The Potential Role of DNA Methylation in Abdominal Aortic Aneurysms
Abstract
:1. Introduction
2. Results
| Experiment | Group | Smoking * | n | Age (Years ± SD) |
|---|---|---|---|---|
| DNA methylation (450k BeadChip) | Control | Yes | 10 | 55.7 ± 8.0 |
| Control | No | 11 | 67.0 ± 11.2 | |
| AAA | Yes | 11 | 65.8 ± 5.2 | |
| AAA | No | 9 | 77.3 ± 9.3 | |
| Gene Expression (Q-RT-PCR) | Control | Yes | 10 | 58.0 ± 9.0 |
| Control | No | 10 | 67.3 ± 13.0 | |
| AAA | Yes | 14 | 68.1 ± 4.8 | |
| AAA | No | 12 | 78.3 ± 6.1 |
| Case ID | Age (Years) | Sex | Cause of Death | Smoking * | Classification |
|---|---|---|---|---|---|
| ME0503 | 54 | Male | Cardiac arrest | Unknown | Control |
| ME0501 | 69 | Female | Head trauma | Unknown | Control |
| ME0105 | 78 | Male | Cardiac arrest | Unknown | Control |
| ME0505 | 59 | Female | Cardiovascular | Unknown | Control |
| ELM0065 | 77 | Male | NA | No | AAA |
| ELM0038 | 65 | Female | NA | No | AAA |
| ELM0056 | 61 | Male | NA | No | AAA |
| ELM0060 | 77 | Male | NA | Yes | AAA |
| ELM0041 | 62 | Male | NA | Yes | AAA |
| ELM0063 | 71 | Male | NA | Yes | AAA |
2.1. Genome-Wide Analysis of DNA Methylation in Peripheral Blood Mononuclear Cells of AAA Patients and Non-Aneurysmal Controls
| CpGI * Name and Location | Gene | Gene Context of the CpGI |
|---|---|---|
| chr4: 190962111–190962689 | null | NA |
| chr6: 2891929–2892182 | SERPINB9 | Body |
| chr6: 41068475–41069343 | ADCY10P1 | Body |
| chr6: 168435835–168436086 | KIF25 | Body |
| chr9: 124987743–124991086 | LHX6 | Body |
| chr11: 396685–397462 | PKP3 | Body |
| chr11: 75139454–75139817 | KLHL35 | Body, Promotera |
| chr17: 152117–152438 | RPH3AL | Body |
| chr17: 19099818–19100138 | null | NA |
| chr19: 1033605–1035236 | CNN2 | Body |
| chr19: 37786692–37787110 | null | NA |
| chr19: 612989–614068 | HCN2 | Body |
| chr19: 49001748–49003087 | LMTK3 | Body |
| chr20: 32254811–32255989 | NECAB3 | Body |
| chr21: 38630052–38630507 | DSCR3 | Body |
| chrX: 72298626–72299108 | PABPC1L2A | 1st Exon |
| Gene | p Value (Diagnosis) | p Value (Age) | p Value (Smoking) |
|---|---|---|---|
| ADCY10P1 | 0.0221 | 0.06702 | 0.04543 |
| CNN2 | 0.006514 | 0.032 | 0.02625 |
| KLHL35 | 0.009153 | 0.009907 | 0.007803 |
| SERPINB9 | 0.00309 | 0.02121 | 0.1016 |
2.2. Real-Time Quantitative RT-PCR to Measure mRNA Levels of CNN2 and SERPINB9 in PBMC of AAA Patients and Non-Aneurysmal Controls
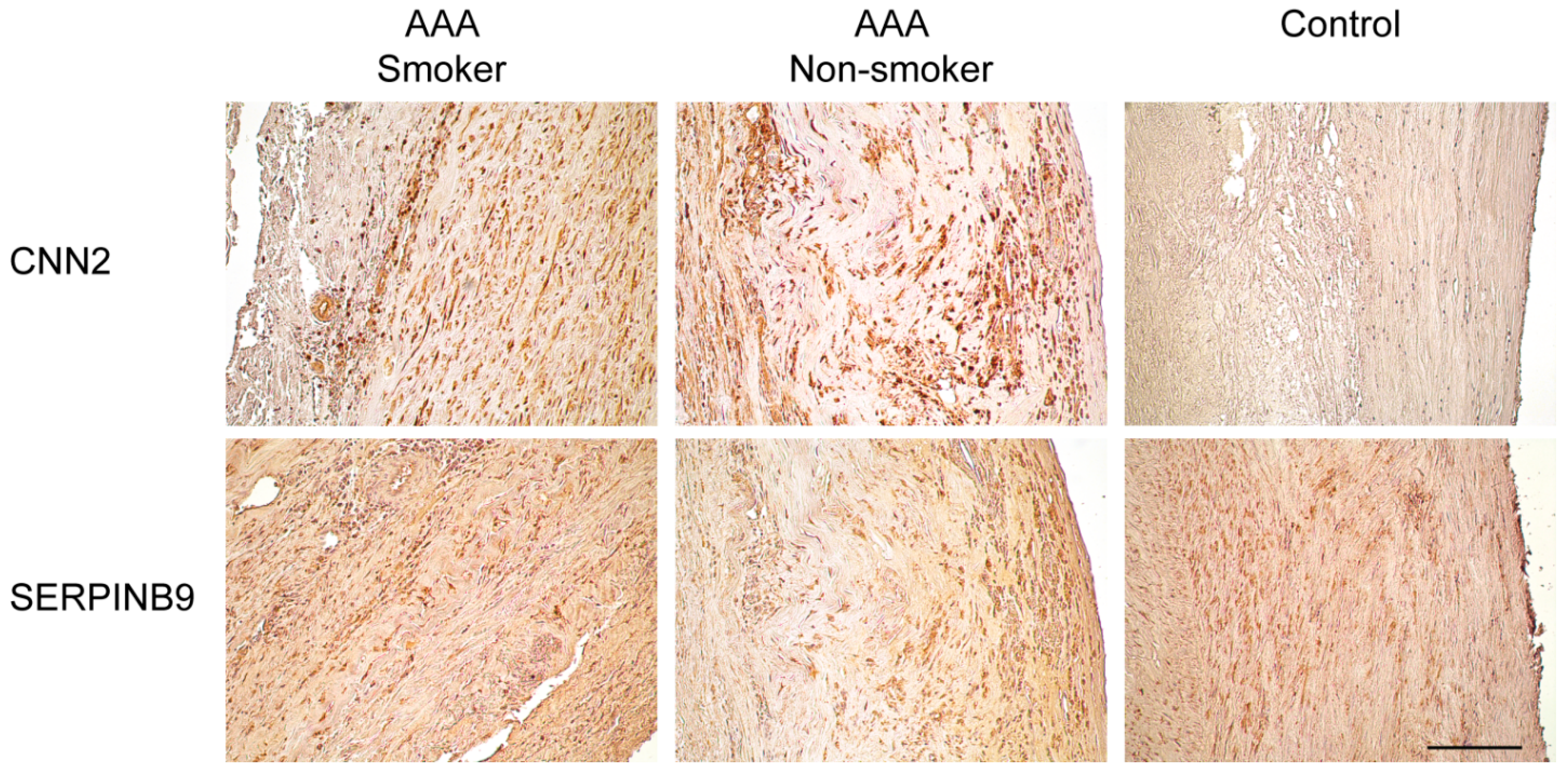
2.3. Immunohistological Staining of CNN2 and SERPINB9 in the Aortic Wall of AAA Patients and Non-Aneurysmal Controls

| Gene Symbol | Gene ID | Protein Symbols | Full Name | Catalog Number | Supplier | Species | IHC Dilution |
|---|---|---|---|---|---|---|---|
| CNN2 | 1265 | CNN2 | Calponin 2 | TA503688 | OriGene, Rockville, MD, USA | Mouse monoclonal | 1:234 |
| SERPINB9 | 5272 | CAP3, PI9, SERPINB9 | Serpin peptidase inhibitor, clade B (ovalbumin), member 9 | TA312970 | OriGene, Rockville, MD, USA | Rabbit polyclonal | 1:4667 |
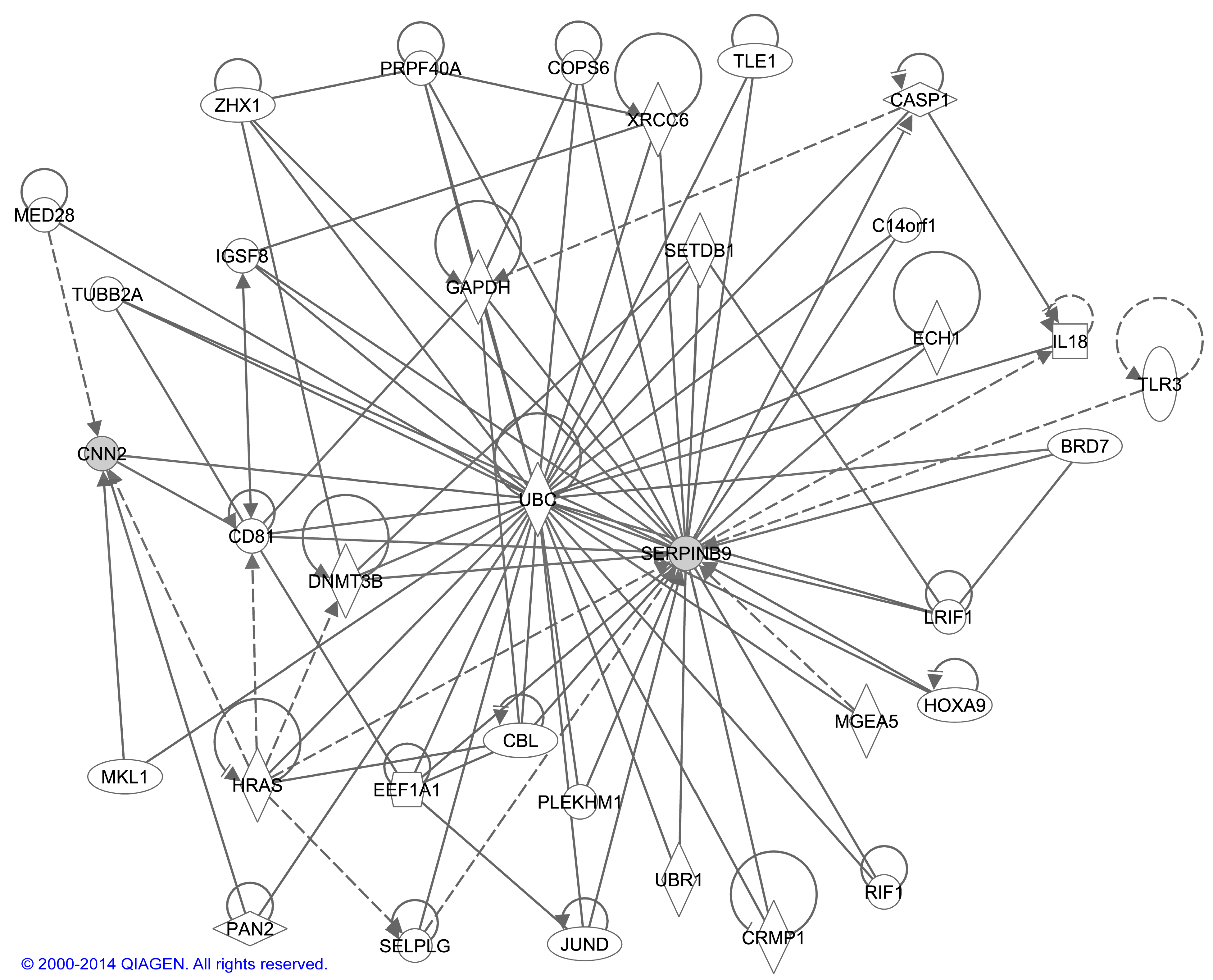
2.4 Bioinformatic Analysis of Interactions between CNN2 and SERPINB9
3. Discussion
4. Experimental Section
4.1. Samples
4.1.1. Processing of Blood Samples for DNA and RNA Isolation
4.1.2. Human Aortic Tissue Samples
4.2. Microarray-Based DNA Methylation Study
4.3. Real-Time Quantitative Reverse Transcriptase PCR
4.4. Immunohistochemical Analysis of Aortic Tissue
4.5. Bioinformatic Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuivaniemi, H.; Platsoucas, C.D.; Tilson, M.D., III. Aortic aneurysms: An immune disease with a strong genetic component. Circulation 2008, 117, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A. Abdominal aortic aneurysm. Ann. Intern. Med. 2009. [Google Scholar] [CrossRef]
- Krishna, S.M.; Dear, A.E.; Norman, P.E.; Golledge, J. Genetic and epigenetic mechanisms and their possible role in abdominal aortic aneurysm. Atherosclerosis 2010, 212, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Hinterseher, I.; Tromp, G.; Kuivaniemi, H. Genes and abdominal aortic aneurysm. Ann. Vasc. Surg. 2011, 25, 388–412. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.L.; Yan, M.S.; Marsden, P.A. Epigenetics and cardiovascular disease. Can. J. Cardiol. 2013, 29, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Zaina, S.; Heyn, H.; Carmona, F.J.; Varol, N.; Sayols, S.; Condom, E.; Ramirez-Ruz, J.; Gomez, A.; Goncalves, I.; Moran, S.; et al. DNA methylation map of human atherosclerosis. Circ. Cardiovasc. Genet. 2014, 7, 692–700. [Google Scholar] [CrossRef]
- Yang, X.; Han, H.; de Carvalho, D.D.; Lay, F.D.; Jones, P.A.; Liang, G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 2014, 26, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Crudele, V.; Soricelli, A.; Al-Omran, M.; Vitale, N.; Infante, T.; Mancini, F.P. Primary prevention of atherosclerosis: A clinical challenge for the reversal of epigenetic mechanisms? Circulation 2012, 125, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q. The critical importance of epigenetics in autoimmunity. J. Autoimmun. 2013, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M.; Neddermann, D.; Piorunska-Stolzmann, M.; Jagodzinski, P.P. Role of epigenetic mechanisms in the development of chronic complications of diabetes. Diabetes Res. Clin. Pract. 2014, 105, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Semi, K.; Yamada, Y. Epigenetic regulation leading to induced pluripotency drives cancer development in vivo. Biochem. Biophys. Res. Commun. 2014, 455, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Heyn, H.; Esteller, M. DNA methylation profiling in the clinic: Applications and challenges. Nat. Rev. Genet. 2012, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Breitling, L.P.; Yang, R.; Korn, B.; Burwinkel, B.; Brenner, H. Tobacco-smoking-related differential DNA methylation: 27K Discovery and replication. Am. J. Hum. Genet. 2011, 88, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, M.J.; Thompson, S.G.; Brown, L.C.; Powell, J.T. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br. J. Surg. 2012, 99, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Stackelberg, O.; Bjorck, M.; Larsson, S.C.; Orsini, N.; Wolk, A. Sex differences in the association between smoking and abdominal aortic aneurysm. Br. J. Surg. 2014, 101, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Keliher, E.; Marinelli, B.; Leuschner, F.; Robbins, C.S.; Gerszten, R.E.; Pittet, M.J.; Swirski, F.K.; Weissleder, R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Boumpas, D.T.; Eleftheriades, E.G.; Molina, R.; Barez, S.; Atkinson, J.; Older, S.A.; Balow, J.E.; Tsokos, G.C. c-Myc proto-oncogene expression in peripheral blood mononuclear cells from patients with primary Sjögren’s syndrome. Arthritis Rheum. 1990, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, H.; Okada, M.; Kaneko, H.; Hishikawa, T.; Sekigawa, I.; Hashimoto, H. Possible role of DNA hypomethylation in the induction of SLE: Relationship to the transcription of human endogenous retroviruses. Clin. Exp. Rheumatol. 2003, 21, 733–738. [Google Scholar] [PubMed]
- Nile, C.J.; Read, R.C.; Akil, M.; Duff, G.W.; Wilson, A.G. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008, 58, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, E.S.; Moon, C.M.; Park, J.J.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Genetic polymorphisms of IL-23R and IL-17A and novel insights into their associations with inflammatory bowel disease. Gut 2011, 60, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Costa-Reis, P.; Sullivan, K.E. Genetics and epigenetics of systemic lupus erythematosus. Curr. Rheumatol. Rep. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Sandoval, J.; Heyn, H.; Moran, S.; Serra-Musach, J.; Pujana, M.A.; Bibikova, M.; Esteller, M. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics 2011, 6, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Breitling, L.P. Current genetics and epigenetics of smoking/tobacco-related cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hu, G.; Hanai, J.; Yadlapalli, G.; Lin, Y.; Zhang, B.; Galloway, J.; Bahary, N.; Sinha, S.; Thisse, B.; et al. A critical role for calponin 2 in vascular development. J. Biol. Chem. 2006, 281, 6664–6672. [Google Scholar] [CrossRef]
- Albinsson, S.; Nordstrom, I.; Hellstrand, P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J. Biol. Chem. 2004, 279, 34849–34855. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Salerno Uriarte, J.A.; de Luca Tupputi Schinosa, L.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur. Heart J. 2008, 29, 1439–1445. [Google Scholar] [CrossRef]
- Sutton, V.R.; Vaux, D.L.; Trapani, J.A. Bcl-2 prevents apoptosis induced by perforin and granzyme B, but not that mediated by whole cytotoxic lymphocytes. J. Immunol. 1997, 158, 5783–5790. [Google Scholar] [PubMed]
- Bird, C.H.; Sutton, V.R.; Sun, J.; Hirst, C.E.; Novak, A.; Kumar, S.; Trapani, J.A.; Bird, P.I. Selective regulation of apoptosis: The cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol. Cell. Biol. 1998, 18, 6387–6398. [Google Scholar] [PubMed]
- Bots, M.; van Bostelen, L.; Rademaker, M.T.; Offringa, R.; Medema, J.P. Serpins prevent granzyme-induced death in a species-specific manner. Immunol. Cell Biol. 2006, 84, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Sukhova, G.K.; Foster, D.; Kisiel, W.; Libby, P.; Schonbeck, U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1β-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J. Exp. Med. 2000, 191, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Q.; Desprat, R.; Fu, H.; Olivier, E.; Lin, C.M.; Lobell, A.; Gowda, S.N.; Aladjem, M.I.; Bouhassira, E.E. DNA methylation supports intrinsic epigenetic memory in mammalian cells. PLoS Genet. 2006, 2, e65. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Ladd-Acosta, C.; Wen, B.; Wu, Z.; Montano, C.; Onyango, P.; Cui, H.; Gabo, K.; Rongione, M.; Webster, M.; et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 2009, 41, 178–186. [Google Scholar] [CrossRef]
- Sullivan, K.E.; Suriano, A.; Dietzmann, K.; Lin, J.; Goldman, D.; Petri, M.A. The TNFα locus is altered in monocytes from patients with systemic lupus erythematosus. Clin. Immunol. 2007, 123, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bock, C. Analysing and interpreting DNA methylation data. Nat. Rev. Genet. 2012, 13, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.; Whitlock, E.P.; Beil, T.L.; Lederle, F.A. Screening for abdominal aortic aneurysm: A best-evidence systematic review for the U.S: Preventive services task force. Ann. Intern. Med. 2005, 142, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hinterseher, I.; Erdman, R.; Donoso, L.A.; Vrabec, T.R.; Schworer, C.M.; Lillvis, J.H.; Boddy, A.M.; Derr, K.; Golden, A.; Bowen, W.D.; et al. Role of complement cascade in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Lillvis, J.H.; Erdman, R.; Schworer, C.M.; Golden, A.; Derr, K.; Gatalica, Z.; Cox, L.A.; Shen, J.; Vander Heide, R.S.; Lenk, G.M.; et al. Regional expression of HOXA4 along the aorta and its potential role in human abdominal aortic aneurysm. BMC Physiol. 2011, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Pahl, M.C.; Derr, K.; Gäbel, G.; Hinterseher, I.; Elmore, J.R.; Schworer, C.M.; Peeler, T.C.; Franklin, D.P.; Gray, J.L.; Carey, D.J.; et al. MicroRNA expression signature in human abdominal aortic aneurysms. BMC Med. Genomics 2012, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Hinterseher, I.; Erdman, R.; Elmore, J.R.; Stahl, E.; Pahl, M.C.; Derr, K.; Golden, A.; Lillvis, J.H.; Cindric, M.C.; Jackson, K.; et al. Novel pathways in the pathobiology of human abdominal aortic aneurysms. Pathobiology 2013, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Koenig, S.N.; Huang, N.; Cheng, J.; Beceiro, S.; Guggilam, A.; Kuivaniemi, H.; Partida-Sanchez, S.; Garg, V. Inhibition of Notch1 signaling reduces abdominal aortic aneurysm in mice by attenuating macrophage-mediated inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing, 3.1.1. R Foundation for Statistical Computing, Vienna, Austria, 2014. [Google Scholar]
- Schalkwyk, L.C.; Pidsley, R.; Wong, C.C.Y.; Toulemeimat, N.; Defrance, M.; Teschendorff, A.; Maksimovic, J. wateRmelon: Illumina 450 Methylation Array Normalization and Metrics, R package version 1.4.0. 2013.
- Hansen, K.D. IlluminaHumanMethylation450kanno.ilmn12.hg19: Annotation for Illumina’s 450k Methylation Arrays, R package version 0.2.1. 2013.
- Smyth, G.K. limma: Linear Models for Microarray Data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R.C., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005; pp. 397–420. [Google Scholar]
- Triche, T., Jr. IlluminaHumanMethylation450k.db: Illumina Human Methylation 450k Annotation Data, R package version 2.0.7. 2012.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2010; p. 213. [Google Scholar]
- Tromp, G.; Gatalica, Z.; Skunca, M.; Berguer, R.; Siegel, T.; Kline, R.A.; Kuivaniemi, H. Elevated expression of matrix metalloproteinase-13 in abdominal aortic aneurysms. Ann. Vasc. Surg. 2004, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryer, E.J.; Ronning, K.E.; Erdman, R.; Schworer, C.M.; Elmore, J.R.; Peeler, T.C.; Nevius, C.D.; Lillvis, J.H.; Garvin, R.P.; Franklin, D.P.; et al. The Potential Role of DNA Methylation in Abdominal Aortic Aneurysms. Int. J. Mol. Sci. 2015, 16, 11259-11275. https://doi.org/10.3390/ijms160511259
Ryer EJ, Ronning KE, Erdman R, Schworer CM, Elmore JR, Peeler TC, Nevius CD, Lillvis JH, Garvin RP, Franklin DP, et al. The Potential Role of DNA Methylation in Abdominal Aortic Aneurysms. International Journal of Molecular Sciences. 2015; 16(5):11259-11275. https://doi.org/10.3390/ijms160511259
Chicago/Turabian StyleRyer, Evan J., Kaitryn E. Ronning, Robert Erdman, Charles M. Schworer, James R. Elmore, Thomas C. Peeler, Christopher D. Nevius, John H. Lillvis, Robert P. Garvin, David P. Franklin, and et al. 2015. "The Potential Role of DNA Methylation in Abdominal Aortic Aneurysms" International Journal of Molecular Sciences 16, no. 5: 11259-11275. https://doi.org/10.3390/ijms160511259