Novel Tertiary Amino Containing Blinding Composite Membranes via Raft Polymerization and Their Preliminary CO2 Permeation Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Various Experimental Parameters on RAFT Polymerization of DMAEMA
2.1.1. Polymerization of DMAEMA Using Dithio and Trithio Compounds as a Source of CTA
| Run | CTA | Time (h) | Monomer/RAFT/Initiator (M/R/I) | Conversion c (%) | Mn a (GPC) | Mn b (Theoretical) | MW/Mn (PDI) |
|---|---|---|---|---|---|---|---|
| 1 | DBTTC | 6 | 100:1:0.25 | 55.4 | 42,218 | 8998 | 2.20 |
| 2 | DBTTC | 6 | 100:1:0.33 | 61.4 | 36,090 | 9903 | 2.41 |
| 3 | BDATC | 6 | 100:1:0.25 | 31.3 | 15,894 | 5201 | 1.37 |
| 4 | BDATC | 6 | 100:1:0.33 | 43.0 | 12,783 | 7032 | 1.42 |
| 5 | BDATC | 6 | 200:1:0.25 | 20.8 | 42,472 | 6880 | 1.54 |
| 6 | BDATC | 10 | 100:1:0.25 | 43.0 | 19,001 | 7308 | 1.57 |
| 7 | MTTCD | 6 | 100:1:0.25 | 52.1 | 21,415 | 8544 | 1.81 |
| 8 | CPTCD | 6 | 60:1:0.25 | 30.6 | 5230 | 3225 | 1.26 |
| 9 | CPTCD | 6 | 100:1:0.25 | 45.5 | 8325 | 7484 | 1.27 |
| 10 | CPTCD | 6 | 100:1:0.33 | 45.5 | 7879 | 7491 | 1.29 |
| 11 | CPTCD | 6 | 200:1:0.25 | 25.17 | 9570 | 8250 | 1.32 |
| 12 | CPTCD | 6 | 300:1:0.25 | 35.7 | 18,833 | 17,160 | 1.36 |
| 13 | CPTCD | 12 | 100:1:0.25 | 47.1 | 8132 | 7740 | 1.30 |
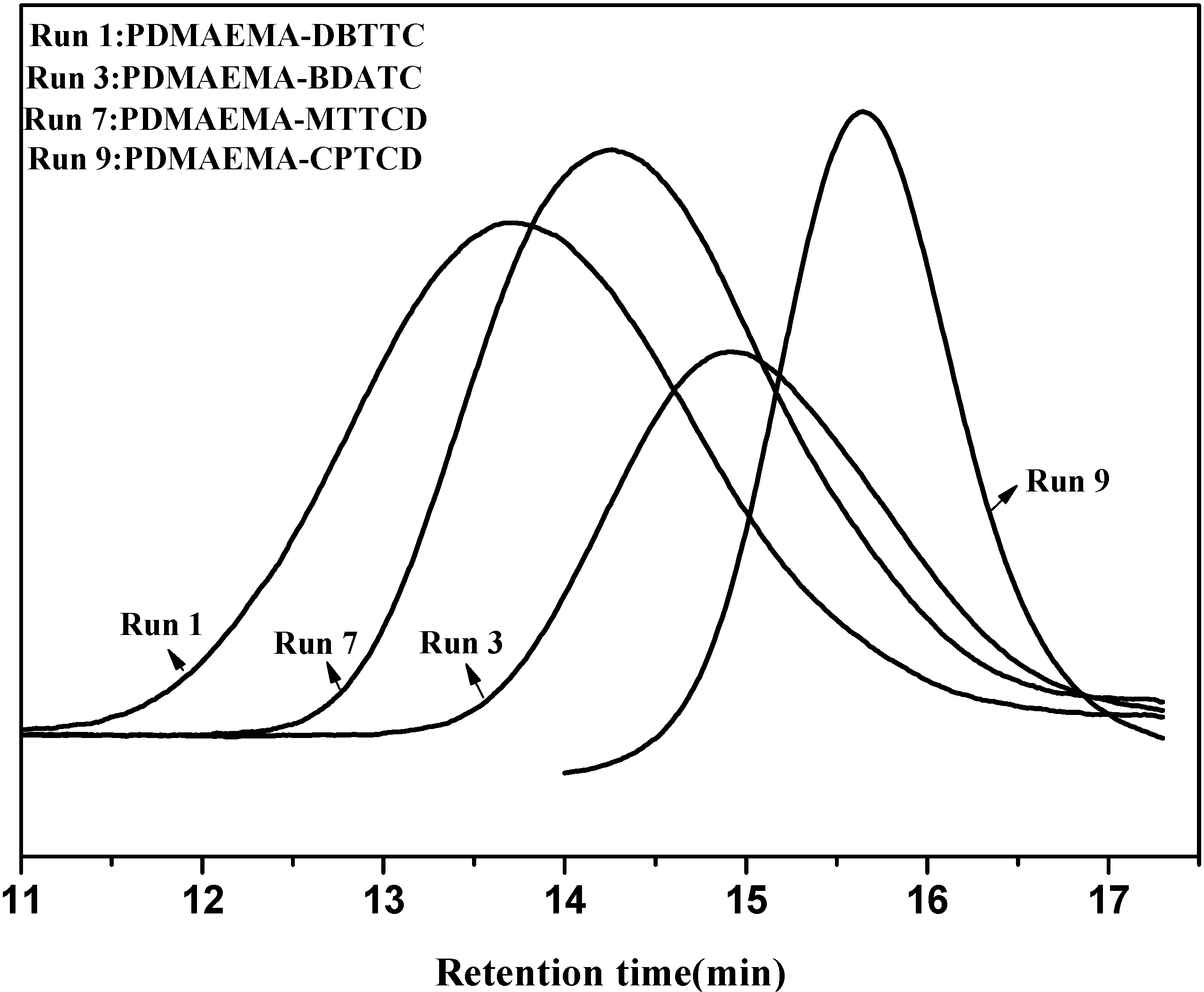
2.1.2. Kinetics of DMAEMA Polymerization in the Presence of CPTCD

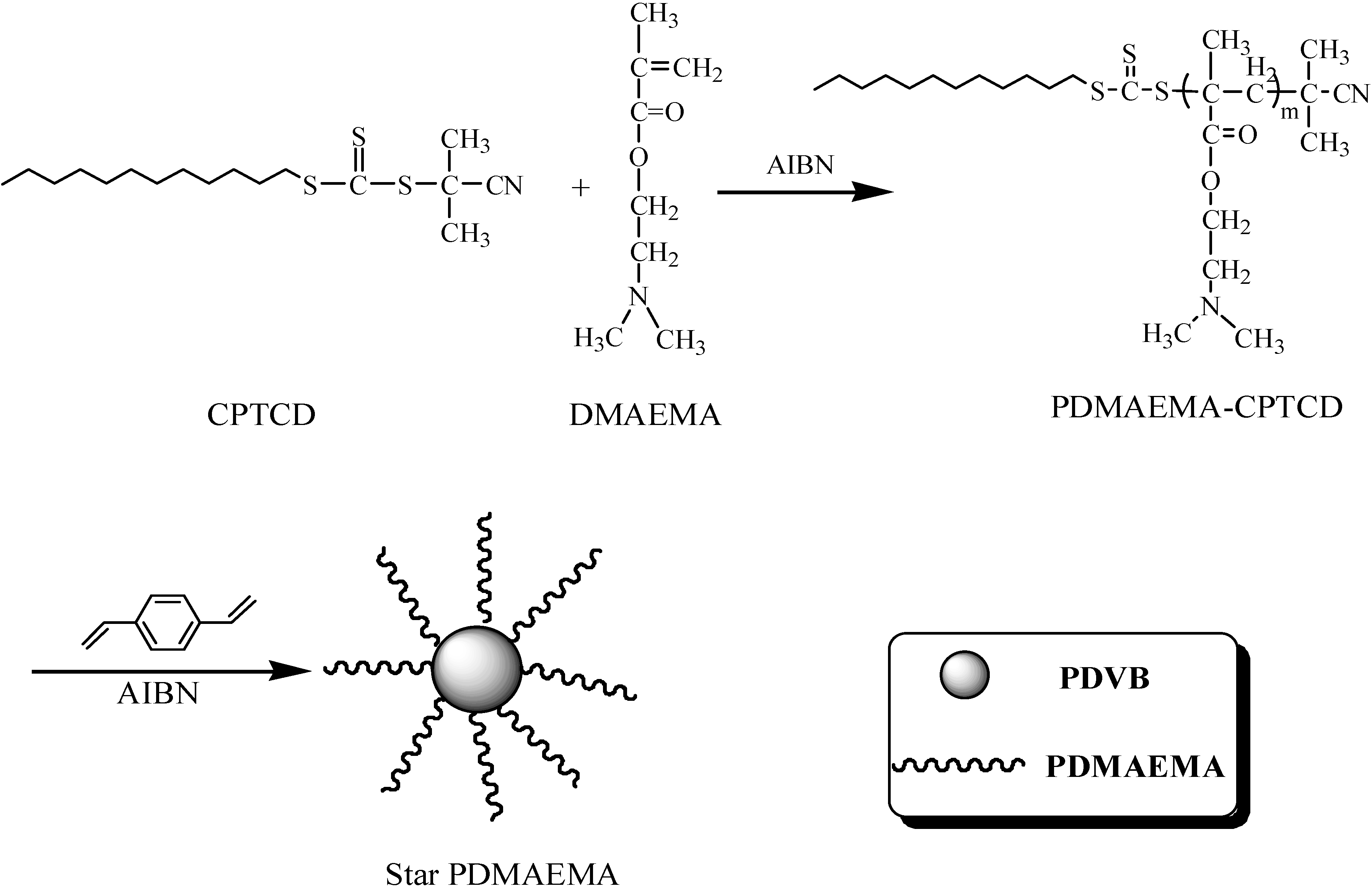
2.2. Star Polymers with PDMAEMA Arms
2.2.1. Influence of DVB/PDMAEMA-CPTCD Ratio
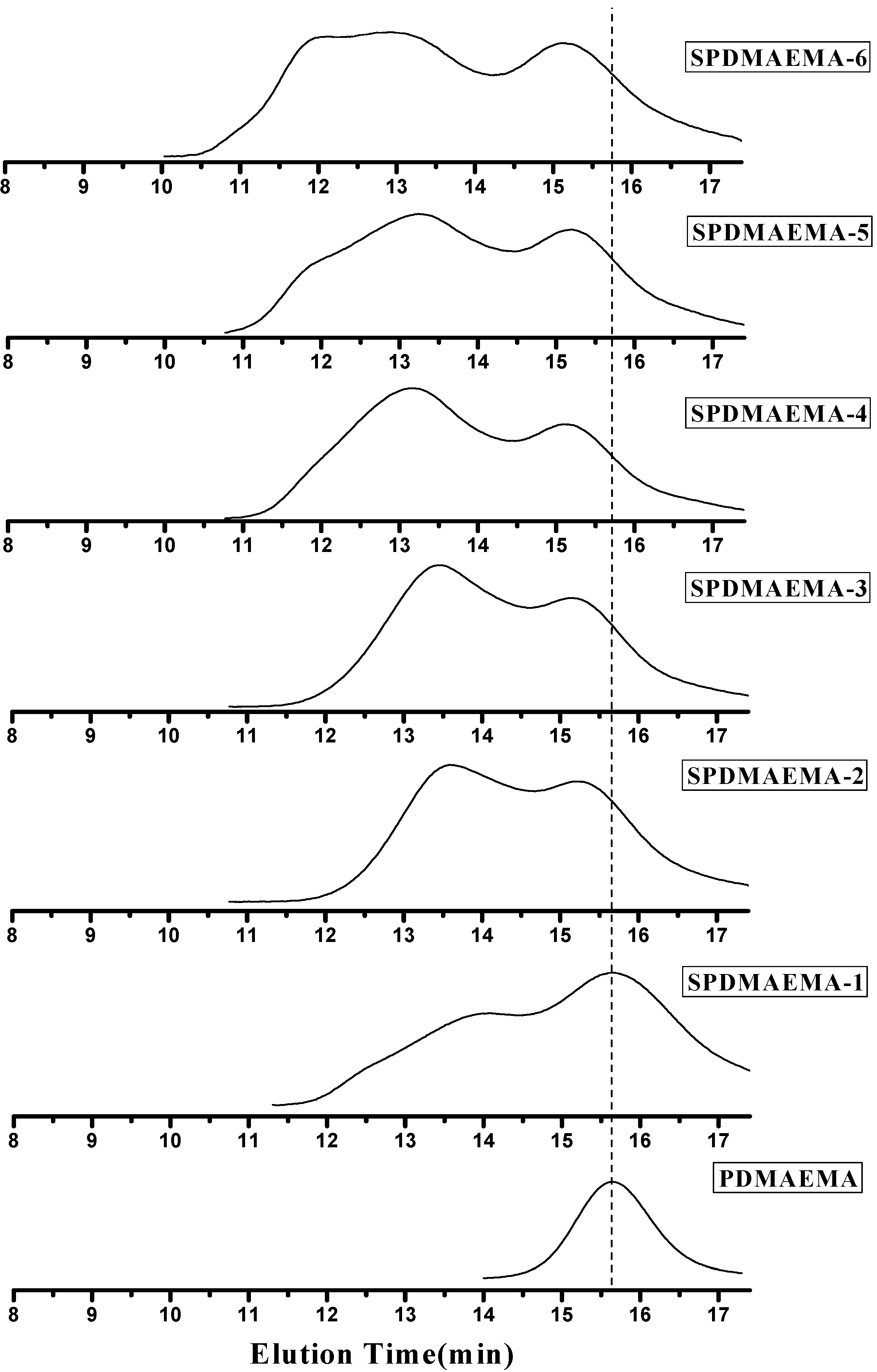
| Run | Sample | DVB/PDMAEMA Molar Ration | Mn (GPC) b | MW (GPC) b | PDI |
|---|---|---|---|---|---|
| 1 | SPDMAEMA-1 | 25 | 46,725 | 78,498 | 1.68 |
| 2 | SPDMAEMA-2 | 35 | 55,448 | 86,611 | 1.56 |
| 3 | SPDMAEMA-3 | 40 | 64,458 | 109,048 | 1.69 |
| 4 | SPDMAEMA-4 | 45 | 91,150 | 191,446 | 2.10 |
| 5 | SPDMAEMA-5 | 50 | 96,692 | 230,403 | 2.38 |
| 6 | SPDMAEMA-6 | 55 | 117,354 | 322,242 | 2.75 |
| 7 c | SPDMAEMA-7 | 60 | - | - | - |
| 8 d | SPDMAEMA-8 | 45 | 114,356 | 163,529 | 1.43 |
2.2.2. Separation of Star Polymers from Linear Polymer Contaminant
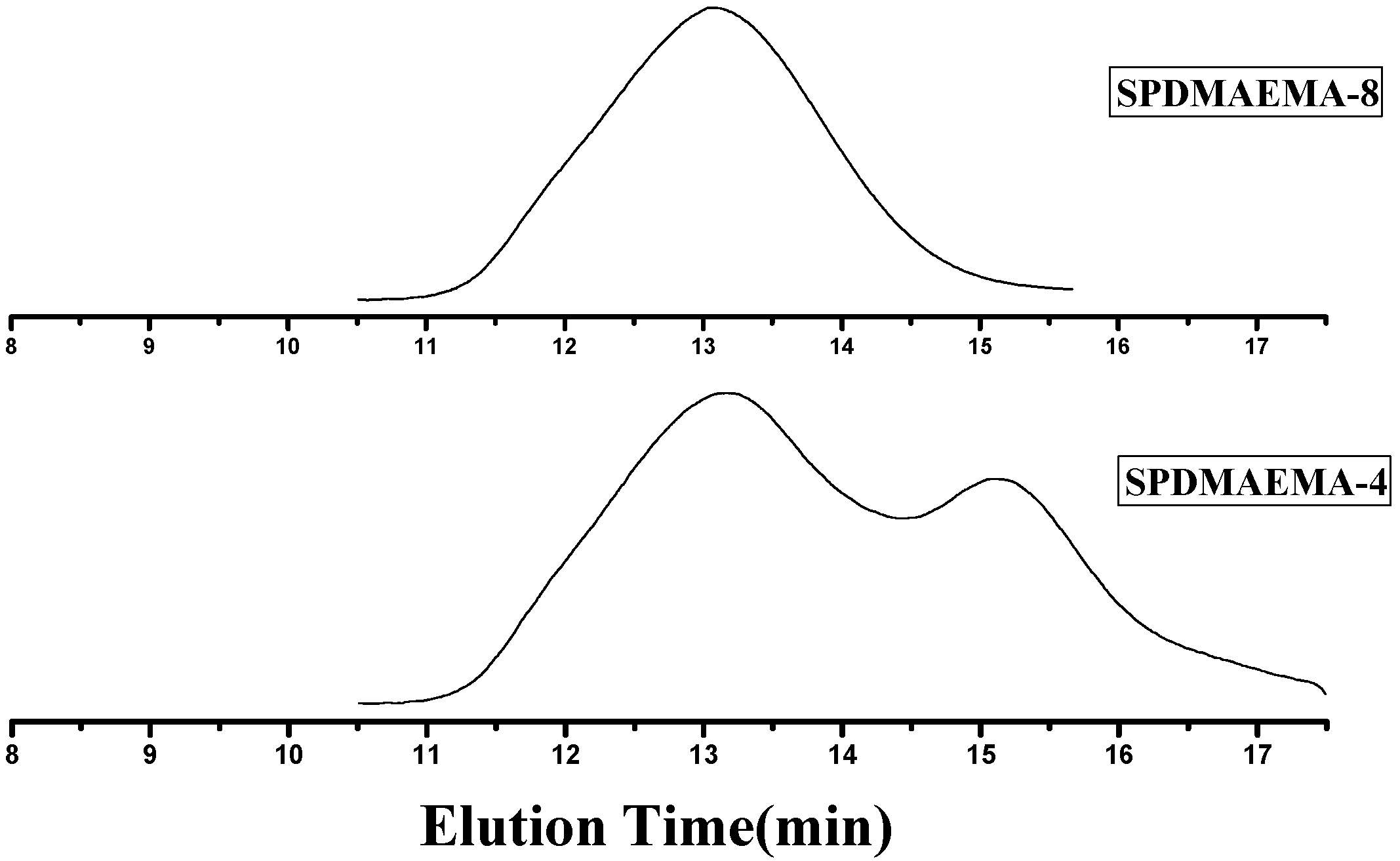
2.3. Characterization
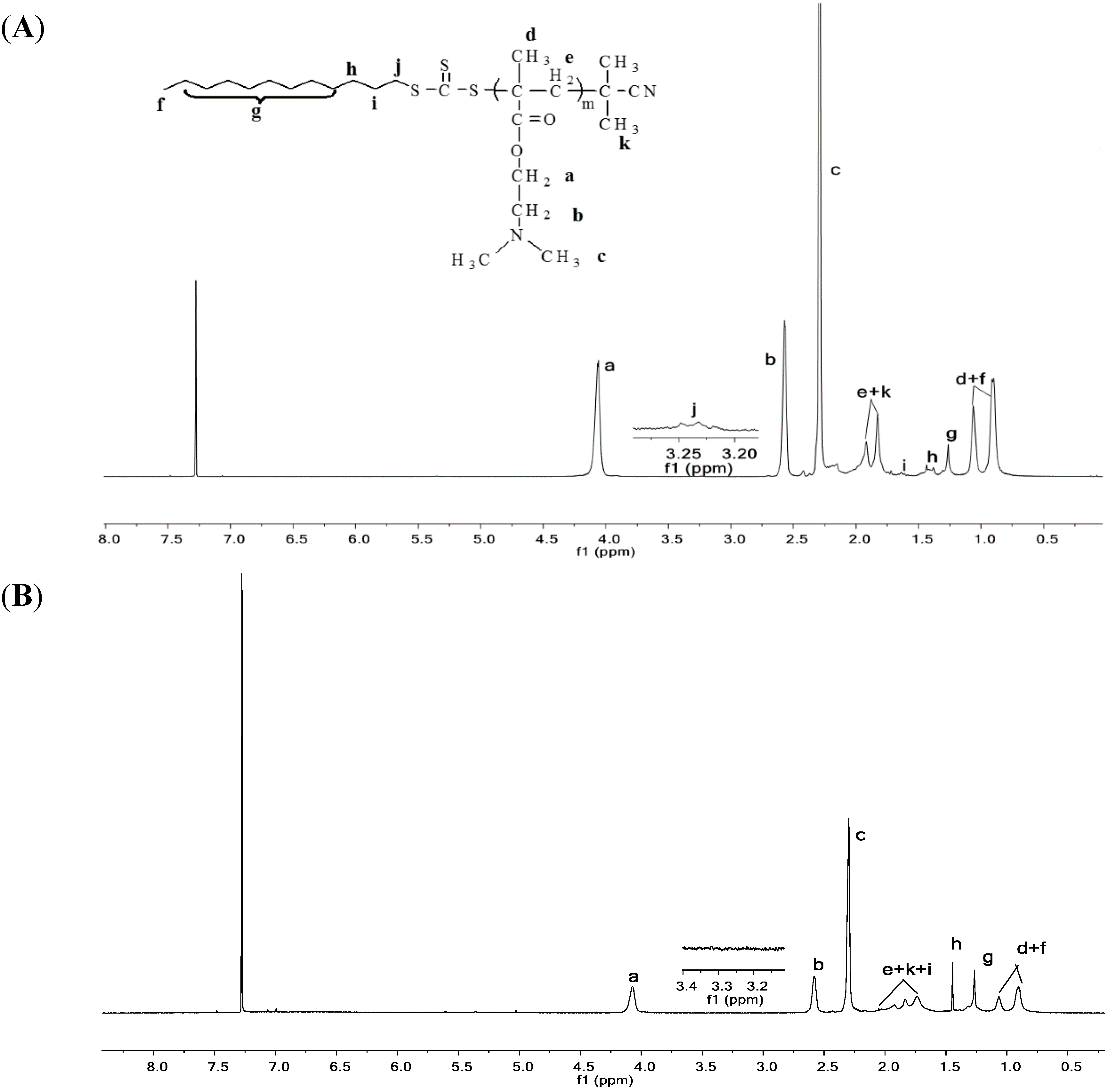
2.3.2. IR Analysis

2.3.3. XRD Analysis

2.4. CO2 Separation Performance of the Blending Composite Membranes with Different Cs/SPDMAEMA-8 Volume Ratios under the Wet Condition

3. Experimental Section
3.1. Materials
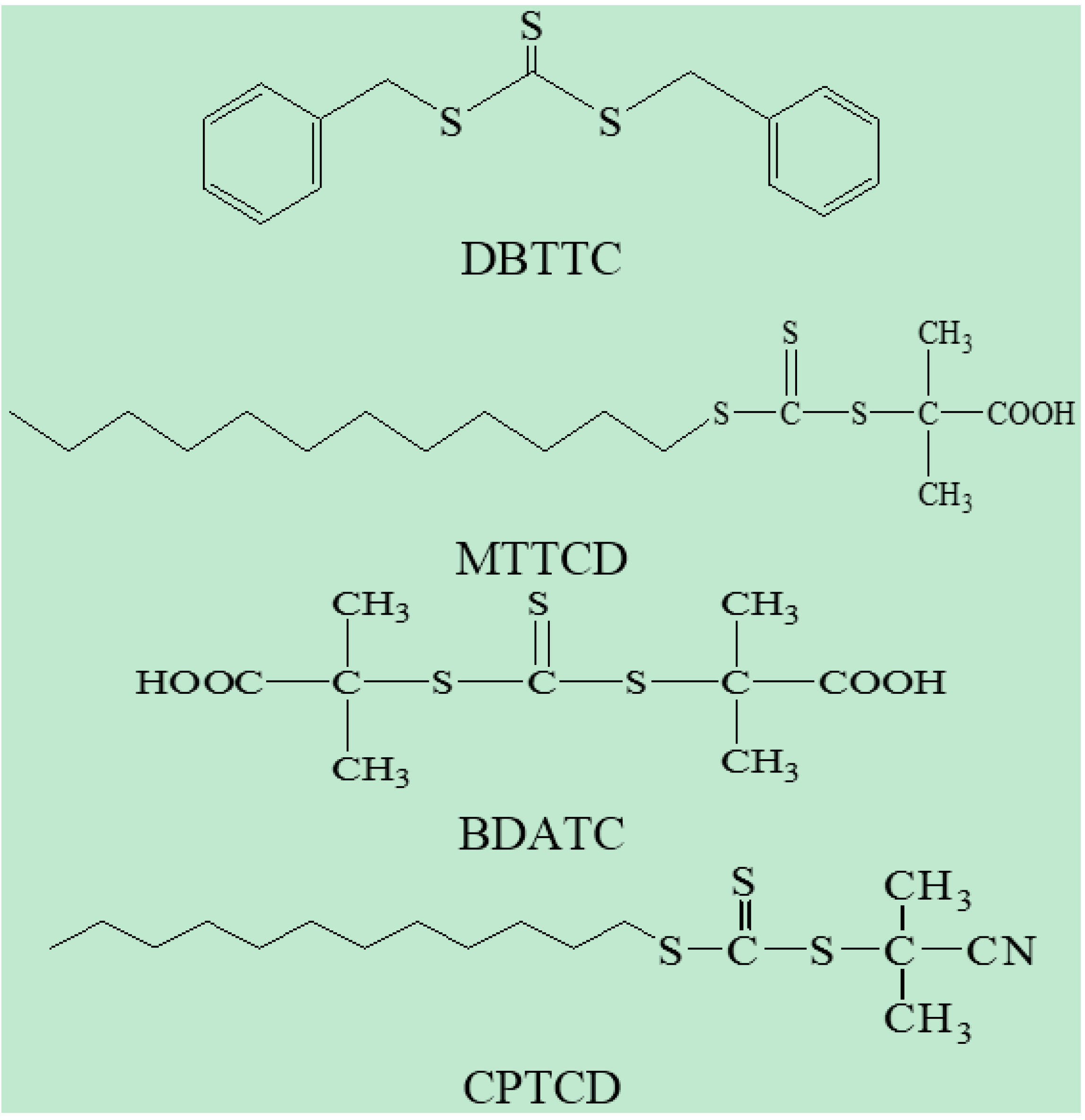
3.2. Polymerization
3.2.1. Preparation of Linear PDMAEMA Macro Transfer Agent
3.2.2. Preparation of Star Polymers
3.3. The Blinding Composite Membranes Preparation
3.4. Measurements and Instruments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen, J.; Li, J.; Jiang, T.; Ruan, H. Preparation of PMAM-b-PNVP and its hydrolysate by RAFT Polymerization: Structure characterization and its CO2 permeation performance. Adv. Mater. Res. 2013, 652–654, 386–397. [Google Scholar] [CrossRef]
- El-Azzami, L.A.; Grulke, E.A. Carbon dioxide separation from hydrogen and nitrogen by fixed facilitated transport in swollen chitosan membranes. J. Membr. Sci. 2008, 322, 225–234. [Google Scholar] [CrossRef]
- Blinova, N.V.; Svec, F. functinalized polyaniline-based composite membranes with vastly improved performance for separation of carbon dioxide from methane. J. Membr. Sci. 2012, 423–424, 514–521. [Google Scholar] [CrossRef]
- Saedi, S.; Madaeni, S.S.; Seidi, F.; Shamsabadi, A.A.; Laki, S. Fixed facilitated transport of CO2 through integrally-skinned asymmetric polyetherfulfone membrane using a novel synthesized poly (acrylonitrile-co-N,N-Dimethylaminopropyl acrylamide). Chem. Eng. J. 2014, 236, 263–273. [Google Scholar] [CrossRef]
- Matsuyama, H.; Terada, A.; Nakagawara, T.; Kitamura, Y.; Teramoto, M. Facilitated transport of CO2 through polyethylenimine/poly (vinyl alcohol) blend membrane. J. Membr. Sci. 1999, 163, 221–227. [Google Scholar] [CrossRef]
- Zhao, Y.; Ho, W.W.S. Steric Hindrance effect on amine demonstrated in solid polymer membranes for CO2 transport. J. Membr. Sci. 2012, 415–416, 132–138. [Google Scholar] [CrossRef]
- Andrew, S.; Stevens, G.W.; Kentish, S. Facilitated transport behavior of humidified gases through thin-film composite polyamide membranes for carbon dioxide capture. J. Membr. Sci. 2013, 429, 349–354. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Cai, Y.; Wang, J.X.; Wang, S.C. Novel CO2 selectively permeating membranes containing PETEDA dendrimer. J. Membr. Sci. 2007, 290, 250–258. [Google Scholar] [CrossRef]
- Dipak, R.; Takeshi, M. Oxygen-nitrogen separation. In Encyclopedia of Membrane Science and Technology; Hoek, E.M.V., Tarabara, V.V., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 1668–1692. [Google Scholar]
- Savoji, H.; Rana, D.; Matsuura, T.; Soltanieh, M.; Tabe, S. Novel surface modifying macromolecules (SMMs) blended polysulfone gas separation membranes by phase inversion technique. J. Appl. Polym. Sci. 2012, 124, 2287–2299. [Google Scholar] [CrossRef]
- Savoji, H.; Rana, D.; Matsuura, T.; Soltanieh, M.; Tabe, S. Influence of novel surface modifying macromolecules and coagulation media on the gas permeation properties of different polymeric gas separation membranes. J. Appl. Polym. Sci. 2012, 124, 2300–2310. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.S.; Goh, S.H.; Pramoda, K.P. Transport properties of cross-linked polyimide membranes induced by different generations of diaminobutane (DAB) dendrimers. J. Membr. Sci. 2004, 238, 153–160. [Google Scholar] [CrossRef]
- Yi, C.; Wang, Z.; Li, M.; Wang, J.; Wang, S. Facilitated transport of CO2 through polyvinylamine/polyethylene glycol blend membranes. Desalination 2006, 193, 90–96. [Google Scholar] [CrossRef]
- Vogtle, F. Dendrimers; Springer-Verlag: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Kovvali, A.S.; Chen, H.; Sirkar, K.K. Dendrimer membranes: A CO2 selective molecular gate. J. Am. Chem. Soc. 2000, 122, 7594–7600. [Google Scholar] [CrossRef]
- Duan, S.H.; Kouketsu, T.; Kazama, S.; Yamada, K.; Nagai, K.; Freeman, B.D. Formation and characterization of PAMAM dendrimer composite membrane for CO2 separation. In Proceeding of the International Congress on Membranes and Membrane Processes, Seoul, Korea, 21–28 August 2005.
- Zheng, G.; Pan, C. Preparation of star polymers based on polystyrene or poly (styrene-b-N-isopropyl acrylamide) and divinylbenzene via reversible addition-fragmentation chain transfer polymerization. Polymer 2005, 46, 2802–2810. [Google Scholar] [CrossRef]
- Mayadunne, R.T.A.; Jeffery, J.; Moad, G.; Rizzardo, E. Living free radical polymerization with reversible addition-fragmentation chain transfer: Approaches to star polymers. Macromolecules 2003, 36, 1505–1513. [Google Scholar] [CrossRef]
- Alhoranta, A.M.; Lehtinen, J.K.; Urtti, A.O.; Butcher, S.J.; Aseyev, V.O.; Tenhu, H.J. Cationic amphiphilic star and linear block copolymers: Synthesis, self-assembly, and in vitro gene transfection. Biomacromolecules 2011, 12, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Luke, A.C.; Qi, L.; John, F.Q.; Elvira, T.; Frank, C.; Greg, G.Q. PH-responsive poly (acrylic acid) core cross-linked star polymers: Morphology transitions in solution and multilayer thin films. Macromolecules 2008, 41, 2620–2626. [Google Scholar] [CrossRef]
- Xie, H.X.; Tao, D.; Xiang, X.Z.; Qu, Y.X.; Bai, X.J.; Wang, L. Synthesis and properties of highly branched star-shaped sulfonated block poly(arylene ether)s as proton exchange membranes. J. Membr. Sci. 2015, 473, 226–236. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Qian, Y.L.; Pang, D.X.; Zhu, B.K.; Xu, Y.Y. Porous membranes modified by hyperbranched polymers II. Effect of the arm length of amphiphilic hyperbranched-star polymers on the hydrophilicity and protein resistance of poly(vinylidene fluoride) membranes. J. Membr. Sci. 2007, 304, 138–147. [Google Scholar] [CrossRef]
- Wodzki, R.; Swiatkowski, M.; Lapienis, G. The application of core functionalized—Star-shaped polymers for cations separation by pertraction in liquid membrane systems. React. Funct. Polym. 2011, 71, 42–48. [Google Scholar] [CrossRef]
- Suda, T.; Yamazaki, K.; Kawakami, H. Syntheses of sulfonated star-hyperbranched polyimides and their proton exchange membrane properties. J. Power Sources 2010, 195, 4641–4646. [Google Scholar] [CrossRef]
- Pelet, J.M.; Putnam, D. High molecular weight poly (methacrylic acid) with narrow polydispersity by RAFT polymerization. Macromolecules 2009, 42, 1492–1499. [Google Scholar] [CrossRef]
- Flory, P.J.; Schaefgen, J.R. Synthesis of multi-chain polymers and investigation of their viscosities. J. Am. Chem. Soc. 1948, 70, 2709–2718. [Google Scholar] [CrossRef]
- Knauss, D.M.; Huang, T. Star-block-linear-block-star triblock (pom-pom) polystyrene by convergent living anionic polymerization. Macromolecules 2002, 35, 2055–2062. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Zou, Y.F.; Pan, C.Y. Synthesis and characterization of star-shaped poly (3,3-dimethyloxetane) with multifunctional oxocarbenium perchlorates as initiators. Macromol. Chem. Phys. 2001, 202, 1094–1099. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.S.; Davis, T.P.; Chien, V.; Fane, A.G. Star-polymer synthesis via radical reversible addition-fragmentation chain-transfer polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2777–2783. [Google Scholar] [CrossRef]
- Morton, M.; Helminiak, T.E.; Gadkary, S.D.; Bueche, F. Preparation and properties of monodisperse branched polystyrene. J. Polym. Sci. 1962, 57, 471–482. [Google Scholar] [CrossRef]
- Otsu, T. Iniferter concept and living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2121–2136. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Zhang, Z.X.; Ping, Y.; Li, J.; Kang, E.T.; Neoh, K.G. Star-shaped cationic polymers by atom transfer radical polymerization from β-cyclodextrin cores for nonviral gene delivery. Biomacromolecules 2009, 10, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ohno, S.; Matyjaszewski, K. Low polydispersity star polymers via cross-linking macromonomers by ATRP. J. Am. Chem. Soc. 2006, 128, 15111–15113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y. Allyl functionalized telechelic linear polymer and star polymer via RAFT polymerization. Polymer 2006, 47, 5259–5266. [Google Scholar] [CrossRef]
- Zheng, Q.; Pan, C. Preparation and characterization of dendrimer-star PNIPAAM using dithiobenzoate-terminated PPI dendrimer via RAFT polymerization. Eur. Polym. J. 2006, 42, 807–814. [Google Scholar] [CrossRef]
- Gao, H.; Min, K.; Matyjaszewski, K. Gelation in ATRP using structurally different branching reagents: Comparison of Inimer, Divinyl and Trivinyl cross-linkers. Macromolecules 2009, 42, 8039–8043. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Arm-first method as a simple and general method for synthesis of miktoarm star copolymers. J. Am. Chem. Soc. 2007, 129, 11828–11834. [Google Scholar] [CrossRef] [PubMed]
- Kyung-Youl, B.; Masami, K.; Mitsuo, S. Core-functionalized star polymers by transition metal-catalyzed living radical polymerization. 1. Synthesis and Characterization of Star Polymers with PMMA Arms and Amide Cores. Macromolecules 2001, 34, 7629–7635. [Google Scholar] [CrossRef]
- Hovestad, N.J.; van Koten, G.; Bon, S.A.F.; Haddleton, D.M. Copper(I) bromide/n-(n-octyl)-2-pyridylmethanimine-mediated living-radical polymerization of methyl methacrylate using carbosilane dendritic initiators. Macromolecules 2000, 33, 4048–4052. [Google Scholar] [CrossRef]
- Baek, K.; Kamigaito, M.; Sawamoto, M. Star-shaped polymers by metal-catalyzed living radical polymerization. 1. Design of Ru(II)-Based Systems and Divinyl Linking Agents. Macromolecules 2001, 34, 215–221. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.W.; Mccormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Loiseau, J.; Doerr, N.; Suau, J.M.; Egraz, J.B.; Llauro, M.F.; Ladaviere, C. Synthesis and characterization of poly(acrylic acid) produced by RAFT polymerization. Application as a very efficient dispersant of CaCO3, kaolin, and TiO2. Macromolecules 2003, 36, 3066–3077. [Google Scholar] [CrossRef]
- Uzulina, I.; Kanagasabapathy, S.; Claverie, J. Reversible addition fragmentation transfer (RAFT) polymerization in emulsion. Macromol. Symp. 2000, 150, 33–38. [Google Scholar] [CrossRef]
- Liu, L.; Wu, C.; Zhang, J.; Zhang, M.; Liu, Y.; Wang, X.; Fu, G. Controlled polymerization of 2-(Diethylamino)ethylmethacrylate and its block copolymer with N-isopropylacrylamide by RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3294–3305. [Google Scholar] [CrossRef]
- Bosman, A.W.; Vestberg, R.; Heumann, A.; Frechet, J.M.J.; Hawker, C.J. A modular approach toward functionalized three-dimensional macromolecules: From synthetic concepts to practical applications. J. Am. Chem. Soc. 2003, 125, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yang, L.; Zhang, X.; Pan, C. Synthesis and characterization of polystyrene-b-tetraaniline stars from polystyrene stars with surface reactive groups prepared by RAFT polymerization. Eur. Polym. J. 2007, 43, 3873–3881. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhou, M.; Yang, S.; Shen, J. Novel Tertiary Amino Containing Blinding Composite Membranes via Raft Polymerization and Their Preliminary CO2 Permeation Performance. Int. J. Mol. Sci. 2015, 16, 9078-9096. https://doi.org/10.3390/ijms16059078
Zhu L, Zhou M, Yang S, Shen J. Novel Tertiary Amino Containing Blinding Composite Membranes via Raft Polymerization and Their Preliminary CO2 Permeation Performance. International Journal of Molecular Sciences. 2015; 16(5):9078-9096. https://doi.org/10.3390/ijms16059078
Chicago/Turabian StyleZhu, Lifang, Mali Zhou, Shanshan Yang, and Jiangnan Shen. 2015. "Novel Tertiary Amino Containing Blinding Composite Membranes via Raft Polymerization and Their Preliminary CO2 Permeation Performance" International Journal of Molecular Sciences 16, no. 5: 9078-9096. https://doi.org/10.3390/ijms16059078







