Preparation and in Vivo Evaluation of a Dutasteride-Loaded Solid-Supersaturatable Self-Microemulsifying Drug Delivery System
Abstract
:1. Introduction
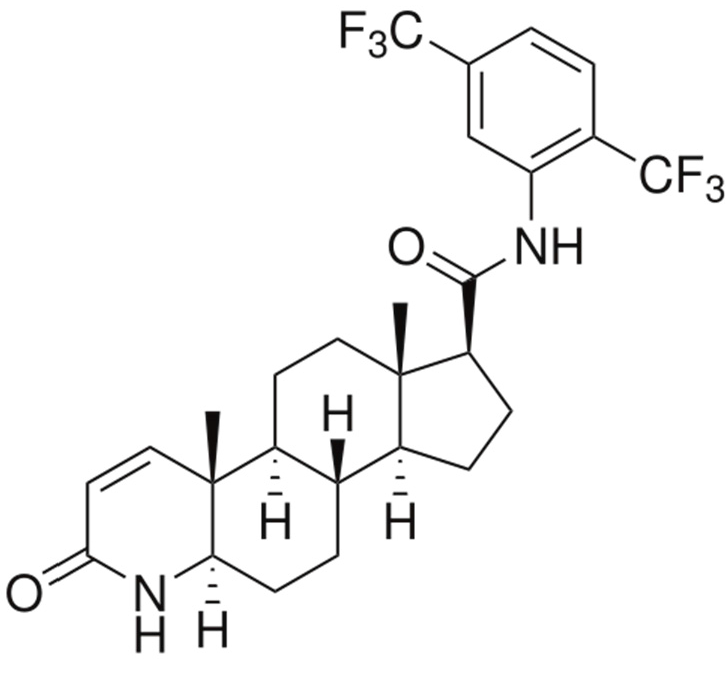
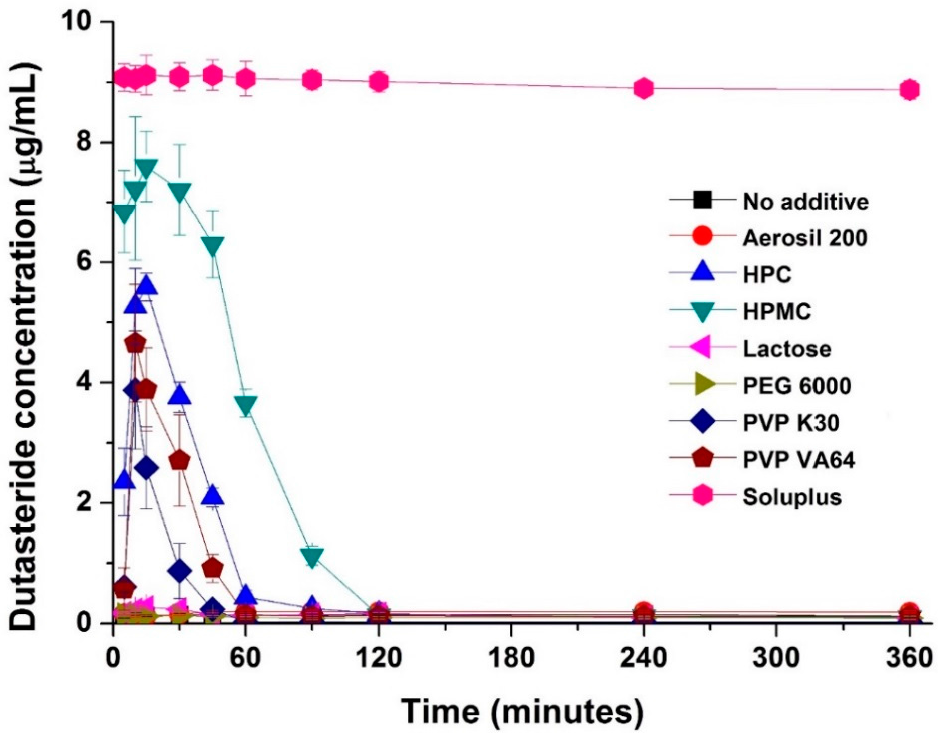
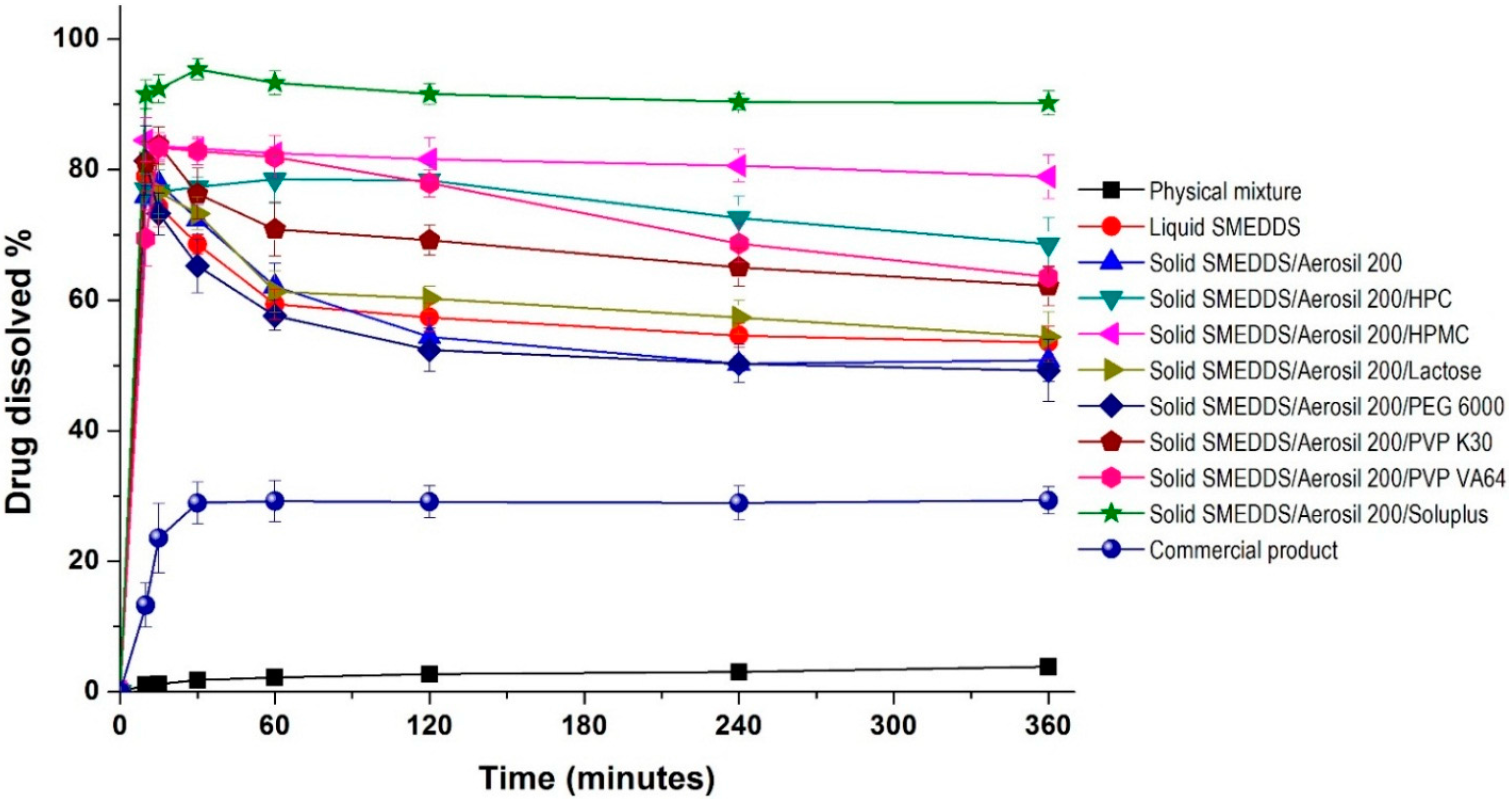
2. Results and Discussion
| Formulation | Volume Mean Particle Size a (μm) | Mean Droplet Size b (nm) |
|---|---|---|
| Liquid SMEDDS | ND | 37.5 ± 3.7 (0.141) d |
| SMEDDS:Aerosil 200 = 1:1 | 7.63 ± 2.01 (1.98) c | 40.9 ± 5.5 (0.189) |
| SMEDDS:Aerosil 200:HPC = 1:1:1 | 8.19 ± 1.93 (1.91) | 133.7 ± 22.6 (0.243) |
| SMEDDS:Aerosil 200:HPMC = 1:1:1 | 7.52 ± 2.11 (2.11) | 94.2 ± 10.5 (0.221) |
| SMEDDS:Aerosil 200:lactose = 1:1:1 | 8.01 ± 1.22 (1.89) | 55.5 ± 7.7 (0.198) |
| SMEDDS:Aerosil 200:PEG6000 = 1:1:1 | 9.02 ± 2.22 (2.12) | 175.7 ± 24.6 (0.246) |
| SMEDDS:Aerosil 200:PVP K30 = 1:1:1 | 8.44 ± 1.56 (1.89) | 69.3 ± 8.5 (0.179) |
| SMEDDS:Aerosil 200:PVA VA64 = 1:1:1 | 8.78 ± 1.88 (1.97) | 50.3 ± 9.6 (0.206) |
| SMEDDS:Aerosil 200:Soluplus = 1:1:1 | 7.49 ± 1.72 (1.95) | 43.9 ± 8.9 (0.195) |
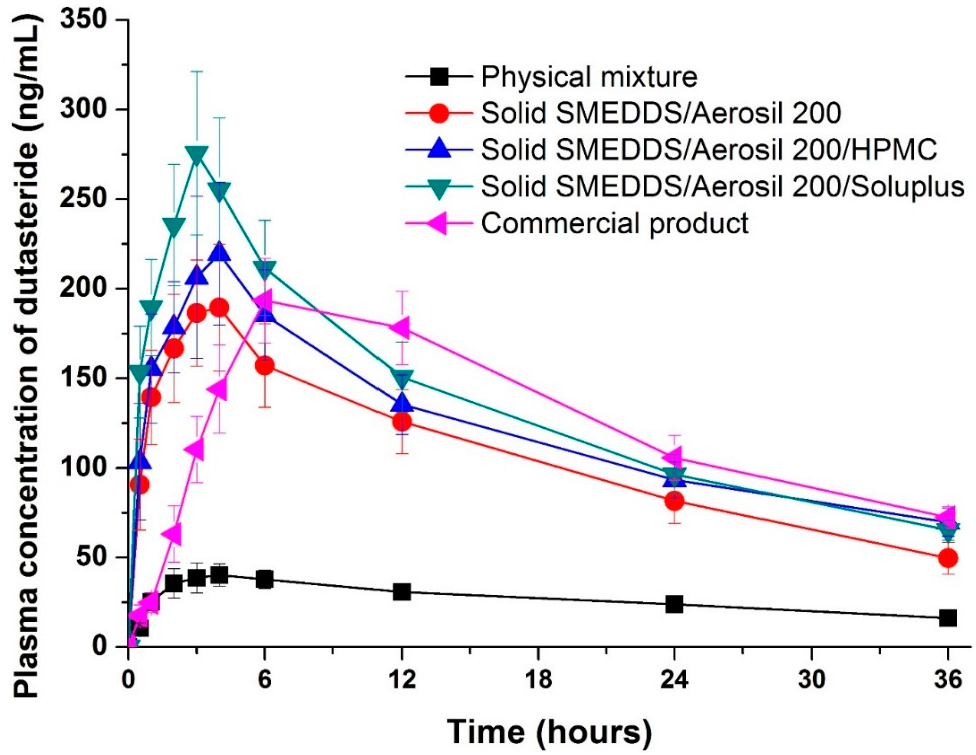
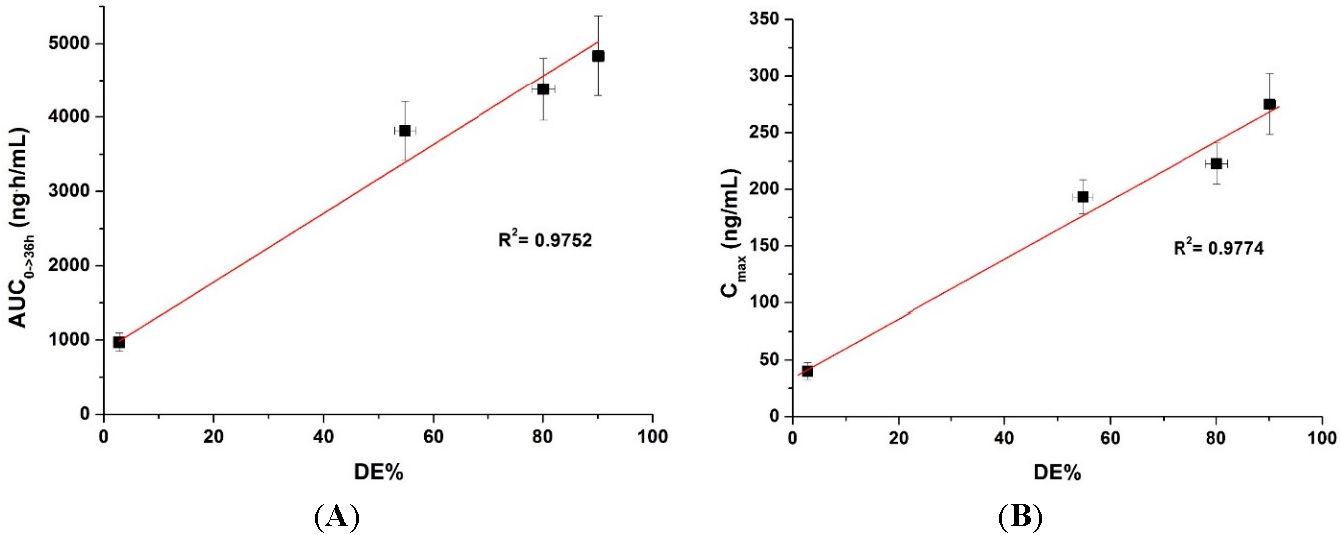
| Formulation | DE a (%) | AUC0→36 h b (ng·h/mL) | Cmax b (ng/mL) | Tmax b (h) |
|---|---|---|---|---|
| Physical mixture | 2.8 ± 0.1 | 970.2 ± 122.5 | 40.2 ± 7.4 | 5.2 ± 1.1 |
| SMEDDS:Aerosil 200 = 1:1 | 54.8 ± 1.9 | 3817.6 ± 399.9 c | 193.3 ± 15.0 c | 4.2 ± 1.1 |
| SMEDDS:Aerosil 200:HPMC = 1:1:1 | 80.1 ± 2.1 | 4379.3 ± 420.8 cd | 222.7 ± 18.1 c−e | 4.0 ± 1.2 |
| SMEDDS:Aerosil 200:Soluplus = 1:1:1 | 90.1 ± 1.1 | 4832.5 ± 540.5 cd | 275.2 ± 26.9 c−f | 3.0 ± 0.7 |
| Commercial product | ND | 4488.9 ± 490.4 cd | 193.2 ± 10.9 c | 6.8 ± 3.0 |
3. Experimental Section
3.1. Materials
3.2. Effect of Hydrophilic Additives on Dutasteride Recrystallization in a Supersaturated Solution
3.3. Preparation of Drug-Loaded Solid-Supersaturatable SMEDDS
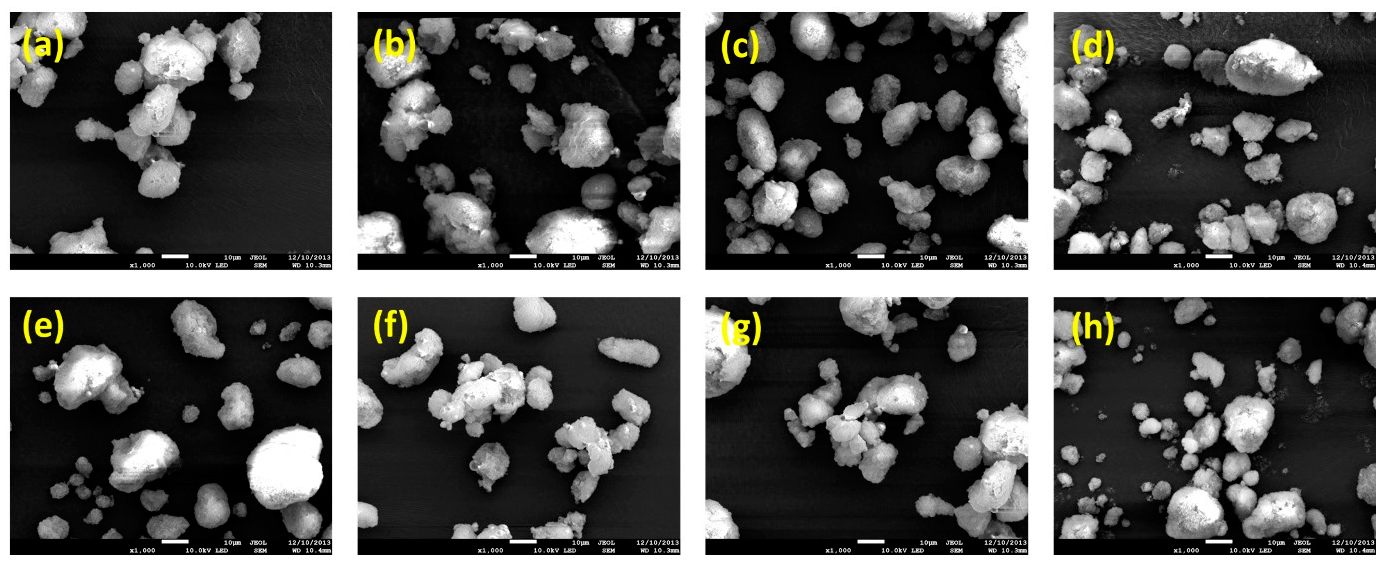
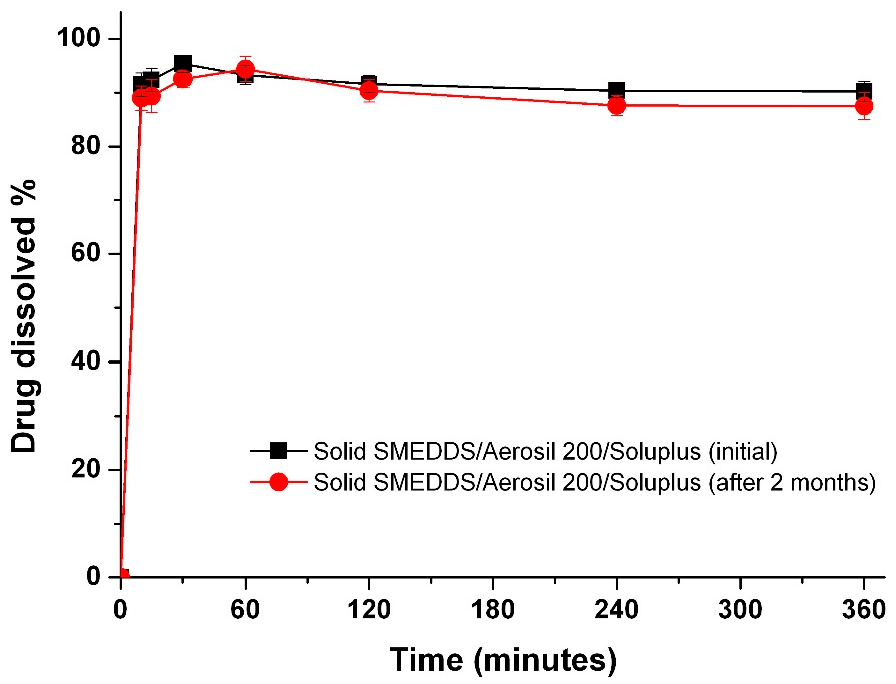
3.4. Characterization of Drug-Loaded Solid-Supersaturatable SMEDDS
3.5. Pharmacokinetic Study in Rats
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evans, H.C.; Goa, K.L. Dutasteride. Drugs Aging 2003, 20, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Baek, I.H.; Kim, M.S. Improved supersaturation and oral absorption of dutasteride by amorphous solid dispersions. Chem. Pharm. Bull. 2012, 60, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choo, G.H.; Hwang, S.J.; Kim, M.S. Quality by design: Screening of critical variables and formulation optimization of Eudragit E nanoparticles containing dutasteride. Arch. Pharm. Res. 2013, 36, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S. Soluplus-coated colloidal silica nanomatrix system for enhanced supersaturation and oral absorption of poorly water-soluble drugs. Artif. Cells Nanomed. Biotechnol. 2013, 41, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Choo, G.H.; Park, S.J.; Hwang, S.J.; Kim, M.S. Formulation and in vivo evaluation of a self-microemulsifying drug delivery system of dutasteride. Drug Res. 2013, 63, 203–209. [Google Scholar] [CrossRef]
- Kim, M.S. Evaluation of in vitro dissolution and in vivo oral absorption of dutasteride-loaded Eudragit E nanoparticles. Drug Res. 2013, 63, 326–330. [Google Scholar] [CrossRef]
- Kim, M.S. Influence of hydrophilic additives on the supersaturation and bioavailability of dutasteride-loaded hydroxypropyl-β-cyclodextrin nanostructures. Int. J. Nanomed. 2013, 8, 2029–2039. [Google Scholar] [CrossRef]
- Cho, W.; Kim, M.S.; Kim, J.S.; Park, J.; Park, H.J.; Cha, K.H.; Park, J.S.; Hwang, S.J. Optimized formulation of solid self-microemulsifying sirolimus delivery systems. Int. J. Nanomed. 2013, 8, 1673–1682. [Google Scholar]
- Trivedi, K.; Patel, P.V.; Pujara, Z. Development and characterization of liquid and solid self-emulsifying drug delivery system of fexofenadine. J. Pharm. Investig. 2013, 43, 385–394. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, E.; Verma, A.; Amin, S. In vitro characterization and pharmacodynamic evaluation of furosemide loaded self nano emulsifying drug delivery system (SNEDDS). J. Pharm. Investig. 2014, 44, 443–453. [Google Scholar] [CrossRef]
- Tang, B.; Cheng, G.; Gu, J.C.; Xu, C.H. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov. Today 2008, 13, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qin, J.; Ma, N.; Chou, X.; Wu, Z. Solid self-microemulsifying dispersible tablets of celastrol: Formulation development, charaterization and bioavailability evaluation. Int. J. Pharm. 2014, 472, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Krupa, A.; Jachowicz, R.; Kurek, M.; Figiel, W.; Kwiecień, M. Preparation of solid self-emulsifying drug delivery systems using magnesium aluminometasilicates and fluid-bed coating process. Powder Technol. 2014, 266, 329–339. [Google Scholar] [CrossRef]
- Parmar, K.; Patel, J.; Sheth, N. Fabrication and characterization of liquid compacts of Embelin for dissolution. J. Pharm. Investig. 2014, 44, 391–398. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.S.; Park, H.J.; Cho, W.K.; Cha, K.H.; Hwang, S.J. Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process. Int. J. Nanomed. 2011, 6, 2997–3009. [Google Scholar]
- Kim, M.S.; Kim, J.S.; Cho, W.; Park, H.J.; Hwang, S.J. Oral absorption of atorvastatin solid dispersion based on cellulose or pyrrolidone derivative polymers. Int. J. Biol. Macromol. 2013, 59, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ye, X.; Shang, X.; Peng, X.; Bao, Q.; Liu, M.; Guo, M.; Li, F. Enhanced oral bioavailability of silybin by a supersaturatable self-emulsifying drug delivery system (S-SEDDS). Colloids Surf. Physicochem. Eng. Asp. 2012, 396, 22–28. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Liu, Y.; Zhao, J.H.; Wang, L.; Feng, N.P. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int. J. Nanomed. 2012, 7, 1115–1125. [Google Scholar]
- Gao, P.; Rush, B.D.; Pfund, W.P.; Huang, T.; Bauer, J.M.; Morozowich, W.; Kuo, M.S.; Hageman, M.J. Development of a supersaturatable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J. Pharm. Sci. 2003, 92, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Akrami, A.; Alvarez, F.; Hu, J.; Li, L.; Ma, C.; Surapaneni, S. Characterization and optimization of AMG 517 supersaturatable self-emulsifying drug delivery system (S-SEDDS) for improved oral absorption. J. Pharm. Sci. 2009, 98, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Guyton, M.E.; Huang, T.; Bauer, J.M.; Stefanski, K.J.; Lu, Q. Enhanced oral bioavailability of a poorly water soluble drug PNU-91325 by supersaturatable formulations. Drug Dev. Ind. Pharm. 2004, 30, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.S.; Cho, W.; Cha, K.H.; Park, H.J.; Park, J.; Hwang, S.J. Supersaturatable formulations for the enhanced oral absorption of sirolimus. Int. J. Pharm. 2013, 445, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Priemel, P.A.; Laitinen, R.; Barthold, S.; Grohganz, H.; Lehto, V.P.; Rades, T.; Strachan, C.J. Inhibition of surface crystallisation of amorphous indomethacin particles in physical drug-polymer mixtures. Int. J. Pharm. 2013, 456, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xia, D.; Zhu, Q.; Zhu, C.; Chen, D.; Gan, Y. Supersaturated polymeric micelles for oral cyclosporine A delivery. Eur. J. Pharm. Biopharm. 2013, 85, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Park, J.H.; Yeom, D.W.; Ahn, B.K.; Lee, K.M.; Lee, S.G.; Woo, H.S.; Choi, Y.W. Enhanced dissolution of celecoxib by supersaturating self-emulsifying drug delivery system (S-SEDDS) formulation. Arch. Pharm. Res. 2013, 36, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.S.; Baek, I.H.; Cho, W.; Hwang, S.J.; Kim, M.S. Preparation and evaluation of solid dispersion of atorvastatin calcium with Soluplus® by spray drying technique. Chem. Pharm. Bull. 2014, 62, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Rhodes, C.T. Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm. Acta Helv. 1972, 47, 594–607. [Google Scholar] [PubMed]
- Song, W.H.; Yeom, D.W.; Lee, D.H.; Lee, K.M.; Yoo, H.J.; Chae, B.R.; Song, S.H.; Choi, Y.W. In situ intestinal permeability and in vivo oral bioavailability of celecoxib in supersaturating self-emulsifying drug delivery system. Arch. Pharm. Res. 2014, 37, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Baek, I.H. Fabrication and evaluation of valsartan polymer surfactant composite nanoparticles using supercritical antisolvent process. Int. J. Nanomed. 2014, 7, 5167–5176. [Google Scholar] [CrossRef]
- Ha, E.S.; Choo, G.H.; Baek, I.H.; Kim, J.S.; Cho, W.; Jung, Y.S.; Jin, S.E.; Hwang, S.J.; Kim, M.S. Dissolution and bioavailability of lercanidipine-hydroxypropylmethyl cellulose nanoparticles with surfactant. Int. J. Biol. Macromol. 2015, 72, 2188–222. [Google Scholar]
- Castile, J.D.; Taylor, K.M.; Buckton, G. The influence of incubation temperature and surfactant concentration on the interaction between dimyristoylphosphatidylcholine liposomes and poloxamer surfactants. Int. J. Pharm. 2001, 221, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.-S.; Choo, G.-H.; Baek, I.-H.; Kim, M.-S. Formulation, characterization, and in vivo evaluation of celecoxib-PVP solid dispersion nanoparticles using supercritical antisolvent process. Molecules 2014, 19, 20325–20339. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.B.; Benameur, H.; Porter, C.J.; Pouton, C.W. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: A mechanistic basis for utility. J. Drug Target 2010, 18, 704–731. [Google Scholar] [CrossRef] [PubMed]
- Overhoff, K.A.; McConville, J.T.; Yang, W.; Johnston, K.P.; Peters, J.I.; Williams, R.O., 3rd. Effect of stabilizer on the maximum degree and extent of supersaturation and oral absorption of tacrolimus made by ultra-rapid freezing. Pharm. Res. 2008, 25, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.K.; Jindal, P. Interaction of terbinafine hydrochloride with nicotinamide in solution and solid state. J. Pharm. Investig. 2014, 44, 23–32. [Google Scholar] [CrossRef]
- Kim, N.A.; Choi, D.H.; Lim, J.Y.; Kim, K.H.; Lim, D.G.; Lee, E.; Park, E.S.; Jeong, S.H. Investigation of polymeric excipients for dutasteride solid dispersion and its physicochemical characterization. Arch. Pharm. Res. 2014, 37, 214–224. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Ha, E.-S.; Choo, G.-H.; Baek, I.-H. Preparation and in Vivo Evaluation of a Dutasteride-Loaded Solid-Supersaturatable Self-Microemulsifying Drug Delivery System. Int. J. Mol. Sci. 2015, 16, 10821-10833. https://doi.org/10.3390/ijms160510821
Kim M-S, Ha E-S, Choo G-H, Baek I-H. Preparation and in Vivo Evaluation of a Dutasteride-Loaded Solid-Supersaturatable Self-Microemulsifying Drug Delivery System. International Journal of Molecular Sciences. 2015; 16(5):10821-10833. https://doi.org/10.3390/ijms160510821
Chicago/Turabian StyleKim, Min-Soo, Eun-Sol Ha, Gwang-Ho Choo, and In-Hwan Baek. 2015. "Preparation and in Vivo Evaluation of a Dutasteride-Loaded Solid-Supersaturatable Self-Microemulsifying Drug Delivery System" International Journal of Molecular Sciences 16, no. 5: 10821-10833. https://doi.org/10.3390/ijms160510821
APA StyleKim, M.-S., Ha, E.-S., Choo, G.-H., & Baek, I.-H. (2015). Preparation and in Vivo Evaluation of a Dutasteride-Loaded Solid-Supersaturatable Self-Microemulsifying Drug Delivery System. International Journal of Molecular Sciences, 16(5), 10821-10833. https://doi.org/10.3390/ijms160510821






