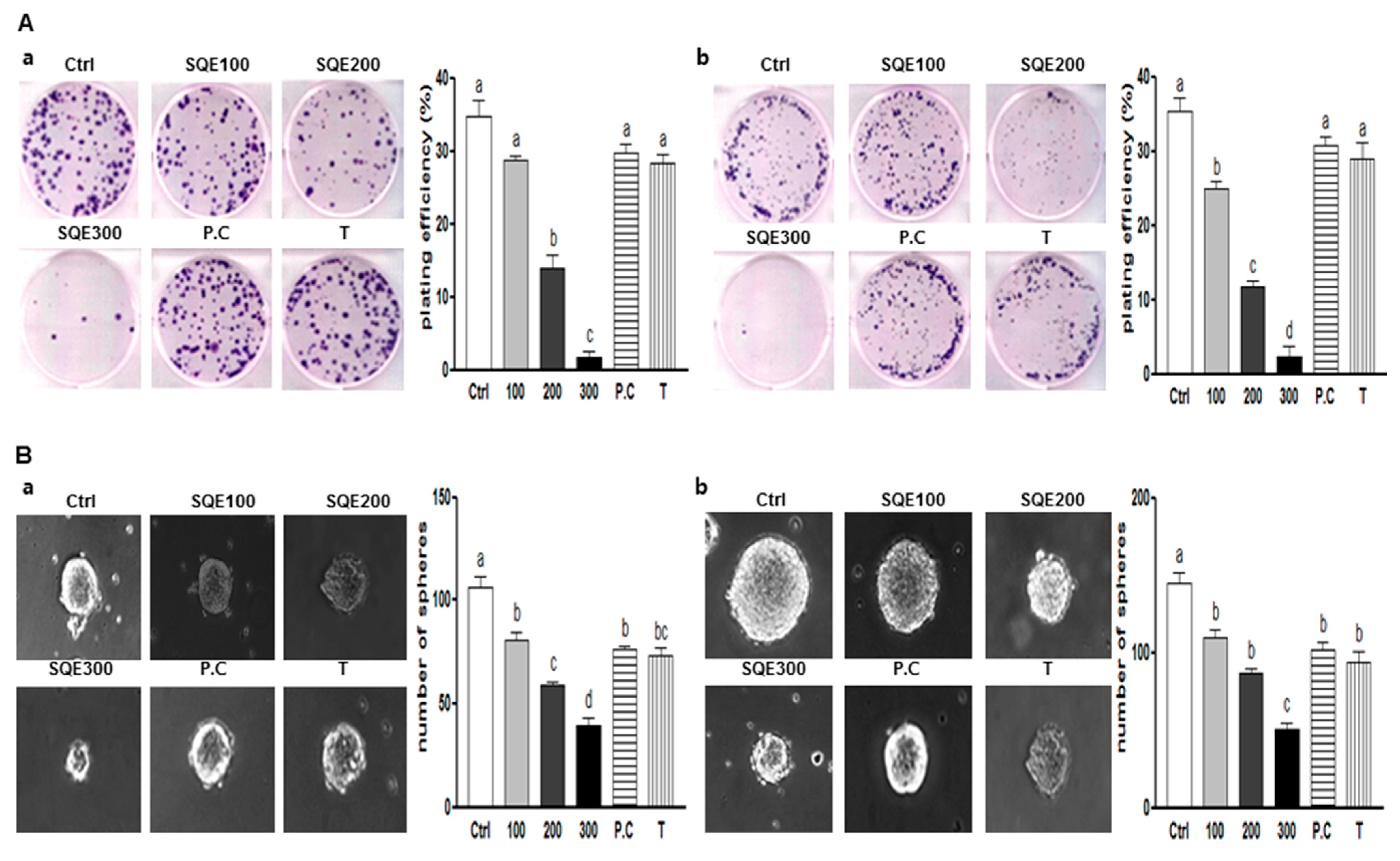
2.2. Effects of SQE (Sasa quelpaertensis Leaf Extract), p-Coumaric Acid, and Tricin on the Self-Renewal Characteristics of Colon CSCs (Cancer Stem Cells)
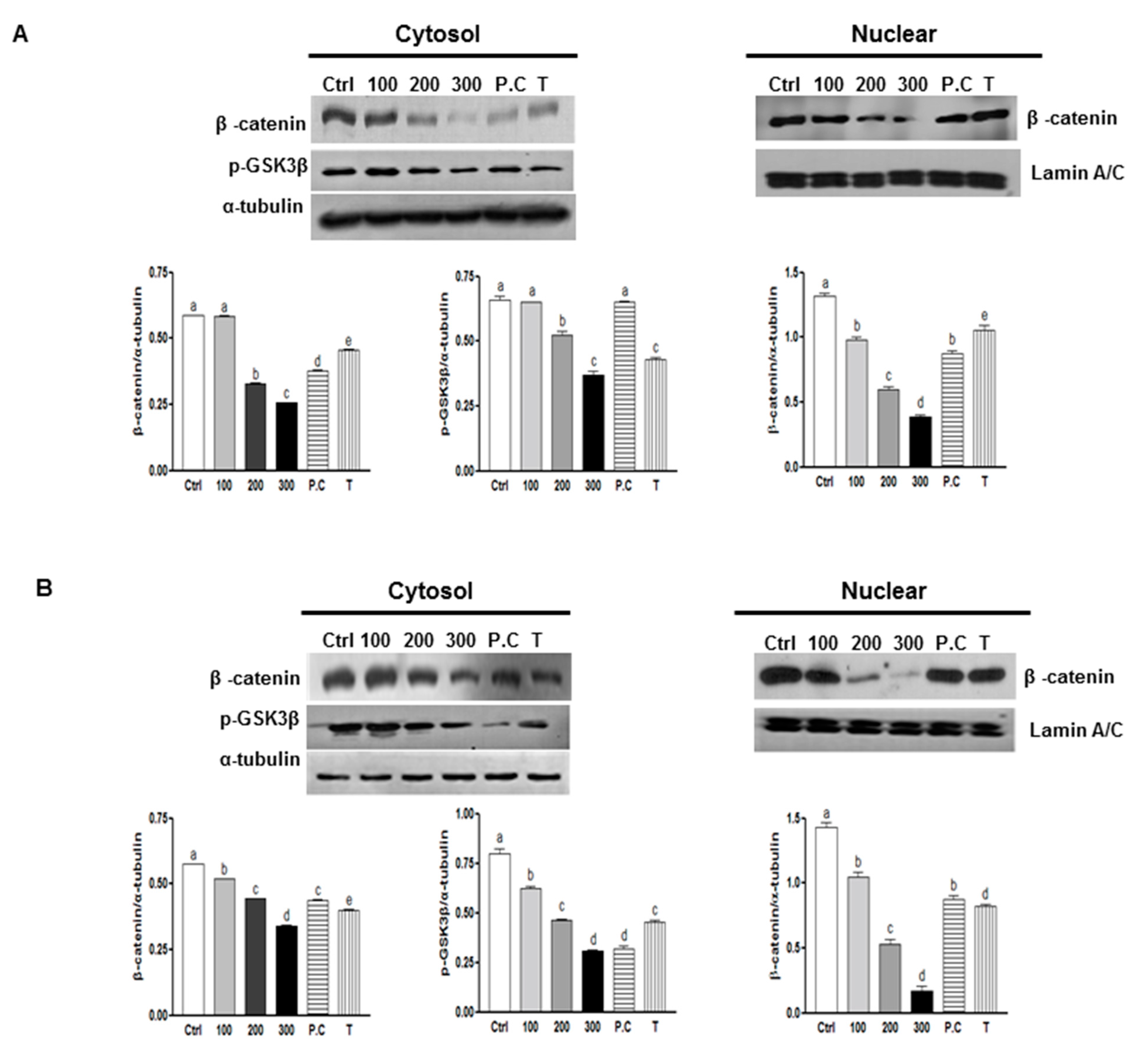
2.3. Effects of SQE, p-Coumaric Acid, and Tricin on Wnt/β-Catenin Signaling
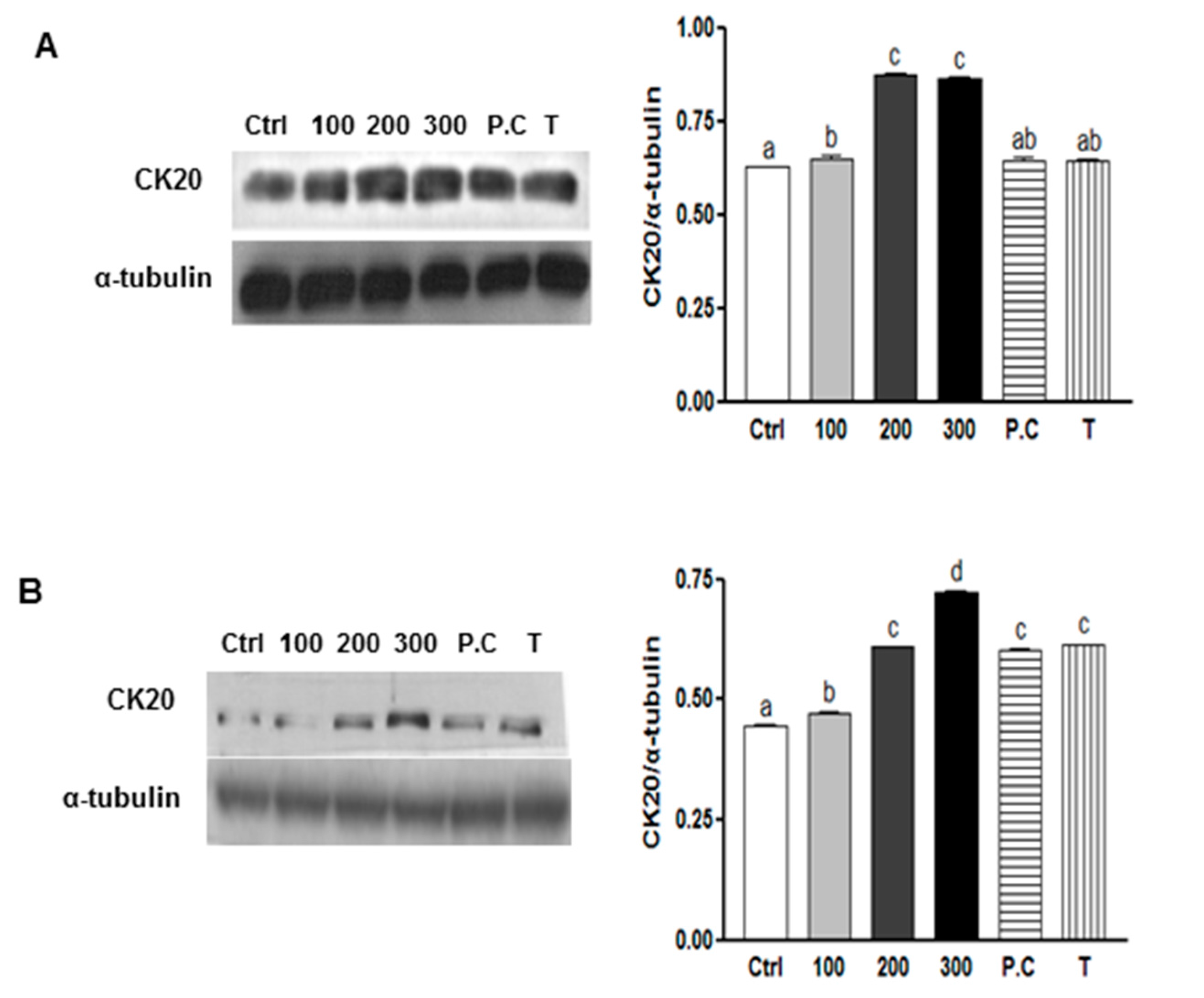
2.4. Effects of SQE, p-Coumaric Acid, and Tricin on Cell Differentiation in Colon CSCs
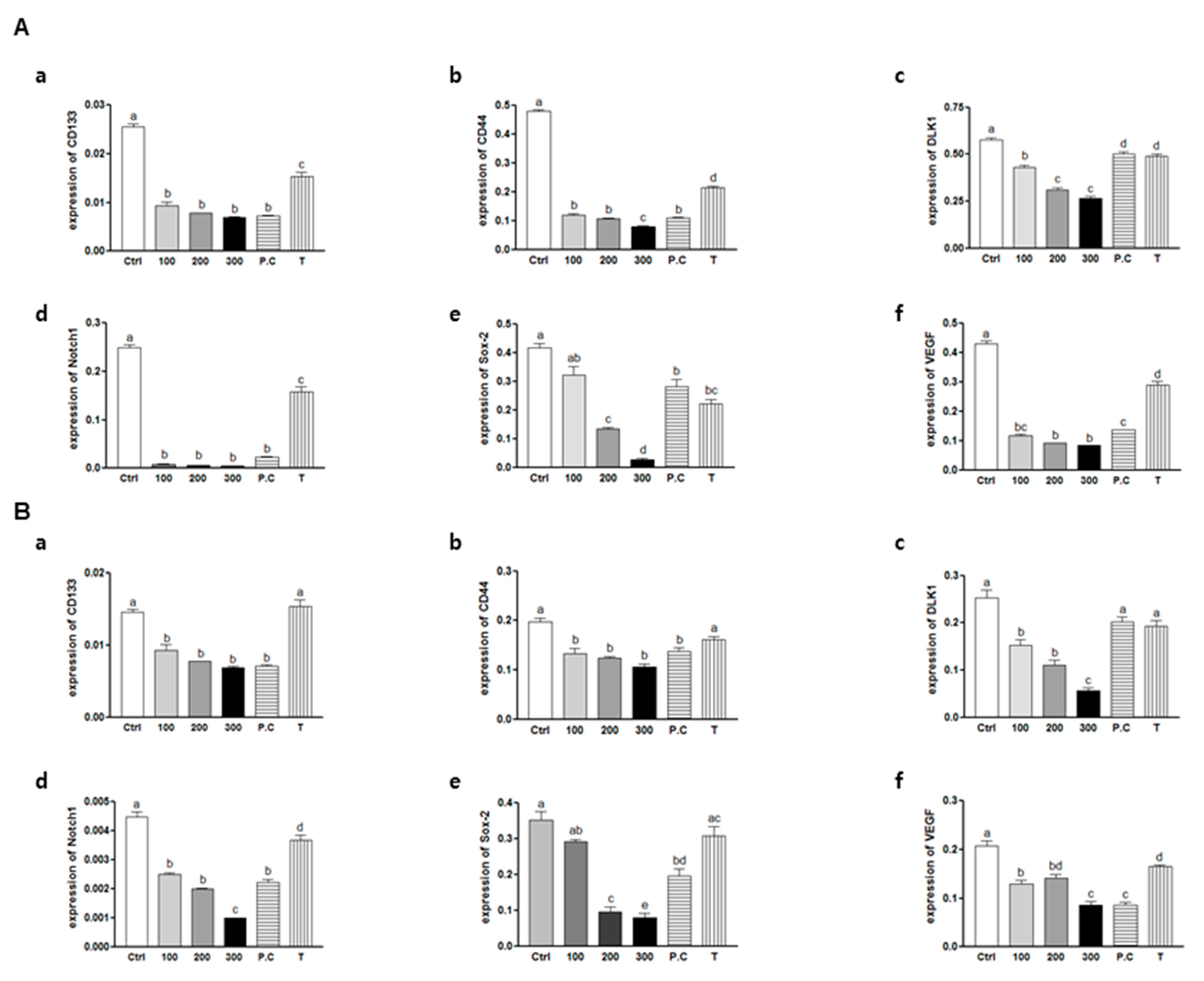
2.5. Effects of SQE, p-Coumaric Acid, and Tricin on the Expression of Stem Cell Markers and VEGF (Vascular Endothelial Growth Factor) in Colon CSCs
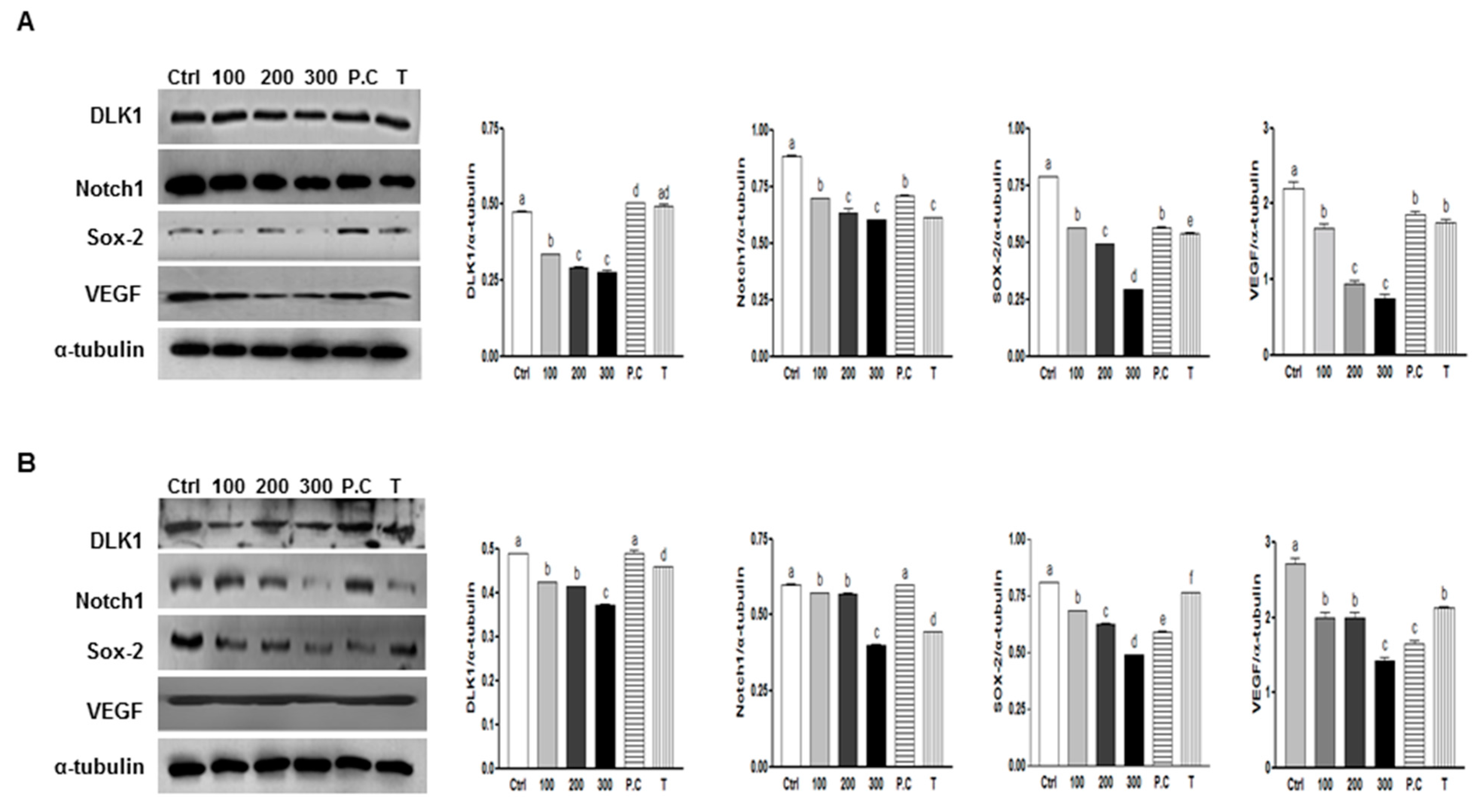
2.6. Effect of Combination of p-Coumaric Acid and Tricin on Clonogenicity and Stem Cell Marker, Notch1

2.7. Effect of SQE on Tumorigenicity and CSC Marker Expression of CD133+CD44+ HT29 Cells in Vivo
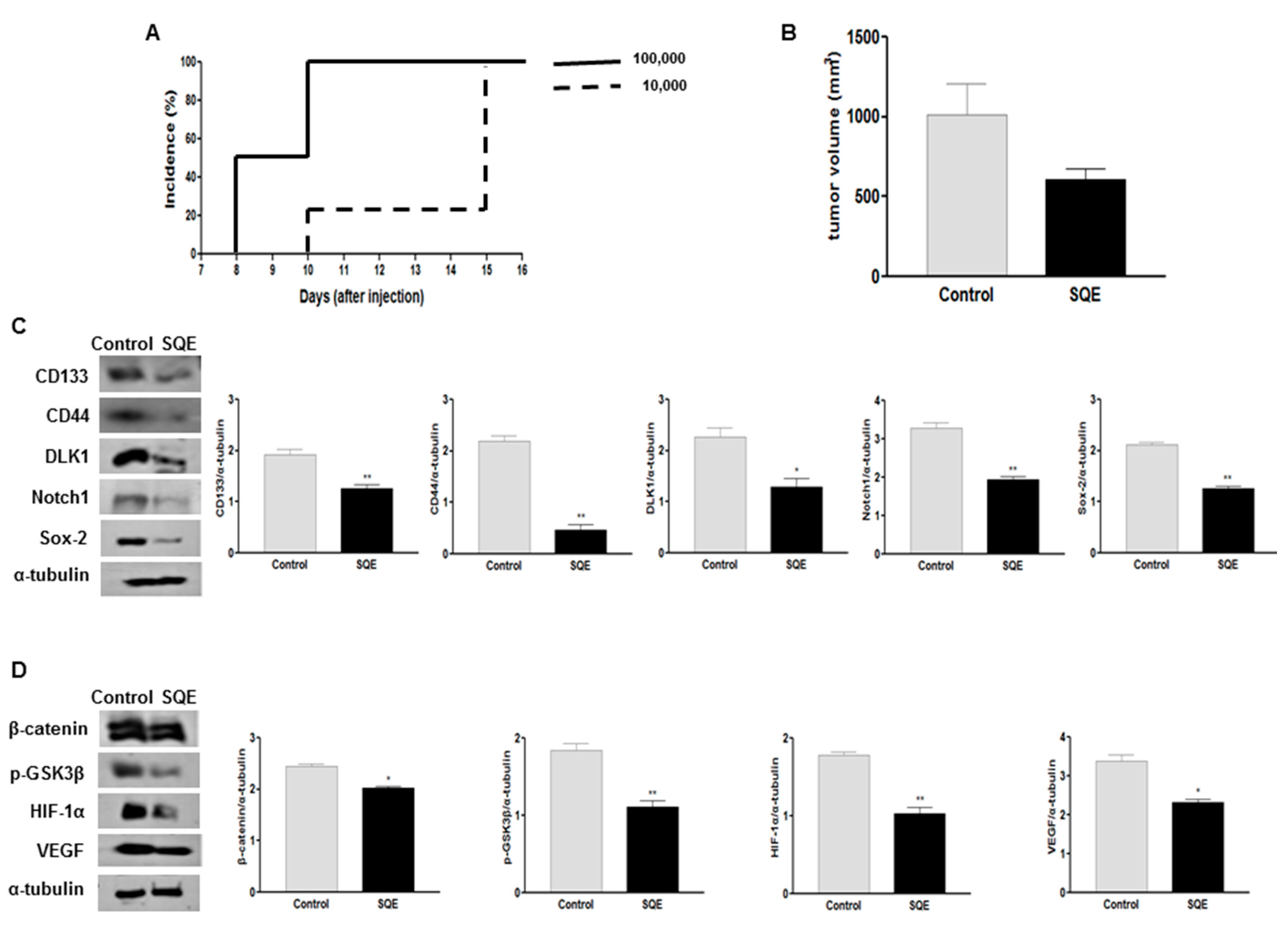
2.8. Discussion
3. Experimental Section
3.1. Preparation of SQE
3.2. Cell Culture
3.3. Isolation of CD133+CD44+ CSCs Using a Fluorescence-Activated Cell Sorting (FACS) System
3.4. Clonogenic Assays
3.5. Sphere Formation Assays
3.6. Antibodies and Western Blot Assays
3.7. Real-Time Quantitative PCR
3.8. In Vivo Tumor Xenograft Model
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| ALDH1 | Aldehyde dehydrogenase 1 |
| bFGF | Basic fibroblast growth factor |
| CSC | Cancer stem cell |
| CK20 | Cytokeratin 20 |
| DLK1 | Delta like 1 homologue |
| DMBA | 7,12-Dimethylbenz[α]anthracene |
| DSS | Dextran sulfate sodium |
| EGF | Epidermal growth factor |
| EPA | Eicosapentaenoic acid |
| FACS | Flow-activated cell sorting |
| GSK3β | Glycogen synthase kinase-3β |
| HEMA | Hydroxyethyl methacylate |
| HIF-1 | Hypoxia-inducible factor-1 |
| HPLC | High performance liquid chromatography |
| mTOR | Mammalian target of rapamycin |
| Notch1 | Notch homolog 1, translocation-associated (Drosophila) |
| PBS | Phosphate buffered saline |
| P.C | P-coumaric acid |
| PE | Plating efficiency |
| PI3K | Phosphorylation of phosphoinositide-3-kinase |
| PVDF | Polyvinylidene fluoride |
| Sox-2 | SRY-related HMG-box-2 |
| SQE | Sasa quelpaertensis leaf extract |
| T | Tricin |
| TCF/LEF | T cell factor/lymphoid enhancer factor |
| VEGF | Vascular endothelial growth factor |
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bary, F. Cancer Incidence and Mortality Worldwide: Iarc Cancer Base; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Andre, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: Aacr workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Ioue, Y.; Miki, C.; Kusunoki, M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann. Surg. Oncol. 2009, 16, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.D.; Kim, Y.J.; Lee, C.N.; Aggarwal, S.; McKinnon, K.; Mesmer, D.; Norton, J.; Birse, C.E.; He, T.; Ruben, S.M.; et al. Expansion of CD133+ colon cancer cultures retaining stem cell properties to enable cancer stem cell target discovery. Br. J. Cancer 2010, 102, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Keysar, S.B.; Jimeno, A. More than markers: Biological significance of cancer stem cell-defining molecules. Mol. Cancer Ther. 2010, 9, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Dalerba, P.; Dylla, S.J.; Park, I.K.; Liu, R.; Wang, X.; Cho, R.W.; Hoey, T.; Gurney, A.; Huang, E.H.; Simeone, D.M.; et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, J.; Guo, J.; Manning, H.C.; Gore, J.C.; Guo, N. Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol. Rep. 2012, 28, 1301–1308. [Google Scholar] [PubMed]
- Haraguchi, N.; Ohkuma, M.; Sakashita, H.; Matsuzaki, S.; Tanaka, F.; Mimori, K.; Kamohara, Y.; Inoue, H.; Mori, M. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann. Surg. Oncol. 2008, 15, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Trumpp, A.; Wiestler, O.D. Mechanisms of disease: Cancer stem cells—targeting the evil twin. Nat. Clin. Pract. Oncol. 2008, 5, 337–347. [Google Scholar] [PubMed]
- Gupta, P.B.; Chaffer, C.L.; Weinberg, R.A. Cancer stem cells: Mirage or reality? Nat. Med. 2009, 15, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Smas, C.M.; Sul, H.S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 1993, 73, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lin, Q.; Zelterman, D.; Yun, Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009, 69, 9271–9280. [Google Scholar]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, S.S.; Pate, K.T.; Anderson, S.; Dizon, D.; Edwards, R.A.; Waterman, M.L.; Lipkin, S.M. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010, 70, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Groden, J.; Joslyn, G.; Samowitz, W.; Jones, D.; Bhattacharyya, N.; Spirio, L.; Thliveris, A.; Robertson, M.; Egan, S.; Meuth, M.; et al. Response of colon cancer cell lines to the introduction of APC, a colon-specific tumor suppressor gene. Cancer Res. 1995, 55, 1531–1539. [Google Scholar] [PubMed]
- Liu, S.; Dontu, G.; Wicha, M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lin, Q.; Glazer, P.M.; Yun, Z. Hypoxic tumor microenvironment and cancer cell differentiation. Curr. Mol. Med. 2009, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, C.; Pouyssegur, J. The role of the hypoxia-inducible factor in tumor metabolism growth and invasion. Bull. Cancer 2006, 93, E73–E80. [Google Scholar] [PubMed]
- Okabe, S.; Takeuchi, K.; Takagi, K.; Shibata, M. Stimulatory effect of the water extract of bamboo grass (folin solution) on gastric acid secretion in pylorus-ligated rats. Jpn. J. Pharmacol. 1975, 25, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Lim, H.S.; Heo, Y.R. Sasa borealis leaves extract improves insulin resistance by modulating inflammatory cytokine secretion in high fat diet-induced obese C57/BL6J mice. Nutr. Res. Pract. 2010, 4, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Reilly, R.T.; Sacchi, N. Sasa health exerts a protective effect on Her2/NeuN mammary tumorigenesis. Anticancer Res. 2004, 24, 2879–2884. [Google Scholar] [PubMed]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Anti-obesity properties of a Sasa quelpaertensis extract in high-fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2012, 76, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Fojii, M.; Yamaguchi, R. [Pharmacological studies on bamboo grass. IV. Toxicological and pharmacological effects of the extract (FIII) obtained from Sasa albomarginata Makino et Shibata (author’s transl)]. (in Japanese). Yakugaku Zasshi 1979, 99, 663–668. [Google Scholar] [PubMed]
- Kitamura, K.; Saitoh, T.; Matsuo, A.; Suyama, Y. Development of microsatellite markers for the dwarf bamboo species Sasa cernua and Sasa kurilensis (Poaceae) in northern japan. Mol. Ecol. Resour. 2009, 9, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Maeda, H. Cancer preventive effect of kumaizasa bamboo leaf extracts administered prior to carcinogenesis or cancer inoculation. Anticancer Res. 2010, 30, 111–118. [Google Scholar] [PubMed]
- Tsunoda, S.; Yamamoto, K.; Sakamoto, S.; Inoue, H.; Nagasawa, H. Effects of Sasa Health, extract of bamboo grass leaves, on spontaneous mammary tumourigenesis in SHN mice. Anticancer Res. 1998, 18, 153–158. [Google Scholar] [PubMed]
- Kim, M.; Kim, Y.S.; Kim, K.M.; Ko, H.C.; Kim, S.J.; Kim, J.H.; Kim, Y. Combination of Sasa quelpaertensis Nakai leaf extract and cisplatin suppresses the cancer stemness and invasion of human lung cancer cells. Integr. Cancer Ther. 2014, 13, 529–540. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Lee, S.I.; Choi, S.W.; Moon, S.W.; Boo, Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by alpha-melanocyte stimulating hormone. Br. J. Dermatol. 2008, 159, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.M. Apoptotic effect of Sasa quelpaertensis Nakai human colon cancer HT29. J. Life Sci. 2014, 24, 1012–1018. [Google Scholar] [CrossRef]
- Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; de Sousa, E.M.F.; van der Heijden, M.; Cameron, K.; de Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Pap, M.; Cooper, G.M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 1998, 273, 19929–19932. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Pan, F.; Jiang, H.; Chen, J.F.; Pei, L.; Xie, F.W.; Liang, H.J. Highly enriched CD133+CD44+ stem-like cells with CD133+CD44high metastatic subset in HCT116 colon cancer cells. Clin. Exp. Metastasis 2011, 28, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Guruvayoorappan, C.; Kuttan, G. Beta-carotene inhibits tumor-specific angiogenesis by altering the cytokine profile and inhibits the nuclear translocation of transcription factors in B16F-10 melanoma cells. Integr. Cancer Ther. 2007, 6, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Tsuchida, R.; Malkin, D.; Koren, G.; Baruchel, S.; Yeger, H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 2008, 26, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Jogi, A.; Ora, I.; Nilsson, H.; Lindeheim, A.; Makino, Y.; Poellinger, L.; Axelson, H.; Pahlman, S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc. Natl. Acad. Sci. USA 2002, 99, 7021–7026. [Google Scholar] [CrossRef] [PubMed]
- Couvelard, A.; O’Toole, D.; Turley, H.; Leek, R.; Sauvanet, A.; Degott, C.; Ruszniewski, P.; Belghiti, J.; Harris, A.L.; Gatter, K.; et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: Negative correlation of microvascular density and vegf expression with tumour progression. Br. J. Cancer 2005, 92, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.; Iveson, T. Management of advanced colorectal cancer: State of the art. Br. J. Cancer 2006, 95, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Boman, B.M.; Wicha, M.S. Cancer stem cells: A step toward the cure. J. Clin. Oncol. 2008, 26, 2795–2799. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Francipane, M.G.; Medema, J.P.; Stassi, G. Colon cancer stem cells: Promise of targeted therapy. Gastroenterology 2010, 138, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Li, B.X.; Liang, Y.; Peng, R.Q.; Ding, Y.; Xu, D.Z.; Zhang, X.; Pan, Z.Z.; Wan, D.S.; Zeng, Y.X.; et al. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIb. J. Transl. Med. 2009, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kida, K.; Maeda, H. Immunostimulation-mediated anti-tumor activity of bamboo (Sasa senanensis) leaf extracts obtained under “vigorous” condition. Evid. Based Complement. Altern. Med. 2010, 7, 447–457. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.M. Antiproliferative and apoptotic effects of Sasa quelpaertensis Nakia in human cancer cells. J. Life Sci. 2014, 24, 903–909. [Google Scholar]
- Ryu, M.J.; Cho, M.; Song, J.Y.; Yun, Y.S.; Choi, I.W.; Kim, D.E.; Park, B.S.; Oh, S. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem. Biophys. Res. Commun. 2008, 377, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.J.; Thibodeau, S.; Noel, D.; Fujita, N.; Tsuruo, T.; Gauthier, R.; Arguin, M.; Vachon, P.H. Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J. Cell. Biochem. 2009, 107, 639–654. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, F.; Witte, T.R.; Hardman, W.E.; Claudio, P.P. Omega-3 eicosapentaenoic acid decreases CD133 colon cancer stem-like cell marker expression while increasing sensitivity to chemotherapy. PLoS 2013, 8, e69760. [Google Scholar]
- Lee, H.A.; Park, S.; Kim, Y. Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells. Oncol. Rep. 2013, 30, 1869–1877. [Google Scholar] [PubMed]
- Kim, K.M.; Kim, Y.S.; Lim, J.Y.; Min, S.J.; Ko, H.C.; Kim, S.J.; Kim, Y. Intestinal anti-inflammatory activity of Sasa quelpaertensis leaf extract by suppressing lipopolysaccharide-stimulated inflammatory mediators in intestinal epithelial Caco-2 cells co-cultured with RAW 264.7 macrophage cells. Nutr. Res. Pract. 2015, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Toll, A.D.; Boman, B.M.; Palazzo, J.P. Dysplastic lesions in inflammatory bowel disease show increased positivity for the stem cell marker aldehyde dehydrogenase. Hum. Pathol. 2012, 43, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Saito, T.; Uchiyama, M.; Akiya, S. Studies on the anti-tumor activity of polysaccharides. I. Isolation of hemicelluloses from yakushima-bamboo and their growth inhibitory activities against sarcoma-180 solid tumor. Chem. Pharm. Bull. 1968, 16, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, B.; Wang, J.; Liu, Y.; Yu, L.; Jiang, Y. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int. Immunopharmacol. 2007, 7, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Natural antioxidants: Therapeutic prospects for cancer and neurological diseases. Mini Rev. Med. Chem. 2009, 9, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Anticancer antioxidant regulatory functions of phytochemicals. Curr. Med. Chem. 2011, 18, 2315–2338. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kakuda, Y.; Yeung, D. Antioxidative properties of lycopene and other carotenoids from tomatoes: Synergistic effects. Biofactors 2004, 21, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, S.J.; Lim, J.Y.; Kim, H.R.; Kim, S.-J.; Kim, Y. Sasa quelpaertensis Leaf Extract Inhibits Colon Cancer by Regulating Cancer Cell Stemness in Vitro and in Vivo. Int. J. Mol. Sci. 2015, 16, 9976-9997. https://doi.org/10.3390/ijms16059976
Min SJ, Lim JY, Kim HR, Kim S-J, Kim Y. Sasa quelpaertensis Leaf Extract Inhibits Colon Cancer by Regulating Cancer Cell Stemness in Vitro and in Vivo. International Journal of Molecular Sciences. 2015; 16(5):9976-9997. https://doi.org/10.3390/ijms16059976
Chicago/Turabian StyleMin, Soo Jin, Ji Ye Lim, Haeng Ran Kim, Se-Jae Kim, and Yuri Kim. 2015. "Sasa quelpaertensis Leaf Extract Inhibits Colon Cancer by Regulating Cancer Cell Stemness in Vitro and in Vivo" International Journal of Molecular Sciences 16, no. 5: 9976-9997. https://doi.org/10.3390/ijms16059976
APA StyleMin, S. J., Lim, J. Y., Kim, H. R., Kim, S.-J., & Kim, Y. (2015). Sasa quelpaertensis Leaf Extract Inhibits Colon Cancer by Regulating Cancer Cell Stemness in Vitro and in Vivo. International Journal of Molecular Sciences, 16(5), 9976-9997. https://doi.org/10.3390/ijms16059976





