A New and Practical Synthetic Method for the Synthesis of 6-O-Methyl-scutellarein: One Metabolite of Scutellarin in Vivo
Abstract
:1. Introduction
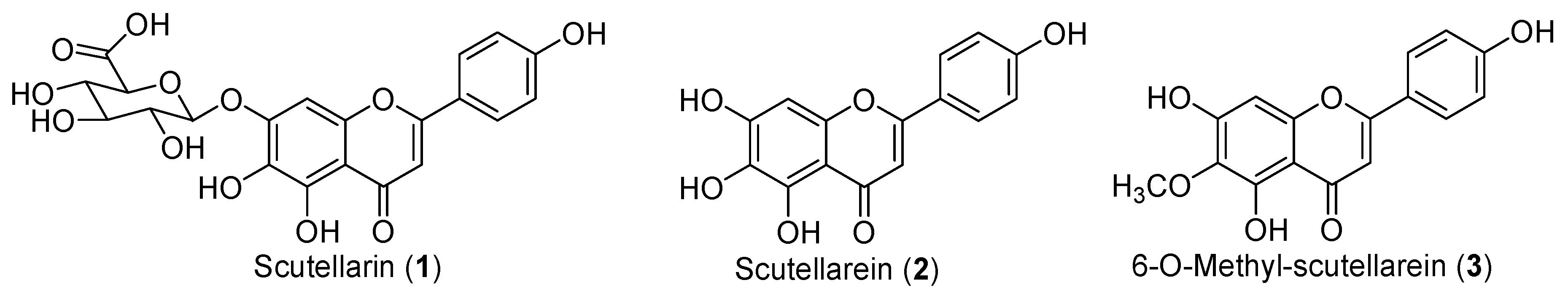
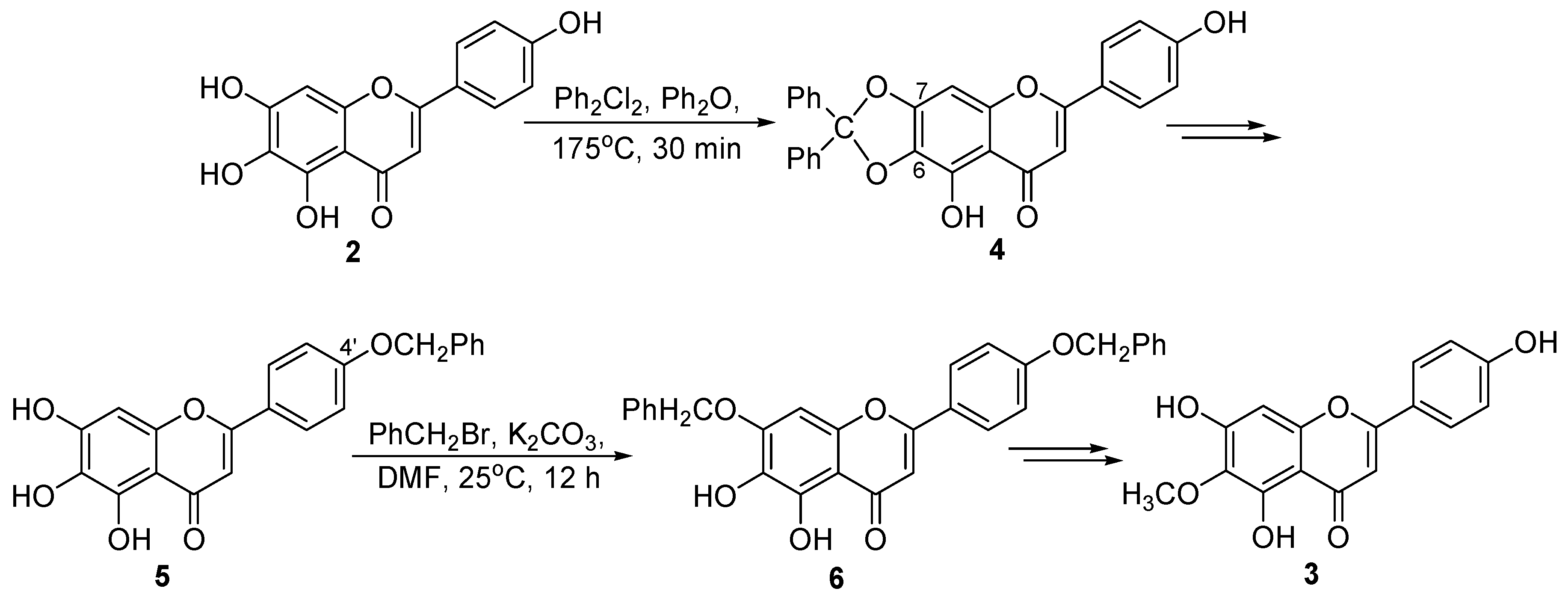
2. Results and Discussion
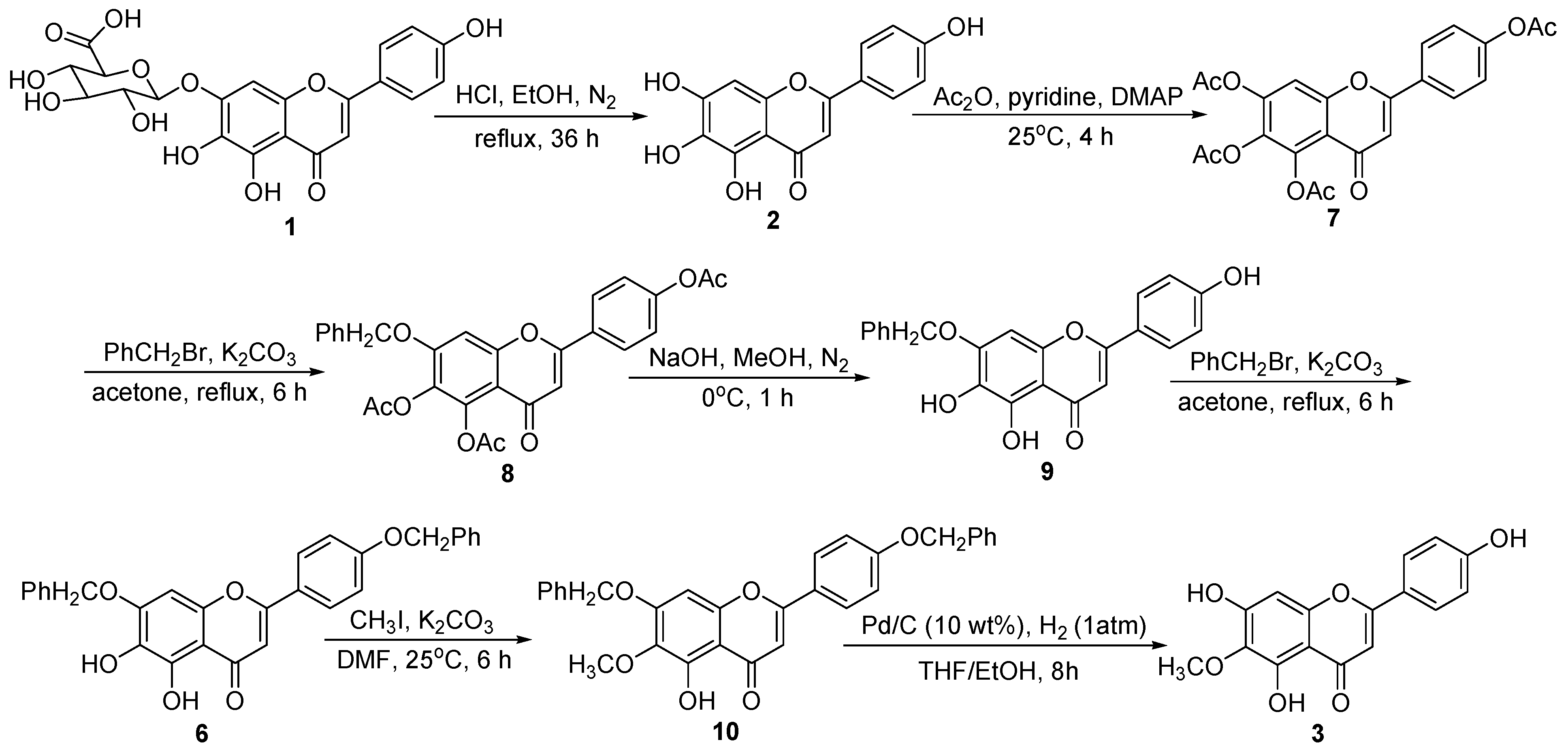
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.W.; Feng, T.M.; Shan, L.C.; Cai, B.Z.; Chu, W.F.; Niu, H.L.; Lu, Y.J.; Yang, B.F. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother. Res. 2008, 22, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; He, W.; Lu, W.H.; Zeng, F.D. Effects of scutellarin on liver function after brain ischemia/reperfusion in rats. Acta Pharmacol. Sin. 2003, 24, 1118–1124. [Google Scholar] [PubMed]
- Hong, H.; Liu, G.Q. Scutellarin attenuates oxidative glutamate toxicity in PC12 cells. Planta Med. 2004, 70, 427–431. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Tang, R.; Liu, J.; Xu, H. Effect of scutellarin on nitric oxide production in early stages of neuron damage induced by hydrogen peroxide. Pharmacol. Res. 2005, 51, 205–210. [Google Scholar]
- Hao, X.; Cheng, G.; Yu, J.E.; He, Y.; An, F.; Sun, J.; Cui, F. Study on the role of hepatic first-pass elimination in the low oral bioavailability of scutellarin in rats. Pharmazie 2005, 60, 477–478. [Google Scholar] [PubMed]
- Huang, J.M.; Weng, W.Y.; Huang, X.B.; Ji, Y.H.; Chen, E. Pharmacokinetics of scutellarin and its aglycone conjugated metabolites in rats. Eur. J. Drug Metab. Pharmacokinet. 2005, 30, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Qiu, F.; Zhu, S.; Zhang, T.; Qu, G.; Yao, X. Isolation and identification of ten metabolites of breviscapine in rat urine. Biol. Pharm. Bull. 2007, 30, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, X.; Zhong, D. Absorption and disposition of scutellarin in rats: A pharmacokinetic explanation for the high exposure of its isomeric metabolite. Drug Metab. Dispos. 2011, 39, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.H.; Li, S.H.; Lan, K.; Yang, J.Y.; Zhou, J. Study on the pharmacokinetics of scutellarin in dogs. Yao Xue Xue Bao 2003, 38, 371–373. [Google Scholar] [PubMed]
- Chen, X.; Cui, L.; Duan, X.; Ma, B.; Zhong, D. Pharmacokinetics and metabolism of the flavonoid scutellarin in humans after a single oral administration. Drug Metab. Dispos. 2006, 34, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.H.; Zhou, Z.; Zhi, X.J.; Ma, L.L.; Chen, X.H. Pharmacokinetics and absolute bioavailability of breviscapine in beagle dogs. Chin. J. Pharm. 2003, 34, 618–620. [Google Scholar]
- Huang, J.; Li, N.; Yu, Y.; Weng, W.; Huang, X. Determination of aglycone conjugated metabolites of scutellarin in rat plasma by HPLC. J. Pharm. Biomed. Anal. 2006, 40, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-Z.; Shi, Z.-H.; Li, N.-G.; Tang, H.; Shi, Q.-P.; Tang, Y.-P.; Yang, J.-P.; Duan, J.-A. Efficient synthesis of 6-O-methyl-scutellarein from scutellarin via selective methylation. Lett. Org. Chem. 2013, 10, 733–737. [Google Scholar] [CrossRef]
- Li, N.-G.; Song, S.-L.; Shen, M.-Z.; Tang, Y.-P.; Shi, Z.-H.; Tang, H.; Shi, Q.-P.; Fu, Y.-F.; Duan, J.-A. Mannich bases of scutellarein as thrombin-inhibitors: Design, synthesis, biological activity and solubility. Bioorg. Med. Chem. 2012, 20, 6919–6923. [Google Scholar] [CrossRef] [PubMed]
- Li, N.-G.; Shen, M.-Z.; Wang, Z.-J.; Tang, Y.-P.; Shi, Z.-H.; Fu, Y.-F.; Shi, Q.-P.; Tang, H.; Duan, J.-A. Design, synthesis and biological evaluation of glucose-containing scutellarein derivatives as neuroprotective agents based on metabolic mechanism of scutellarin in vivo. Bioorg. Med. Chem. Lett. 2013, 23, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Li, N.-G.; Wang, J.-X.; Liu, X.-R.; Lin, C.-J.; You, Q.-D.; Guo, Q.-L. A novel and efficient route to the construction of the 4-oxa-tricyclo[4.3.1.0]decan-2-one scaffold. Tetrahedron Lett. 2007, 48, 6586–6589. [Google Scholar] [CrossRef]
- Tisdale, E.J.; Slobodov, I.; Theodorakis, E.A. Unified synthesis of caged Garcinia natural products based on a site-selective Claisen/Diels-Alder/Claisen rearrangement. Proc. Natl. Acad. Sci. USA 2004, 101, 12030–12035. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-H.; Li, N.-G.; Tang, Y.-P.; Li, W.; Yin, L.; Yang, J.-P.; Tang, H.; Duan, J.-A. Metabolism-based synthesis, biologic evaluation and SARs analysis of O-methylated analogs of quercetin as thrombin inhibitors. Eur. J. Med. Chem. 2012, 54, 210–222. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zhang, W.; Dong, Z.-X.; Gu, T.; Li, N.-G.; Shi, Z.-H.; Kai, J.; Qu, C.; Shang, G.-X.; Tang, Y.-P.; et al. A New and Practical Synthetic Method for the Synthesis of 6-O-Methyl-scutellarein: One Metabolite of Scutellarin in Vivo. Int. J. Mol. Sci. 2015, 16, 7587-7594. https://doi.org/10.3390/ijms16047587
Lin H, Zhang W, Dong Z-X, Gu T, Li N-G, Shi Z-H, Kai J, Qu C, Shang G-X, Tang Y-P, et al. A New and Practical Synthetic Method for the Synthesis of 6-O-Methyl-scutellarein: One Metabolite of Scutellarin in Vivo. International Journal of Molecular Sciences. 2015; 16(4):7587-7594. https://doi.org/10.3390/ijms16047587
Chicago/Turabian StyleLin, Hang, Wei Zhang, Ze-Xi Dong, Ting Gu, Nian-Guang Li, Zhi-Hao Shi, Jun Kai, Cheng Qu, Guan-Xiong Shang, Yu-Ping Tang, and et al. 2015. "A New and Practical Synthetic Method for the Synthesis of 6-O-Methyl-scutellarein: One Metabolite of Scutellarin in Vivo" International Journal of Molecular Sciences 16, no. 4: 7587-7594. https://doi.org/10.3390/ijms16047587




