Histomorphometric Evaluation of the Small Coronary Arteries in Rats Exposed to Industrial Noise
Abstract
:1. Introduction
2. Results
2.1. Data in Totum
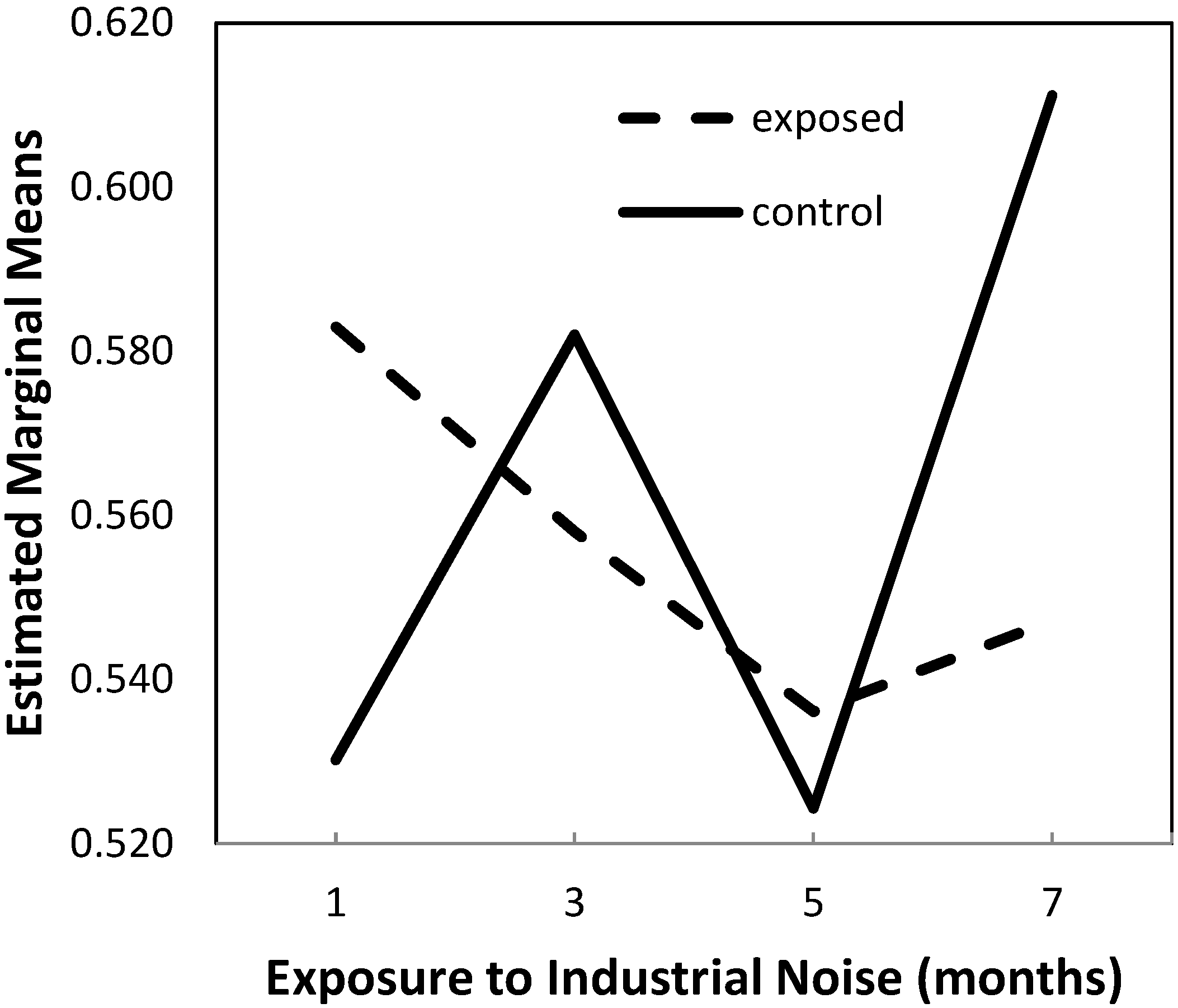
2.1.1. Lumen-to-Vessel Wall Ratio
| Group | Exposure Time (Months) | Mean | Standard Deviation | N |
|---|---|---|---|---|
| IN exposed (group A) | 1 | 0.5828933 | 0.03119288 | 5 |
| 3 | 0.5579890 | 0.02053823 | 5 | |
| 5 | 0.5361110 | 0.04120586 | 5 | |
| 7 | 0.5471695 | 0.04270947 | 5 | |
| Total | 0.5560407 | 0.03675745 | 20 | |
| Control (group B) | 1 | 0.5301545 | 0.04229613 | 5 |
| 3 | 0.5820185 | 0.06370201 | 5 | |
| 5 | 0.5242906 | 0.06810608 | 5 | |
| 7 | 0.6111611 | 0.03569630 | 5 | |
| Total | 0.5619062 | 0.06211472 | 20 |
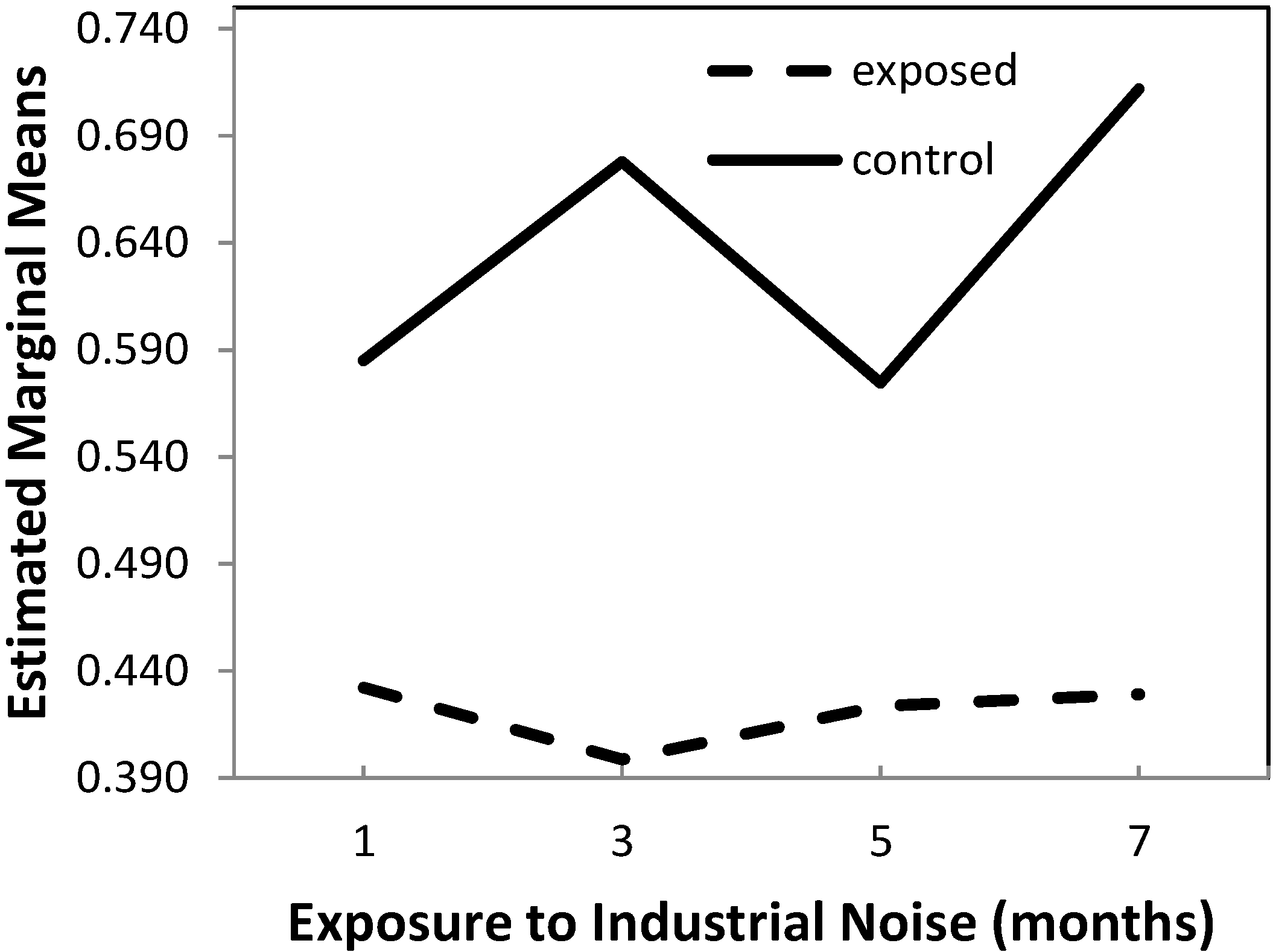
2.1.2. Wall-to-Perivascular Tissue Ratio
| Group | Exposure Time (Months) | Mean | Standard Deviation | N |
|---|---|---|---|---|
| IN exposed (group A) | 1 | 0.4322535 | 0.03050318 | 5 |
| 3 | 0.3986722 | 0.02655070 | 5 | |
| 5 | 0.4234890 | 0.05262512 | 5 | |
| 7 | 0.4292097 | 0.04201934 | 5 | |
| Total | 0.4209061 | 0.03850858 | 20 | |
| Control (group B) | 1 | 0.5849600 | 0.04896260 | 5 |
| 3 | 0.6778928 | 0.01880381 | 5 | |
| 5 | 0.5745753 | 0.07750708 | 5 | |
| 7 | 0.7117764 | 0.01747083 | 5 | |
| Total | 0.6373011 | 0.07454984 | 20 |
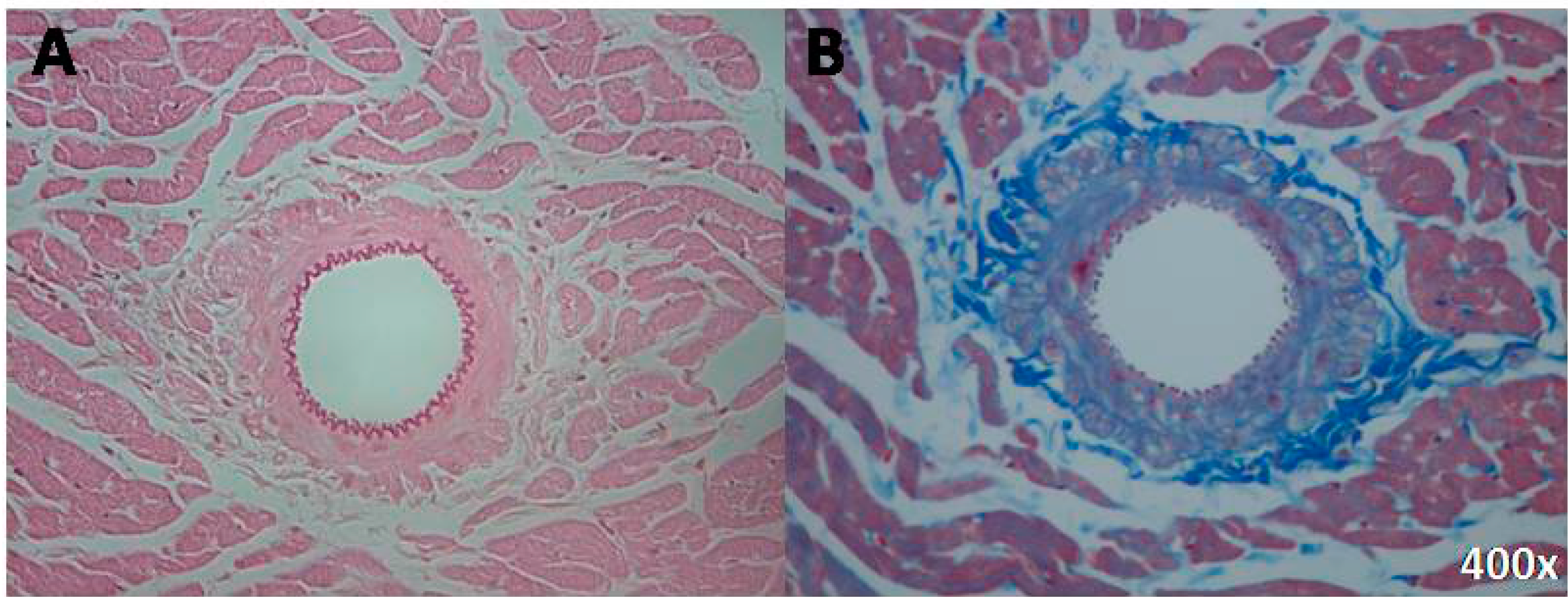
2.2. Data Per Anatomic Region
2.2.1. Lumen-to-Vessel Wall Ratio
2.2.2. Wall-to-Perivascular Tissue Ratio
Right Ventricle
Septum
Left Ventricle
3. Discussion
4. Experimental Section
Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Oliveira, M.J.R.; Monteiro, M.; Ribeiro, A.M.; Pignatelli, D.; Aguas, A.P. Chronic exposure of rats to occupational textile noise cause cytological changes in adrenal cortex. Noise Health 2009, 11, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Antunes, E.; Oliveira, P.; Borrecho, G.; Oliveira, M.J.R.; Brito, J.; Águas, A.; Martins dos Santos, J. Myocardial fibrosis in rats exposed to low-frequency noise. Acta Cardiol. 2013, 68, 241–245. [Google Scholar] [PubMed]
- Antunes, E.; Oliveira, P.; Oliveira, M.J.R.; Brito, J.; Águas, A.; Martins dos Santos, J. Histomorphometric evaluation of the coronary artery vessels in rats submitted to industrial noise. Acta Cardiol. 2013, 68, 285–289. [Google Scholar] [PubMed]
- Antunes, E.; Borrecho, G.; Oliveira, P.; Brito, J.; Águas, A.; Martins dos Santos, J. Immunohistochemical evaluation of cardiac connexin43 in rats exposed to low-frequency noise. Int. J. Clin. Exp. Pathol. 2013, 6, 1874–1879. [Google Scholar] [PubMed]
- Antunes, E.; Borrecho, G.; Oliveira, P.; Matos, A.; Brito, J.; Águas, A.; Martins dos Santos, J. Effects of low-frequency noise on cardiac collagen and cardiomyocyte ultrastructure: An immunohistochemical and electron microscopy study. Int. J. Clin. Exp. Pathol. 2013, 6, 2333–2341. [Google Scholar] [PubMed]
- Martins dos Santos, J.; Grande, N.R.; Castelo Branco, N.A.; Zagalo, C.; Oliveira, P. Vascular lesions and vibroacoustic disease. Eur. J. Anat. 2002, 6, 17–21. [Google Scholar]
- Oliveira, P.; Brito, J.; Mendes, J.; da Fonseca, J.; Águas, A.; Martins dos Santos, J. Effects of large pressure amplitude low frequency noise in the parotid gland perivascular-ductal connective tissue. Acta Med. Port. 2013, 26, 237–242. [Google Scholar] [PubMed]
- Babish, W. Transportation noise and cardiovascular risk: updated review and synthesis of epidemiological studies indicate that the evidence has increased. Noise Health 2009, 8, 1–29. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G.; Janicki, J.S. Myocardial fibrosis. Functional significance and regulatory factors. Cardiovasc. Res. 1993, 27, 13–31. [Google Scholar]
- Assyang, P.; Carré, F.; Chevalier, B.; Delcayre, C.; Mansier, P.; Swynghedauw, B. Compensated cardiac hypertrophy: Arrhythmogenicity and the new myocardial phenotype I. Fibrosis. Cardiovasc. Res. 1997, 34, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Yekkala, K.; Borg, T.K.; Baudino, T.A. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann. N. Y. Acad. Sci. 2006, 1080, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Alves-Pereira, M.; Castelo Branco, N.A.A. Vibroacoustic disease: Biological effects of infrasound and low-frequency noise explained by mechanotransduction cellular signaling. Prog. Biophys. Mol. Biol. 2007, 93, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Diez, J. Mechanisms of cardiac fibrosis in hypertension. J. Clin. Hypertens. 2007, 9, 546–550. [Google Scholar] [CrossRef]
- Rakusan, K.; Wicker, P. Morphometry of the small arteries and arterioles in the rat heart: Effects of chronic hypertension and exercise. Cardiovasc. Res. 1990, 24, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Torry, R.J.; Baumbach, G.L.; Tomanek, R.J. Proportional arteriolar growth accompanies cardiac hypertrophy induced by volume overload. Am. J. Physiol. 1994, 267, 2132–2137. [Google Scholar]
- Khazaei, M.; Fallahzadeh, A.R.; Sharifi, M.R.; Afsharmoghaddam, N.; Javanmard, S.H.; Salehi, E. Effects of diabetes on myocardial capillary density and serum angiogenesis biomarkers in male rats. Clinics 2011, 66, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Komaru, T.; Kanatsuka, H.; Shirato, K. Coronary microcirculation: Physiology and pharmacology. Pharmacol. Ther. 2000, 86, 217–261. [Google Scholar] [CrossRef] [PubMed]
- Amann, K.; Neususs, R.; Ritz, E.; Irzyniec, T.; Wiest, G.; Mall, G. Changes of vascular architecture independent of blood pressure in experimental uremia. Am. J. Hypertens. 1995, 8, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Amann, K.; Wiest, G.; Zimmer, G.; Gretz, N.; Ritz, E.; Mall, G. Reduced capillary density in the myocardium of uremic rats—A stereological study. Kidney Int. 1992, 42, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Mall, G.; Rambausek, M.; Neumeister, A.; Kollmar, S.; Vetterlein, F.; Ritz, E. Myocardial interstitial fibrosis in experimental uremia—Implications for cardiac compliance. Kidney Int. 1988, 33, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Amann, K.; Ritz, E. Reduced cardiac ischaemia tolerance in uraemia—What is the role of structural abnormalities of the heart? Nephrol. Dial. Transplant. 1996, 11, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lousinha, A.; Antunes, E.; Borrecho, G.; Oliveira, M.J.; Brito, J.; Santos, J.M.d. Histomorphometric Evaluation of the Small Coronary Arteries in Rats Exposed to Industrial Noise. Int. J. Mol. Sci. 2015, 16, 10095-10104. https://doi.org/10.3390/ijms160510095
Lousinha A, Antunes E, Borrecho G, Oliveira MJ, Brito J, Santos JMd. Histomorphometric Evaluation of the Small Coronary Arteries in Rats Exposed to Industrial Noise. International Journal of Molecular Sciences. 2015; 16(5):10095-10104. https://doi.org/10.3390/ijms160510095
Chicago/Turabian StyleLousinha, Ana, Eduardo Antunes, Gonçalo Borrecho, Maria João Oliveira, José Brito, and José Martins dos Santos. 2015. "Histomorphometric Evaluation of the Small Coronary Arteries in Rats Exposed to Industrial Noise" International Journal of Molecular Sciences 16, no. 5: 10095-10104. https://doi.org/10.3390/ijms160510095






